Abstract
Previous studies have demonstrated the significance of signaling through the CXCR2 receptor in the process of recovery and regeneration of functional liver mass after hepatic ischemia/reperfusion (I/R). CXCR2 is constitutively expressed on both neutrophils and hepatocytes, however, the cell-specific roles of this receptor is unknown. In the present study, chimeric mice were created through bone marrow transplantation (BMT) using wild-type and CXCR2-knockout mice, yielding selective expression of CXCR2 on hepatocytes (Hep) and/or myeloid cells (My) in the following combinations: Hep+/My+; Hep−/My+; Hep+/My−; Hep−/My−. These tools allowed us to assess the contributions of myeloid and hepatocyte CXCR2 in the recovery of the liver after I/R injury. Flow cytometry confirmed adoption of the donor phenotype in neutrophils. Interestingly, Kupffer cells from all chimeras lacked CXCR2 expression. Recovery/regeneration of hepatic parenchyma was assessed by histologic assessment and measurement of hepatocyte proliferation. CXCR2Hep+/My+ mice showed the least amount of liver recovery and hepatocyte proliferation, while CXCR2Hep−/My− mice had the greatest liver recovery and hepatocyte proliferation. CXCR2Hep+/My− mice had enhanced liver recovery with hepatocyte proliferation similar to CXCR2Hep−/My− mice. Myeloid expression of CXCR2 directly regulated CXC chemokine expression levels following hepatic I/R, such that mice lacking myeloid CXCR2 had markedly increased chemokine expression compared to mice expressing CXCR2 on myeloid cells.
Conclusion
The data suggest that CXCR2 on myeloid cells is the predominant regulator of liver recovery and regeneration after I/R injury, while hepatocyte CXCR2 plays a minor, secondary role. These findings suggest that myeloid cell-directed therapy may significantly impact liver regeneration after liver resection or transplantation.
Keywords: chemokines, neutrophils, hepatocytes, hepatic regeneration, bone marrow transplant
Hepatic ischemia/reperfusion (I/R) injury is a cause of significant morbidity and mortality after liver transplantation, hemorrhagic shock, and extended liver resection for cancer (1–4). I/R injury is a biphasic response comprised of an acute injury phase resulting from oxidative stress and a later phase resulting from an intense inflammatory response that culminates in the hepatic recruitment of neutrophils and subsequent neutrophil-dependent parenchymal injury (5–10). An emerging interest has been focused on the processes of liver recovery, repair, and regeneration after I/R injury, which are highly relevant to transplantation and shock.
Our laboratory has previously characterized the recovery, repair and regenerative responses after liver I/R injury in a murine model. The reparative process spans several days and involves the clearance of dead tissue and the replacement of functional liver mass via hepatocyte proliferation (11–13). Interestingly, we recently discovered that CXC chemokines that contain the amino-terminal ELR motif play an important role in recovery, repair and regeneration of the liver after I/R (12–14). These same chemokines are potent neutrophil chemoattractants and have been shown by us and others to contribute significantly to the inflammatory response induced by liver I/R (15, 16). CXC chemokines bind to the receptors CXCR1 and CXCR2, which are expressed on a variety of cell types, including myeloid cells, endothelial cells, and hepatocytes (17, 18). In neutrophils, these receptors are responsible for cell-priming, chemotaxis, and induction of respiratory burst (5, 19, 20). In hepatocytes, we have shown that these receptors can regulate cell proliferation and cell death (12, 13). Our studies with knockout mice for CXCR1 or CXCR2 demonstrate that signaling through CXCR2 is a major regulatory component of the reparative and regenerative response, whereas CXCR1 appears to play a minor counterbalancing role (12, 13).
Despite our current understanding of the importance of signaling through CXCR2 during the recovery response to hepatic I/R, the cell-specific roles for CXCR2 in this response are unknown. Therefore, in the current study, we employed bone marrow transplantation to create chimeric mice to allow us to examine the functional roles of hepatocyte versus myeloid expression of CXCR2 in the reparative and regenerative response after I/R injury.
