Abstract
Individuals who report subjective cognitive complaints but perform normally on neuropsychological tests may be at increased risk for pathological cognitive aging. The current study examined the effects of the presence of subjective cognitive complaints on functional brain activity during a working memory task in a sample of middle-aged postmenopausal women. Twenty-three postmenopausal women aged 50–60 completed a cognitive complaint battery of questionnaires. Using 20% of items endorsed as the threshold, twelve women were categorized as cognitive complainers (CC) and 11 were non-complainers (NC). All subjects then took part in a functional MRI scanning session during which they completed a visual-verbal N-back test of working memory. Results showed no difference in working memory performance between CC and NC groups. However, the CC group showed greater activation relative to the NC group in a broad network involved in working memory including the middle frontal gyrus (BA 9 and 10), the precuneus (BA 7), and the cingulate gyrus (BA 24 and 32). The CC group recruited additional regions of the working memory network compared to the NC group as the working memory load and difficulty of the task increased. This study showed brain activation differences during working memory performance in a middle-aged group of postmenopausal women with subjective cognitive complaints but without objective cognitive deficit. These findings suggest that subjective cognitive complaints in postmenopausal women may be associated with increased cortical activity during effort-demanding cognitive tasks.
Keywords: cognitive complaints, menopause, working memory, fMRI, neuroimaging
1. Introduction
Subjective cognitive complaints have been associated with the menopause transition (e.g., (Weber et al., 2012). One study showed that 60% of women reported subjective cognitive changes after menopause (Mitchell and Woods, 2001). By contrast, data showing objective cognitive impairments after menopause are inconsistent (see (Hogervorst and Bandelow, 2010) for a meta-analysis). Schaafsma et al. (Schaafsma et al., 2010) found that subjective complaints were associated with menopause-related physical symptoms, psychological factors, and objective impairment. In a study of perimenopausal women, Weber et al. (2012) found that subjective complaints were related to working memory and attention performance as well as depression, anxiety, somatic complaints, and sleep disturbance. Understanding the nature of subjective cognitive complaints is important because older adults with subjective cognitive complaints have been shown to convert to dementia at higher rates than those without complaints (Reisberg et al., 2010).
The current study examined differences in brain activation during a working memory task in postmenopausal women not on hormone therapy with and without cognitive complaints. We hypothesized that postmenopausal women who reported subjective cognitive complaints would show increased frontal activation during the working memory task relative to women who did not report subjective cognitive complaints. In addition, we hypothesized that there would be no differences in cognitive performance between the two participant groups as well as no differences in depression or menopause-related physical symptoms.
2. Method
2.1 Participants
Participants were 23 healthy, cognitively normal, not depressed, postmenopausal women and were recruited and screened using criteria similar to our prior studies (e.g., Dumas et al. 2012; see Supplementary Materials). Participants completed a cognitive complaint inventory (CCI) after Saykin et al. (Saykin et al., 2006) to assess subjective cognitive complaints. Participants were categorized as cognitive complainers (CC) if they endorsed more than 20% of the items on the CCI based on our prior work demonstrating that those clinically characterized as CC tended to endorse >20% of these items. Those who endorsed less than 20% were categorized as non-complainers (NC).
2.2 Cognitive Testing: Working Memory and Episodic Memory
Participants completed an MRI scan with structural and functional imaging and an extensive cognitive battery that included an episodic memory task. The main fMRI task of interest was a visually presented verbal N-back task used to probe working memory circuitry (see Supplementary Materials). fMRI acquisition and preprocessing procedures were similar to our prior studies (Dumas et al., 2010, Dumas et al., 2012). After completion of the MRI session, participants completed an episodic memory test, the Buschke Selective Reminding Task (SRT; (Buschke, 1973) that required subjects to immediately recall words that were read to them.
