Abstract
Our elderly population is growing and declines in cognitive abilities, such as memory, can be costly, because it can interfere with a person’s ability to live independently. The NMDA receptor is very important for many different forms of memory and this receptor is negatively affected by aging. This review examines the progress that has been made recently in characterizing selective vulnerabilities of different subunits and splice variants of the NMDA receptor to normal aging in C57BL/6 mice. Evidence is also presented for changes in the relationships of NMDA receptors to plasticity across aging. Recent interventions show that enhancing NMDA receptors in aged individuals is associated with improvements in memory, but mouse models of neurodegenerative diseases suggest that finding the right balance between too little and too much NMDA receptor activity will be the key to enhancing memory without inducing pathology.
Keywords: frontal cortex, GluN1 (NR1; ζ1) subunit, GluN2B (NR2B; ε2) subunit, glutamate receptor, hippocampus, splice variants
Aging & cognition
Our population is aging. The percentage of the population in the USA that is over the age of 65 years is projected to increase to 19% by 2030 [101]. With this increase will come a rising financial burden to both families and society, unless we take measures to enhance the ‘health span’ of our elderly population. Many different cognitive functions in people show declines during aging [1]. One of the cognitive abilities that is affected early in aging is memory. Humans in their late 40s already show significant declines in recall of information as compared with their recall 10 years earlier [1]. These declines in memory can range in severity from normal age-related memory declines and mild cognitive impairment to the degenerative disorder Alzheimer’s disease (AD), which induces dementia and severe declines in cognitive functions [2–4]. The causes of decreased memory during normal aging or whether the aging brain has the ability to compensate for some declines in a way that is beneficial for memory are not yet understood. A better understanding of the mechanisms underlying these deficits will allow us to better develop interventions that could prevent or repair cognitive deficits during aging. This will benefit the vast majority of aged individuals and should also help to delay some of the debilitating effects of AD, by reducing the effects of normal aging.
The NMDA receptor
The NMDA receptor, a type of glutamate receptor, is expressed in high density in the cerebral cortex and hippocampus and is very important in the initiation steps of learning and memory [5,6]. NMDA receptors play a role in the performance of many different memory tasks, including those using spatial, reference, working and passive avoidance memory, and are crucial to long-term potentiation (LTP) in many different brain regions, a cellular phenomenon that is believed to be involved in at least some types of memory [7–9].
NMDA receptor subunits
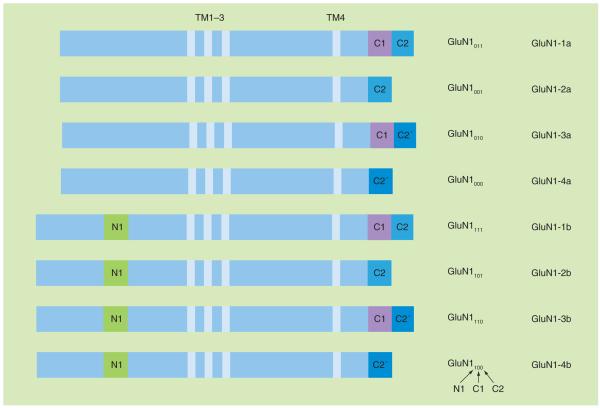
The NMDA receptor complex is believed to be comprised of four subunits; two GluN1 subunits and two other subunits from two regulatory families, GluN2A–D and GluN3A–B [9–12]. There are eight different splice variants of the GluN1 subunit that exist in the brain (Figure 1)[12–17]. These are generated by alternative splicing of one N-terminal (N1; exon 5) and two C-terminal (C1 and C2; exons 21 and 22, respectively) cassettes in the mRNA [10,13,14,16]. The C2 cassette contains a translational stop codon and, in its absence, an additional sequence, identified as C2′, with the next stop codon becomes part of the mature mRNA [14]. We will use three subscripts to indicate the presence (1), absence (0) or either condition (X) of the N1, C1 and C2 cassettes, in that order (Figure 1). The X is necessary because our hybridization probes only identified the N terminal or C terminal splice forms, not individual splice variants [18]. The eight different splice variants are important to NMDA receptor function, as there is heterogeneity between variants with respect to agonist and antagonist affinity, modulation and regional and developmental expression patterns [12,15,19].