MATERIALS AND METHODS
Generation of Chimeric CXCR2 Mice
This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines. Male BALB/c and CXCR2−/− (BALB/c background) were obtained from The Jackson Laboratory (Bar Harbor, ME). Chimeric mice were generated using bone marrow transplantation (BMT). For all experiments, mice were exposed to whole body irradiation at 6–8 weeks of age. Mice received 9.5Gy γradiation, given as a split dose of 4.75Gy and 4.75Gy, separated by three hours. Immediately thereafter, 10 × 106 cells of wild-type or CXCR2−/− bone marrow was injected via the tail vein. These experiments generated chimeric mice with hepatocytes (Hep) and myeloid cells (My) either positive (wild-type) or negative (CXCR2−/−) for CXCR2 in the following combinations: Hep+/My+; Hep−/My+; Hep+/My−; Hep−/My−. The mice were then kept in a pathogen free environment with ad libitum access to food and water and a twelve-hour light-dark cycle for a total of four weeks before undergoing procedures.
Hepatic Ischemia/Reperfusion Model
Partial hepatic ischemia was induced as previously described (15). All mice underwent either sham or I/R operation. Briefly, mice were anesthetized using sodium pentobarbital (65mg/kg). A midline laparotomy was performed and an atraumatic vascular occlusion clip was used to interrupt blood supply to the left lateral and median lobes of the liver. The caudal lobes retained intact portal and arterial inflow and venous outflow, preventing intestinal venous congestion. After 90 minutes of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Sham control mice underwent the same protocol without vascular occlusion. Mice were sacrificed after the indicated periods of reperfusion, and blood and liver samples were taken for analysis.
Blood and Tissue Analysis
Blood was obtained via intracardiac puncture at the time of sacrifice. Serum was then analyzed for alanine aminotransferase (ALT) as a measure of hepatocellular injury. Measurements of serum ALT were made using a diagnosis kit via bioassay (Wiener Laboratories, Rosario, Argentina). Serum levels of TNFα, keratinocyte chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) were measured by enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Hepatic tissue was harvested at time of sacrifice for measurement of neutrophil accumulation and histologic analysis. Liver neutrophil accumulation was determined via myeloperoxidase (MPO) content. MPO content was assessed using methods described elsewhere (21). For histologic analysis, tissue samples were fixed in 10% neutral-buffered formalin (Richard Allen Scientific, Kalamazoo, MI), processed, and embedded in paraffin prior to staining with hematoxylin-eosin. Quantitative morphometric analysis of hepatocellular necrosis was performed in a blinded fashion with histologic sections at low power (10X) using Photoshop image analysis software (Adobe Systems, Inc., San Jose, CA). Necrotic area was expressed as percentage of total area examined.
Proliferating Cell Nuclear Antigen Staining
Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) was performed on paraffin-embedded liver tissue with anti-PCNA antibody using a Dako ARK Peroxidase kit (Dako, Copenhagen, Denmark). Briefly, a three-step peroxidase method was performed according to the manufacturer’s instructions. PC-10 monoclonal antibody (Santa Cruz Biotechnology) was used at a dilution of 1:50 for 15 minutes at room temperature. The sections were counterstained with hematoxylin. Evaluation of PC-10 immunostaining was performed based on the percentage of positive nuclei of 400–600 hepatocytes from 4–6 highest positive fields at high power (400X), and was expressed as PCNA labeling index.
Isolation and flow cytometric analysis of neutrophils and Kupffer cells
Analysis of blood leukocytes was conducted similarly as described (22). 50–100uL of whole blood was added to glass tubes with 4mL ACK lysing buffer and mixed by inversion. Samples were incubated at room temperature for 5 minutes and then spun down for 5 minutes at 400g. Supernatant was decanted and 0.5mL FACS buffer was used as a wash, followed by centrifugation at 400g × 5 minutes. Cells (0.5–1.5 × 106/tube) were then placed in polystyrene tubes containing 1μg anti-CD16/32 and 5% rat serum to block nonspecific antibody labeling. Alexa Fluor-700 rat anti-mouse CD11b (Clone: M1/70, BD Biosciences, San Diego, CA), FITC rat anti-mouse Ly6-G (Clone: 1A8, BD Biosciences, San Diego, CA), and Allophycocyanin Rat IgG2A anti-mouse CXCR2 (Lot: LMC0711011, R&D, Minneapolis, MN) or Allophycocyanin conjugated rat anti-mouse IgG2A Isotype Control (Lot: KEU1111011 R&D, Minneapolis, MN) were used as antibodies. Labeling antibodies (0.5–1μg antibody/tube) were added and incubated at 4°C for 20 min. Cells were washed two times with cold-fluorescence activated cell sorter buffer (PBS with 1% BSA and 0.1% azide), and fixed with 250μL of 1% paraformaldehyde. Samples were analyzed with an LSR II flow cytometer and FACS Diva software (BD Biosciences, Mountain View, CA).