2.3 fMRI, Cognitive, and Behavioral Analyses
Statistical analyses were performed using a 2 (Group: CC versus NC) × 4 (Working Memory Load: 0-, 1-, 2-, 3-back) random effects ANOVA using standard ANOVA procedures in Brain Voyager. We hypothesized that group differences in activation would increase as the working memory load increased. The contrast vector for the overall interaction was as follows: −6, +1, +2, +3 for the 0-, 1-, 2- and 3-back conditions in the CC group and +6, −1, −2, −3 for the 0-, 1-, 2-, 3-back conditions for the NC group. The hemodynamic response function was accounted for in these models. In an effort to correct for multiple comparisons, we used the cluster-level statistical threshold estimator from Brain Voyager QX to estimate a minimum cluster size threshold based on the approach of Forman et al. (Forman et al., 1995). The starting p-value used in this procedure was p < 0.005. This procedure estimated a minimum cluster size of 13 voxels in functional space (3×3×3) or 321 mm clusters at an alpha level of 0.005 for the analyses below. Statistical analyses for cognitive and mood measures were performed using independent samples t-tests for the CC and NC groups.
3. Results
No differences between the CC and NC groups were found for age, education, years since menopause, years on estrogen, BMI, menopause symptoms, sleep disturbance (largest t(21) = 1.60; smallest p = .12 on the BMI; see Table 1). While the partial SCID interview confirmed that no subjects had current Major Depressive Disorder, there was a group difference in Beck Depression Inventory (BDI-II) scores (t(21) = 2.50, p = .02; See Table 1) with the CC group scoring higher than the NC group. These scores on the BDI were significantly below the clinical range. By definition, the CC group endorsed more items on the CCI than the NC group (t(21) = 7.06, p < .001.
Table 1.
Demographic, menopause symptom, and cognitive performance data (means and standard deviations) for the cognitive complainer (CC) and non-complainer (NC) groups. Differences were found for Cognitive Complaint Index (t(21) = 7.06, p < .001) and for the Beck Depression Inventory score (t(21) = 2.50, p = .02). No group differences on any menopause symptom or cognitive performance measures were found.
| Group | ||
|---|---|---|
| CC N=12 | NC N=11 | |
| Age (y) | 57.1 (2.3) | 56.8 (1.9) |
| BMI (kg/m2) | 25.0 (3.5) | 23.7 (2.4) |
| Education (y) | 16.0 (2.4) | 16.7 (1.9) |
| Years since menopause (y) | 6.4 (4.3) | 7.3 (3.5) |
| Prior postmenopausal hormone use (yes/no) | 4/12 | 2/11 |
| Duration of hormone use (y) | 0.79 (1.8) | 0.64 (1.5) |
| Pittsburgh Sleep Quality Inventory Total score | 2.83 (3.3) | 3.73 (2.2) |
| Menopause Symptom Checklist | .33 (.15) | .24 (.12) |
| Beck Depression Inventory | 5.3 (2.3) | 3.0 (2.6) |
| Cognitive Complaint Index (%) | 29.3 (9.6) | 8.1 (5.1) |
| N-back performance | ||
| 0-back percent correct | 88.2 (6.1) | 89.0 (6.3) |
| 1-back percent correct | 85.4 (9.4) | 89.1 (3.3) |
| 2-back percent correct | 72.5 (15.0) | 76.3 (15.0) |
| 3-back percent correct | 71.3 (16.8) | 66.0 (16.4) |
| SRT performance | ||
| Total Immediate Recall | 74.8 (16.1) | 72.5 (12.6) |
For the imaging analysis we first examined brain activation patterns related to working memory maintenance and updating during the N-back task performance to establish the general N-back brain activation pattern across all subjects. Overall, there was bilateral frontal, parietal, insular, and cerebellar activation as well as anterior cingulate activation that increased as the working memory load increased as has been described in prior studies using the N-back task (See Supplementary Materials; (Cohen et al., 1997).
Our primary analysis of interest was the interaction between group and working memory load. This analysis showed increased activation for the CC compared to the NC group as a function of increasing working memory load in the left middle frontal gyrus (BA 10), right middle frontal gyrus (BA 9), right cingulate gyrus (BA 24), left cingulate gyrus (BA 32), right and left insula (BA 13), and right precuneus (BA 7; See Figure 1). Decreased activation was seen in the right tail of the caudate across working memory load for the CC relative to the NC group.
Figure 1.