Figure 1. Schematic diagram of the eight splice variants of the GluN1 subunit and the nomenclature used to identify each.
There is one N-terminal cassette, N1, and two C-terminal cassettes, C1 and C2. In the absence of C2, there is an additional sequence, C2′, with a new stop codon. The arrows at the bottom of the figure indicate the cassette identified by each subscript. Subscripts: 1 indicates the presence of the cassette, 0 indicates the absence of the cassette. Please note that a 0 in the C2 position (third subscript) indicates the presence of the C2′ sequence. An X is used in the text to indicate both the presence and absence of a cassette.
TM: Transmembrane domain.
The GluN2A and GluN2B subunits of the NMDA receptor are highly expressed in the cerebral cortex and hippocampus [20,21]. The GluN2B subunit has higher affinity for the transmitter, glutamate, and provides longer channel open times than the GluN2A subunit, which confers faster kinetics and greater calcium-sensitive desensitization to NMDA receptors [17,22–24]. The GluN2B subunit is highly expressed at birth and declines during development, while GluN2A subunit-containing receptors increase across the same time frame [25,26].
NMDA receptors & aging
The NMDA receptor shows declines in binding densities and functions with increasing age in many species. This has been described in detail in a recent review [7]. Rats have provided a rich array of details on age-related changes in NMDA receptor binding and electrophysiological properties and related changes in behavior, including memory [7,27,28]. With the use of C57BL/6 mice, it has been possible to identify that the NMDA receptor is more susceptible to aging than other glutamate receptors and that the subunits comprising the receptor also show different susceptibilities to the aging process [7]. This review will expand on what the authors have learned more recently about the effects of aging on the GluN1, GluN2A and GluN2B subunits and the splice variants of the GluN1 subunit, and how the relationships between some of these subunits and memory change with advanced age in these mice. The authors were unable to detect aging changes in the GluN2C or GluN2D subunits in the hippocampus or cortex of C57BL/6 mice [Magnusson KR, Unpublished Data] and no other aging studies were found for GluN2C or GluN2D or GluN3A or GluN3B, so these subunits will not be considered further. The authors will also examine information that has been garnered about NMDA receptors from transgenic and knockout mouse models of altered lifespan and age-related disease. The goal is to identify potential targets involving the NMDA receptor that could enhance memory in aged individuals without exacerbating age-related neurodegenerative disorders.
The GluN2B subunit & aging
The GluN2B subunit is the component of the NMDA receptor that is most affected by the aging process, showing greater declines in mRNA and protein expression than the GluN1 or GluN2A subunits [20,21,29,30]. It shows dramatic declines in mRNA expression across development from birth to adulthood [25,26]. In the frontal lobes of C57BL/6 mice, it appears that the decline in mRNA during adult aging may be a continuation of this developmental decrease [29]. This suggests that the age-related decreases in the GluN2B subunit may be programmed. The changes across development and aging in the mRNA for the GluN2B subunit demonstrated a significant correlation with declines in the density of glutamate binding to NMDA receptors [29], suggesting that alterations in the Glu2B subunit are likely to account for some of the changes in binding density for the NMDA receptor. Declines in the protein expression of both the GluN2B and GluN1 subunits in the frontal lobe of mice are significantly associated with poor performance in a spatial, reference (long-term) memory task across aging [21]. Following fractionation of brain tissue into whole-tissue homogenate, light membranes (e.g., endoplasmic reticulum and the Golgi apparatus) and synaptic membranes, the GluN2B subunit protein showed similar declines across fractions in the hippocampus, suggesting that the alterations in mRNA account for the aging changes in protein subunit expression in this region [30]. However, in the frontal lobe, there was a greater decrement in GluN2B subunit protein expression within the synaptic membrane than the other fractions [30]. This suggests that there is an additional effect of aging on the localization of the GluN2B subunit within the synaptic environ ment. In middle-aged mice, high expression of the GluN2B and GluN1 subunits within the synaptic membrane of the hippocampus is associated with good performance in a spatial reference memory task involving the Morris water maze, as would be expected [30]. Unexpectedly, however, this relationship is reversed in aged mice; despite declines from young adult levels, those aged animals that retain the most GluN2B and GluN1 subunits are the worst learners [30]. This suggests that there is a change in the NMDA receptor or associated signaling complex that no longer makes the receptor good for memory. It is not known exactly when this switch occurs or the cause. Since the GluN2A subunit is relatively spared during aging, it seems likely that there is a imbalance in the GluN2A:GluN2B ratio in the aged animal, leading to more NMDA receptors that are less responsive to glutamate and that desensitize faster than in the young adult [8]. Although the transgenic mice that over express the GluN2B subunit do show declines in protein expression across ages (from 3 to 18 months), they still maintain a higher expression level and superior learning in fear conditioning and spatial reference and working memory than the wild-type mice [31]. This indicates that maintaining higher levels of the GluN2B subunit than are seen in normal aged mice is beneficial for memory.