For the isolation of Kupffer cells, chimeric mice were anesthetized with sodium pentobarbital (65mg/kg) and a midline laparotomy was performed. The portal vein was cannulated using a 22G angiogath (Abbocath-T, Hospira Venisystems, Lake Forest, IL), and the liver was flushed with 10mL of phos-buffered saline (PBS). Liver leukocytes were isolated using a gentleMACS™ dissociator (Miltenyi Biotec, Auburn, CA), and the instructions provided by the manufacturer. The isolated cells were resuspended in 7mL HBSS and carefully layered on 7mL Lympholyte M (Cedarlane Laboratories, Hornby, Ontario, Canada). Tubes were spun at 1500g for 20 minutes at 25°C. The cell layer was collected and placed into FACS tubes with HBSS and spun down at 450g for 5 min at room temperature. Analysis of cell surface antigen expression in situ was performed as described elsewhere (23). Phycoerythrin-conjugated anti-CD68 (Clone: FA-11, Biolegend, San Diego, CA), Pacific Blue anti-mouse F4/80 (Clone: BM8, Biolegend, San Diego, CA), Alexa Fluor-700 Rat anti-mouse CD11b (Clone: M1/70, BD Biosciences, San Diego, CA), FITC rat anti-mouse Ly-6G (Clone: 1A8, BD Biosciences, San Diego, CA), and Allophycocyanin Rat IgG2A anti-mouse CXCR2 (Lot: LMC0711011, R&D, Minneapolis, MN) or Allophycocyanin conjugated rat anti-mouse IgG2A Isotype Control (Lot: KEU1111011 R&D, Minneapolis, MN) were purchased for cell staining. Cells (0.5–1.5 × 106/tube) were then placed in polystyrene tubes containing 1μg anti-CD16/32 and 5% rat serum to block nonspecific antibody labeling. Labeling antibodies (0.5–1μg antibody/tube) were then added and incubated at 4°C for 20 min. Cells were washed two times with cold-fluorescence activated cell sorter buffer (PBS with 1% BSA and 0.1% azide) and fixed with 250μL of 1% paraformaldehyde. Samples were analyzed with an LSR II flow cytometer and FACS Diva software (BD Biosciences, Mountain View, CA). For some experiments, isolated Kupffer cells were plated and treated with TNFα (10 ng/ml) or LPS (100 ng/ml) for 30 minutes prior to staining and FACS analysis for CXCR2 expression.
Statistical Analysis
All data are expressed as the mean ± standard error of the mean (SEM). Data were analyzed with a one-way analysis of variance with subsequent Student’s t-test. Differences were considered significant when P < 0.05.
RESULTS
Bone marrow transplantation effectively modulates neutrophil expression of CXCR2, but is associated with significant pathology in CXCR2−/− mice
The role of neutrophils in the pathogenesis of hepatic I/R injury is well known (5, 19). We have shown that CXCR2 is important for acute neutrophil recruitment, and that mice nullizygous for CXCR2 have a lesser degree of hepatic injury acutely after I/R than wild-type mice (12). CXCR2 chimeric mice (CXCR2Hep+/My+, CXCR2Hep−/My+, CXCR2Hep−/My−, CXCR2Hep+/My−) were generated as described in the Experimental Procedures. Mice receiving bone marrow from wild-type mice (CXCR2Hep+/My+, CXCR2Hep−/My+) retained expression of CXCR2 on neutrophils, whereas mice receiving bone marrow from CXCR2−/− mice (CXCR2Hep−/My−, CXCR2Hep+/My−) lacked neutrophil CXCR2 expression (Figure 1A). Expression of CXCR2 on Kupffer cells has not been previously studied. Examination of liver Kupffer cells from all chimeric phenotypes as well as normal wild-type (BALB/c) and CXCR2 −/− mice demonstrated no expression of CXCR2 (Figure 1B). In order to determine if CXCR2 expression might be inducible, Kupffer cells were isolated and stimulated with either LPS 100ng/mL or TNFα 10ng/mL for 30 minutes. Surface expression of CXCR2 was determined by flow cytometry. Stimulation of Kupffer cells did not induce the expression of CXCR2 (Supplemental Figure 1).