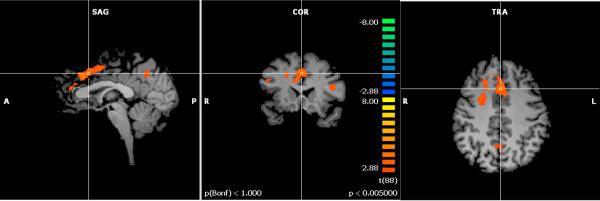
Activation map for cognitive complainers (CC) – non-complainers (NC) as working memory load was parametrically increased from 0-back to 3-back (p < .005). Orange colors represent activation that is greater for the CC group relative to the NC group as the working memory load increased.
No group differences between the CC and NC groups were found for N-back performance during the MRI or SRT episodic memory performance outside the scanner
4. Discussion
The current study found that middle-aged postmenopausal women with subjective cognitive complaints showed increased activation in working memory-related brain regions as working memory load increased compared to women with no subjective complaints. There were no group differences in working memory performance during the MRI or episodic memory performance after the MRI session. The only group difference was that the CC group reported more subjective cognitive complaints than the NC group (by definition) and had slightly elevated scores on the BDI at screening. The BDI scores were in the normal range and below the clinical depression cut off score of 10 and did not correlate with any activated clusters during the fMRI.
The increased activation observed for the CC group was in task-relevant regions that were part of a larger working memory network activated by the N-back task in general. Thus, activation increased with increased task difficulty for the CC group compared to the NC group. We hypothesize that in middle-aged women there may be a number of mechanisms underlying the increased task-related activation response and it may take a cognitive “stressor” such as increased working memory load to bring out this increased activation response in women with subjective cognitive complaints. The underlying mechanism responsible for the increased activation for the CC group cannot be determined from the current study. While we favor a compensation explanation for the increased activation for CC compared to NC group, the increased activation may also be less efficient use of neural resources for the CC group.
Cognitive complaints at menopause have been associated with increased autonomic instability, sleep disturbance, and depression (e.g., Schaafsma et al. 2011). In the current study, our CC and NC groups did not differ on any of these dimensions; thus these factors are not likely to be an explanation for our findings. The small numerical difference that did exist on the BDI measure was substantially below the clinical range and was not correlated with activation during the N-back task. This difference in depression scores was also seen in Saykin et al. (2006) and likewise did not correlate with the hippocampal morphology differences reported in the CC and NC groups. Thus, women who report more cognitive complaints may be more inclined to endorse more items on a depression measure. Additionally, the small sample size in this study may have accounted for some of the lack of significant group differences and correlations on the imaging, cognitive, and behavioral measures. However, when the pattern of means was examined, they showed numerically better performance for the CC group. Larger samples are needed to assess the reliability of these findings.
No study of subjective cognitive complaints to date has followed a group of middle-aged postmenopausal women longitudinally. Thus, the predictive usefulness of cognitive complaints after menopause for MCI and dementia risk is unknown. However, when considered together with data from prior studies indicating increased risk for the development of objective cognitive impairment is decades long, it is reasonable to hypothesize that cognitive complaints after menopause may be an important phenotype to follow to assess the future risk of cognitive decline.
Supplementary Material
Acknowledgements
This work was supported by NIA K01 AG030380, R01 AG021476, R01 AG19771, P30 AG10133, NCRR-00109, DoE SC 0001753, and NCI R01 CA101318. The authors wish to thank the research nursing staff of the University of Vermont GCRC for their hard work and support of this study and our volunteers for their dedication to clinical research. The authors also thank Jay Gonyea, Scott Hipko, and Trevor Andrews, Ph.D. from the University of Vermont MRI Center for Biomedical Imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement The authors have no conflicts of interest to disclose.
6. References
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–607. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Estradiol treatment altered anticholinergic-related brain activity in postmenopausal women. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66:56–71. doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2001;14:252–261. doi: 10.3109/13697137.2010.516848. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma M, Homewood J, Taylor A. Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric. 2010;13:84–98. doi: 10.3109/13697130903009187. [DOI] [PubMed] [Google Scholar]
- Weber M, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012;19:735–741. doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