GluN1 subunit splice variants & aging
The effects of aging on the GluN1 subunit exhibit a great deal of variability between aging studies in C57BL/6 mice, showing significant declines in some studies, but not in others [18,32]. This may mean that the effects of aging on this subunit are more environmental than genetic, which may mean that interventions will be more practical for this subunit. The authors hypothesized that the variability was due to differential effects of aging on the eight splice variants of the receptor. For mRNA, the GluN1X11 (GluN1-1) splice variants show declines with increasing age in the frontal lobe and hippocampus, regardless of whether animals have had a behavioral experience or not and whether the GluN1 subunit mRNA as a whole showed significant declines or not [18]. It seems likely that these splice variants are affected earlier in the aging process than the others. The mRNA for the GluN10XX (GluN1-a) splice variants, which lack the N-terminal cassette, is increased in aged animals only following a behavioral experience, involving 23 days of reference and working memory testing in the Morris water maze [18]. This increase appeared to be associated with better performance in both reference and working memory tasks in old mice [18]. Blocking the expression of these splice variants also impairs spatial reference memory in young animals, providing further evidence for its importance to this cognitive function [33]. The 23-day behavioral experience downregulated the mRNA expression of the GluN1X10 (GluN1-3) splice variants and exacerbated the aging differences [18]. It is not clear whether the effects of the behavioral experience were due to learning or just physical activity. The GluN11XX (GluN1-b), GluNX01 (GluN1-2) and GluN1X00 (GluN1-4) are not significantly changed during the aging process [18,32], showing a heterogeneity in the effects of aging on the GluN1 splice variant mRNA.
With respect to the protein cassettes of the GluN1 subunit, there was no effect of aging or experience on the expression of the N-terminal cassette [34]. The authors were unable to determine whether the protein for the variants lacking this cassette showed an increase in response to behavioral experience, like the GluN10XX (GluN1-a) mRNA, due to the lack of a specific antibody. There was no significant effect of behavioral experience on any of the C-terminal cassettes [34]. There were significant declines in the protein expression of both the C2 and C2′ cassettes with increased age, despite the fact that the epitope identifying all of the GluN1 splice variants did not show age-related decreases [34]. This is a conundrum. Every GluN1 splice variant should express either the C2 or C2′ cassette. Thus, if the total number of GluN1 subunits does not decline with increased age and one sees a decline in, for example, the C2 cassette expression, one would expect to see an increase in the expression of the C2′ cassette (Figure 1). The fact that both C2 and C2′ cassettes decline in expression suggests that there may be damage and/or post-translational modifications to the C terminal tail of the GluN1 subunits, which could alter the ability of the antibodies to identify the cassettes. The splice variants expressing the C2′ cassette appear to become more important for good spatial reference memory in middle-aged mice than they were in young mice. This significant relationship between the C2′ cassette and memory is lost again in the aged mice [34]. There was no significant effect of age on the protein expression of the C1 cassettes; however, there were two populations of aged mice, low and high expressers of the C1 cassette. High expression of the C1 cassette-containing GluN1 subunits in aged mice appeared to be related to good performance at the end of the probe trials for spatial reference memory – that is, they were associated with closer proximity to the platform location [34]. However, the old mice expressing low levels of the C1 cassette were the ones that performed more like young mice – that is, widening their search when the platform is missing [34]. Those with higher levels of the C1 cassette showed perseveration in the absence of the goal and a less accurate search near the end of the probe trials [34], suggesting that retention of the C1 cassette-containing NMDA receptors in aged animals reduces flexibility and precision. These changes in protein expression of the splice cassettes of the GluN1 subunit provide more examples of heterogeneity in the effects of aging on NMDA receptor components and support the idea that the NMDA receptor in an older animal does not have the same role in memory as it does in younger mice.