Figure 1.
CXCR2 expression on (A) neutrophils and (B) Kupffer cells in chimeric mice. Expression of CXCR2 was analyzed using flow cytometry. An isotype control was used for all experiments. Representative panels from wild-type (WT) and CXCR2-knockout (KO) mice are shown. (A) CXCR2 expression on neutrophils from WT mice and absence of expression in KO mice was confirmed. Neutrophils from chimeric mice receiving WT bone marrow (Hep−/My+) had positive staining for CXCR2, whereas mice receiving KO bone marrow (Hep+/My−) did not. (B) Kupffer cells lacked CXCR2 expression regardless of genotype. There was an absence of CXCR2 expression on both WT and KO mice, and none of the Kupffer cells from chimeric mice (Hep−/My+ and Hep+/My−) expressed CXCR2. All panels are representative of three or more independent experiments.
While bone marrow transplantation yielded the appropriate myeloid and hepatocyte expression phenotypes, some chimeric mice displayed pathologies that precluded their use in I/R experiments. Survival of the different chimeric mice after bone marrow transplantation was not significantly different amongst the different groups (Figure 2A). At the time of laparotomy for hepatic I/R, a number of pathologies were noted in the different chimeras that prevented their use in experiments (Figure 2B). These pathologies and their frequencies are shown in Table 1. A low frequency of hepatic lesion and infection was observed in CXCR2Hep+/My+, CXCR2Hep−/My+, and CXCR2Hep+/My− (Table 1). In contrast, CXCR2Hep−/My− mice had a high frequency of splenomegaly and hepatic lesions that limited their use in I/R experiments (Table 1). Splenomegaly was observed in 24% of Hep−/My− mice (Figure 3A). In those animals, closer examination of splenic histology revealed extensive hematopoiesis (Figure 3B). Hepatic lesions were observed in 30% of Hep−/My− mice. These lesions were found to be focal areas of organizing necrosis (Figure 3C). The incidence and frequency of splenomegaly and hepatic lesions we observed in CXCR2Hep−/My− mice are consistent with the CXCR2-knockout phenotype (24).
Figure 2.
Survival and experimental viability of chimeric mice. (A) Survival following bone marrow transplantation for all phenotypes. (B) Due to pathologic findings at the time of laparotomy, including massive splenomegaly and hepatic lesions, there were significantly fewer Hep−/My− chimeric mice available to use for experimentation. Data are mean ± SEM with n=33–46 per group. *P<0.05 compared to control.
Table 1.
Pathologic findings at time of laparotomy in chimeric mice
| Phenotype | Pathology |
|---|---|
| Hep+/My+ (n=41) | None |
| Hep−/My+ (n=46) | Ventral neck lesion, n=1 (0.02%) |
| Hepatic lesion, n=1 (0.02%) | |
| Hep+/My− (n=36) | Abdominal wall infection, n=1 (0.03%) |
| Hep−/My− (n=33) | Massive splenomegaly, n=8 (24%) |
| Hepatic lesion, n=10 (30%) |
Figure 3.
Massive splenomegaly and hepatic lesions in CXCR2Hep−/My− chimeric mice. Hep−/My− chimeric mice had a higher frequency of pathologic findings including massive splenomegaly (A). Histologic cross sections of mice with massive splenomegaly revealed large areas of extensive hematopoiesis (H&E, 10X) (B). In addition, hepatic lesions were frequently noted, which revealed large areas of organizing necrosis on histologic examination (H&E, 10X) (C).