These results from examining the NMDA receptor during normal aging in mice show heterogeneity in the effects of aging on different subunits and different splice variants. This implies that there is a change in the subunit composition of the NMDA receptors in aged individuals. This could lead to an imbalance between receptors within a population or altered interactions with upstream and downstream elements associated with NMDA receptor signaling. Some of these changes have led to a reversal of the relationship seen in young, in which NMDA receptors are important for good memory. There is also evidence for this switch in other species [7].
Prolonged cultures of primary cortical neurons from BALB/c mice are an interesting model of aging [35]. These cultures show downregulation of GluN1 and GluN2B subunits of the NMDA receptor, similar to the authors’ findings in aged C57BL/6 mice, but they did not test GluN2A, so the authors cannot be sure if the model truly mimics this mouse’s aging phenotype [35]. A deficiency of heme synthesis in younger cultures also produces the same subunit declines and other signs of premature senescence [35]. Although these culture models are unlikely to fully mimic aging within a whole animal, it is interesting that they show similar NMDA receptor subunit susceptibilities during the aging process.
Age-related alterations in NMDA receptor functions
Along with changes in expression of the NMDA receptor, there is abundant evidence for functional changes in the receptor [7]. The late phase of NMDA receptor-dependent LTP, induced by high-frequency stimulation, has been shown in multiple studies to be reduced in aged animals [27,28,36]. Huang and Kandel have discovered a late phase of LTP that is only present in older C57BL/6 mice and is induced by low-frequency stimulation [36]. Following paired-pulse stimulation at 1 Hz in young mice, there is a short-lived LTP that decays within 90 min [36]. Beginning at 6 months of age, this LTP begins to be maintained for longer periods and at 12–18 months it leads to a long-lasting LTP [36]. This LTP is NMDA receptor dependent and is sensitive to protein synthesis inhibitors [36]. It remains to be determined whether this is due to changes in the subunit composition of the receptor populations, but it certainly provides more evidence for the role of NMDA receptors in synaptic plasticity changing during aging. With respect to fear memory, young C57BL/6 mice show moderate impairment of both multi- and one-trial learning with a GluN2B subunit-specific antagonist, RO-25–6981, but 12-month old mice do not show any change [37]. This would fit with previously published work showing declines in GluN2B subunit expression during aging, but the authors did not see any significant decreases in GluN2B protein in the regions that are normally involved in fear memory, such as the amygdala, hippocampus or medial prefrontal cortex, to account for the decreased sensitivity [37].
Sterlemann and colleagues have reported that cognitive impairment in aged mice can be induced by chronic social stress during adolescence in CD1 mice [38]. The mice showed spatial memory deficits 12 months following the stress [38]. After the same post-stress interval, mice also showed impaired LTP and increases in the GluN2B and GluN1 mRNA and GluN2B protein levels in the hippocampus [38]. α1-GABAA receptor mRNA was also decreased by the same stress [38]. It is possible that an imbalance between excitatory and inhibitory neuro transmissions may have contributed to the declines in synaptic plasticity. It would be interesting to know whether these changes were induced at the time of the stress and were maintained over the 12 months or whether they arose during the aging process.