Liver injury and recovery after I/R is impacted by both myeloid and hepatocyte CXCR2
Our previous studies using global knockout mice for CXCR2 suggested that CXCR2 expression on hepatocytes is detrimental to liver recovery after I/R (12). However, these studies were unable to directly assess the function of CXCR2 on different cell types in vivo. In vitro studies demonstrated that hepatocytes exposed to high concentrations of CXC chemokines had increased cytotoxicity (12). Using chimeric mice, we assessed the impact of cell-specific CXCR2 expression on the recovery response after I/R. Seven days after I/R, we examined liver histology, necrotic area, and serum ALT as indices of remaining liver injury and extent of repair/recovery. In CXCR2Hep+/My+ mice, necrotic liver was prominent, with little recovery observed (Figure 4). These mice also had the highest level of circulating ALT (Figure 4). CXCR2Hep−/My+ had a similar degree of liver necrosis and similar ALT levels as CXCR2Hep+/My+ mice (Figure 4). In contrast, CXCR2Hep+/My− mice had reduced hepatocellular necrosis compared to CXCR2Hep+/My+ mice, and had significantly lower ALT levels than both CXCR2Hep+/My+ and CXCR2Hep−/My+ mice (Figure 4). CXCR2Hep−/My− mice had markedly less hepatocellular necrosis than all other chimeric groups and had ALT levels that were significantly lower than both CXCR2Hep+/My+ and CXCR2Hep−/My+ mice (Figure 4).
Figure 4.
Liver injury in chimeric mice after hepatic I/R. Hep−/My− mice displayed the least amount of liver injury as determined by histology, quantitative necrosis, and serum ALT values. Hep−/My+ and Hep+/My− chimeric mice showed intermediate degrees of injury. Data are mean ± SEM with n=3–6 per group. *P<0.05 compared to Hep+/My+ and Hep−/My+ mice; **P<0.05 compared to all other groups.
Myeloid, but not hepatocyte, CXCR2 negatively regulates expression of CXC chemokines and is critical for hepatocyte proliferation after I/R
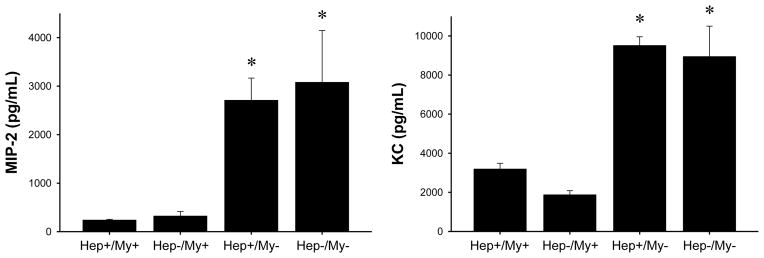
Our previous studies have demonstrated that CXCR2-knockout mice have greatly increased expression of CXC chemokines (12). These studies also showed that CXCR2 mediated proliferative effects of CXC chemokines on hepatocytes in vitro. However, subsequent experiments demonstrated that CXC chemokines are not direct mitogens for hepatocytes and that the proliferative effects of CXC chemokines required factors in the media serum supplement (25). In order to determine the roles of myeloid and/or hepatocyte CXCR2 in CXC chemokine expression and hepatocyte proliferation in vivo, we examined chemokine expression and hepatocyte proliferation in CXCR2 chimeric mice. Because peak chemokine production after I/R injury occurs 8 hours after reperfusion, we used this time point for analysis of MIP-2 and KC. CXCR2Hep+/My+ and CXCRHep−/My+ mice had similar levels of MIP-2 and KC (Figure 5). Furthermore, the levels of MIP-2 and KC were consistent with expression levels previously observed in wild-type mice (12). Interestingly, expression of MIP-2 and KC was increased by up to 15-fold in both CXCR2Hep+/My− and CXCR2Hep−/My− mice (Figure 5), which was similar to the increase previously reported in CXCR2-knockout mice (12).
Figure 5.
CXC chemokine expression in chimeric mice. Serum concentrations of the CXC chemokines MIP-2 and KC were measured after 90 minutes of hepatic ischemia and 8 hours of reperfusion by ELISA. Data are mean ± SEM with n=3–9 per group. *P<0.05 compared to Hep+/My+ and Hep−/My+ mice.
We next evaluated the hepatic regenerative response after I/R using PCNA staining as an index of hepatocyte proliferation. CXCR2Hep+/My+ and CXCRHep−/My+ mice had low numbers of PCNA-positive hepatocytes, whereas CXCR2Hep+/My− and CXCR2Hep−/My− mice had far higher numbers of PCNA-positive hepatocytes (Figure 6). When numbers of PCNA-positive hepatocytes were quantified, CXCR2Hep+/My− and CXCR2Hep−/My− mice demonstrated up to 4-fold increases in proliferation compared to CXCR2Hep+/My+ and CXCRHep−/My+ mice (Figure 6).
Figure 6.
Hepatocyte proliferation and regeneration after hepatic I/R in chimeric mice. Hepatocyte proliferation was determined by immunohistochemical staining for PCNA in liver sections obtained after ischemia and 7 days of reperfusion. Hep+/My+ and Hep−/My+ had low levels of hepatocyte proliferation, whereas Hep+/My− and Hep−/My− mice had markedly increased proliferation of hepatocytes. Data are mean ± SEM with n=5 per group. *P<0.05 compared to Hep+/My+ and Hep−/My+ mice.
DISCUSSION
Our laboratory has previously demonstrated that signaling via CXCR2 is detrimental to liver recovery following hepatic I/R, and that high concentrations of CXC chemokines were cytotoxic to hepatocytes in vitro (12). However, because both myeloid cells as well as hepatocytes are known to constitutively express the CXCR2 receptor, it was previously undefined as to their individual contributions within the schema of hepatic I/R injury. By generating chimeric mice with various combinations of CXCR2 expression on hepatocyte and myeloid cells, we have advanced our understanding of the role of this receptor in recovery, repair, and regeneration processes occurring after liver I/R.
ELR-positive CXC chemokines are key to the pathophysiology of I/R injury, serving as primary chemoattractants that mediate neutrophil recruitment, resulting in subsequent neutrophil-dependent liver injury (15, 16, 26). CXC chemokines are elevated following I/R (15), reaching maximal expression acutely. Previous studies have shown that CXCR2-knockout mice express far higher levels of CXC chemokines than their wild-type counterparts (12). These results suggested that the lack of CXCR2 receptor/ligand interaction interfered with a possible negative feedback process. Whether this negative feedback was occurring through myeloid CXCR2 signaling or hepatocyte CXCR2 signaling was unknown. Our analysis of CXC chemokine levels in CXCR2Hep−/My+ mice following I/R demonstrated levels that were comparable to those of CXCR2Hep+/My+ mice, which are the equivalent of a wild-type control. Conversely, in CXCR2Hep+/My− mice there was a dramatic increase in CXC chemokine levels following I/R, similar to that seen in CXCR2Hep−/My− mice (the equivalent of a CXCR2-knockout). These results indicate that the negative feedback mechanism of CXC chemokine signaling appears to be primarily driven by CXCR2 expressed on myeloid cells, and that hepatocyte CXCR2 is not involved in this regulation.
Kupffer cells have a significant role in the hepatic response to I/R. Their production of proinflammatory mediators, such as TNF-α, propagates the inflammatory response throughout the liver and induces the hepatic expression of CXC chemokines and endothelial adhesion molecules (7). In mice subjected to total body irradiation and bone marrow transplantation, it was important to assess whether or not resident Kupffer cells of the liver expressed CXCR2, as these cells lie at the interface of the vasculature and parenchyma. Previous research analyzing Kupffer cell turnover following bone marrow transplant has demonstrated almost complete replacement of Kupffer cells with those expressing the bone marrow donor cell phenotype at four weeks (27). Interestingly, our data demonstrates that Kupffer cells from all phenotypes of chimeric mice (CXCR2Hep+/My+, CXCR2Hep−/My+, CXCR2Hep+/My−, CXCR2Hep−/My−) lacked expression of CXCR2. In addition, Kupffer cells from normal wild-type BALB/c and CXCR2 −/−mice that did not undergo BMT also lacked expression of CXCR2. Furthermore, stimulation of Kupffer cells with LPS or TNF-α failed to induce expression of CXCR2. These findings demonstrate, for the first time, a lack of CXCR2 expression by Kupffer cells and strongly suggests that the observed effects of myeloid CXCR2 deficiency in the current study is related to a lack of expression of CXCR2 on neutrophils.
Neutrophil, and not hepatocyte, CXCR2 also appears to be a primary regulator of liver recovery and regeneration after I/R injury. Seven days after injury, mice lacking myeloid CXCR2 expression had less hepatocellular injury and increased hepatocyte proliferation compared to those retaining myeloid CXCR2. Myeloid CXCR2 appears to function primarily by contributing to hepatocellular toxicity in the presence of high levels of CXCR2 ligands found after reperfusion. This is supported by the findings that CXCR2Hep+/My− mice had less liver necrosis than CXCR2Hep+/My+ mice and far lower ALT levels than both CXCR2Hep+/My+ and CXCR2Hep−/My+ mice. The greatest repair and recovery, as determined by histological evidence of necrosis and circulating ALT, was found in CXCR2Hep−/My− mice. Hepatocyte proliferation was 3–4-fold greater in mice lacking myeloid CXCR2, suggesting that chemokine signaling in myeloid cells, most likely neutrophils, directly stimulates hepatocyte proliferation. The precise mechanism of this interaction needs to be elucidated.