Other noncognitive functions involving NMDA receptors are also altered during aging. A reduction in the percentage of gonadotrophin hormone-releasing neurons expressing the GluN1 subunit protein prior to the luteinizing hormone surge and expressing the phosphorylated GluN1 subunit during and after the surge in middle-aged mice, as compared with young adults, suggests that reproductive aging may be due to a decrease in glutamate responsiveness [39]. The amplitude of synaptic currents in astrocytes following moderate stimulation shows a decline with increasing age from adulthood [40]. However, the contribution of NMDA receptors to the current grew with age, partly due to changes in other receptors [40]. Middle-aged C57BL/6 mice are more sensitive to the effects of the NMDA receptor channel blocker, MK801, on motor function than young mice [41]. This could fit with subunit imbalances due to differential susceptibilities to aging. The astrocyte and motor changes may also be related to the increased reliance on NMDA receptors in middle-aged C57Bl/6 mice that the author of this article saw for spatial memory [34].
Lessons from mouse models of altered lifespan
The senescence-accelerated family of mice (SAM) consists of three different SAM-resistant (SAMR) and eight SAM-prone (SAMP) strains [42]. The SAMP8 strain exhibits reductions in memory, LTP and NMDA receptor expression from 2 to 12 months [42–44]. The addition of d-serine to hippocampal slices from 6 and 12-month-old SAMP8 mice enhanced the deficient NMDA receptor-dependent LTP. Interestingly, the 2-month-old mice actually show an enhanced LTP over the SAMR1 mice, suggesting that there might be a period of enhanced NMDA receptor function in these mice prior to the memory-related declines [42,43]. Yamada et al. found a progressive accumulation of hippocampal cholinergic neurostimulating peptide and its precursor protein in SAMP8 mice from 2 to 5 months of age [43]. The surge in accumulation of these proteins is described as corresponding to the age of onset of memory declines, a reduction in NMDA receptors and alterations in morphology [43]. Hippocampal cholinergic neurostimulating peptide is normally released from the hippocampus by stimulation of NMDA receptors [43]. Is it possible that the accumulation could be initiated during the period of enhanced LTP?
SAMP6 mice differ from the SAMP8 strain in that they show enhanced working and object recognition memory, as compared with the SAMR1 mice [42]. The object recognition memory involved NMDA receptors and was associated with an increase in the GluN2B subunit expression in these mice at 4 months of age [42]. It is not clear whether the SAMP6 mice maintain higher GluN2B subunit expression throughout aging, but it would be interesting to know whether this represents only a spike in NMDA receptor function, occurring around 4 months of age, or if there is enhanced NMDA receptor-dependent memory throughout the lifespan. The latter would suggest that high GluN2B subunit expression does not contribute to the accelerated aging. The superior working memory of the SAMP6 mice was maintained across 1–8 months of age, but was not effectively blocked by a NMDA receptor antagonist [42]. However, it is possible that an insufficient dose was used, since the highest dose was just starting to impair the SAMR1 mice.
Laron dwarf mice (growth hormone receptor knockout [GHRKO]) mice have a targeted disruption of the mouse growth hormone receptor or binding protein gene [45]. The GHRKO mice have a longer lifespan and delayed cognitive decline as compared with age-matched control siblings [46]. There are significant declines in the density of binding of glutamate to NMDA receptors within the medial prefrontal and motor cortices of control mice between 6 and 24 months of age [47]. The GHRKO mice showed a slight sparing of NMDA receptors within these regions [47]. The effects of aging on the GluN1 subunit mRNA was just the opposite, with GHRKO mice showing greater declines in the frontal lobe and dentate gyrus of the intermediate hippo campus [47]. These contrasting findings could be due to a differential sparing of splice variants with higher agonist affinity, a mismatch between mRNA and protein or changes in other subunits. The sparing of the NMDA receptors may contribute to some of the delay in memory decline in this mouse model of increased lifespan.