In summary, the current study demonstrates that myeloid CXCR2 is the primary regulator of liver recovery and regeneration after hepatic I/R injury. Our data also show that Kupffer cells lack CXCR2 expression suggesting that it is primarily neutrophil CXCR2 that regulates the hepatic recovery response. Hepatocyte CXCR2 appears to play a minor role in this process. CXCR2 continues to be an important therapeutic target and has relevance in a number of clinically relevant liver injuries.
Supplementary Material
Acknowledgments
Financial Support: National Institutes of Health Grants DK56029 and AG025881 to ABL.
List of Abbreviations
- I/R
Ischemia/reperfusion
- BMT
bone marrow transplant
- ALT
alanine aminotransferase
- KC
keratinocyte-derived chemokine
- MIP-2
macrophage inflammatory protein-2
- ELISA
enzyme linked immunosorbent assay
- MPO
myeloperoxidase assay
- PCNA
proliferating cell nuclear antigen
- SEM
standard error of the mean
Contributor Information
Heather L. Van Sweringen, Email: lewishh@ucmail.uc.edu.
Nozomu Sakai, Email: sakainu@ucmail.uc.edu.
Ralph C. Quillin, Email: quillirc@ucmail.uc.edu.
Jeff Bailey, Email: Jeff.Bailey@cchmc.org.
Rebecca Schuster, Email: schustrm@ucmail.uc.edu.
John Blanchard, Email: blanchjn@ucmail.uc.edu.
Holly Goetzman, Email: goetzmh@ucmail.uc.edu.
Charles C. Caldwell, Email: caldwecs@ucmail.uc.edu.
Michael J. Edwards, Email: edwardm6@ucmail.uc.edu.
Alex B. Lentsch, Email: alex.lentsch@uc.edu.
References
- 1.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–458. [PubMed] [Google Scholar]
- 2.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 3.Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442–447. doi: 10.1001/archsurg.1996.01430160100022. [DOI] [PubMed] [Google Scholar]
- 4.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–338. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 6.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 8.Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des. 2006;12:2867–2873. doi: 10.2174/138161206777947597. [DOI] [PubMed] [Google Scholar]
- 9.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–382. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 11.Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, et al. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;289:C826–835. doi: 10.1152/ajpcell.00629.2004. [DOI] [PubMed] [Google Scholar]
- 12.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, et al. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology. 2008;48:1213–1223. doi: 10.1002/hep.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke C, Kuboki S, Sakai N, Kasten KR, Tevar AD, Schuster R, Blanchard J, et al. CXC chemokine receptor-1 is expressed by hepatocytes and regulates liver recovery after hepatic ischemia/reperfusion injury. Hepatology. 53:261–271. doi: 10.1002/hep.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke CN, Kuboki S, Tevar A, Lentsch AB, Edwards M. CXC chemokines play a critical role in liver injury, recovery, and regeneration. Am J Surg. 2009;198:415–419. doi: 10.1016/j.amjsurg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 17.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 20.Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci U S A. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 22.Tschop J, Martignoni A, Reid MD, Adediran SG, Gardner J, Noel GJ, Ogle CK, et al. Differential immunological phenotypes are exhibited after scald and flame burns. Shock. 2009;31:157–163. doi: 10.1097/SHK.0b013e31817fbf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adediran SG, Dauplaise DJ, Kasten KR, Tschop J, Dattilo J, Goetzman HS, England LG, et al. Early infection during burn-induced inflammatory response results in increased mortality and p38-mediated neutrophil dysfunction. Am J Physiol Regul Integr Comp Physiol. 299:R918–925. doi: 10.1152/ajpregu.00132.2010. [DOI] [PubMed] [Google Scholar]
- 24.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 25.Clarke CN, Sakai N, Tevar AD, Edwards MJ, Lentsch AB. CXC chemokines are not direct mitogens for hepatocytes. FASEB J. 2010;24:749.743. [Google Scholar]
- 26.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.