Recent interventions affecting NMDA receptor aging
Over the last two decades, a number of interventions have shown promise for reversing the effects of aging on the NMDA receptor [7]. Many of these have only selective effects on specific subunits, supporting the idea that there are different mechanisms of aging that affect the NMDA receptor components. Some more recent interventions include an intermittent fasting version of caloric restriction, environmental enrichment, ginseng and green tea catechins. A long-term (6–8 months), intermittent fasting diet enhances learning and consolidation in multiple tasks, including rotorod and object recognition and increases synaptic efficiency [48]. This was associated with an increase in mRNA for the GluN2B subunit, but not for GluN2A or GluN1 subunits, in the hippocampus and GluN2B and GluN1 subunits in the perirhinal cortex in the mice fed every other day [48]. In addition, the enhancements in learning and LTP were blocked by a GluN2B subunit-specific antagonist [48]. These assessments were carried out at a single age, so it is not clear whether the intervention up regulated these subunits over the level in young and/or whether it prevented the age-related declines. It does suggest that enhancing the GluN2B subunit during aging can be beneficial for plasticity.
Environmental enrichment in Naval Medical Research Institute mice for 6 months (from 14 to 20 months of age) prevents memory deficits and reduces anxiety in aged animals [49]. This is associated with a reversal in the age-related decrease in NMDA receptor synaptic potentials [49]. Ginsenoside, the major active ingredient in ginseng, was effective in enhancing spatial and passive avoidance memory following 8 months of treatment in female C57Bl/6 mice. This treatment also enhances the phosphorylation of a number of important proteins involved in synaptic plasticity, including the GluN1 subunit, which enhances activity [50]. Administration of green tea catechins for 6 months in female C57BL/6 mice prevented age-related reductions in the GluN1 subunit in the hippocampus, but had no significant effect on the cortex [51]. Each of these treatments suggests that it is possible to intervene into the effects of aging on NMDA receptors and this may contribute to an enhancement of plasticity.
Other potential targets for enhancing NMDA receptor function in old age are proteins that interact with the receptor. Reelin is an extracellular matrix protein that can regulate NMDA receptor homeostasis, through phosphorylation of the GluN2 subunits, and modulate LTP [52]. Age-related decreases in Reelin contribute to cognitive declines during aging and dysfunctional Reelinmediated signaling reduces LTP and promotes abnormal processing of APP and hyperphosphorylation of tau, both of which contribute to the pathologies associated with AD [52]. Deletion of the glycine transporter type 1 enhances synaptic glycine, NMDA receptor function and multiple cognitive functions [53]. The enhanced aversive Pavlovian conditioning that is seen in 3-month-old mutants is still enhanced at 22 months of age and there is no evidence of neurotoxicity caused by enhanced NMDA receptor function in the mutants [53]. Neural cell adhesion molecule is involved in NMDA receptor trafficking and inhibiting extrasynaptic GluN2B-containing NMDA receptors. Deficits in neural cell adhesion molecule can occur during aging and can impair LTP and long-term depression [54]. These deficits can be restored by d-cycloserine, acting primarily through GluN2A subunit-containing NMDA receptors [54]. d-cycloserine is effective in sparing the decline in LTP seen prematurely in neural cell adhesion molecule knockout mice and in old age in both wild-type and knockout mice, suggesting that enhancing GluN2A-containing NMDA receptors is effective in combating age-related declines in synaptic plasticity, potentially by restoring the balance between GluN2B and GluN2A subunits [54]. Targeting these NMDA receptor-related proteins could be effective in indirectly enhancing NMDA receptor function in aged individuals.
There are, of course, many other genes that are involved in cognitive processing, that are affected by aging [55]. It is possible that the interventions mentioned above had effects on other genes besides the NMDA receptor subunits, which led to the enhancements in memory. There is also evidence, however, for redundant memory systems in young animals [56]. This may mean that it is possible to focus on fixing one system in the elderly and restore function to a level that is indistinguishable from performance in the young.
Lessons from AD & Huntington’s disease models
One concern about restoring or preventing NMDA receptor changes during aging is whether this would make individuals more susceptible to excitotoxicity and other age-related diseases. Some information can be gleaned from mouse models of AD and Huntington’s disease (HD). There is evidence that amyloid β (Aβ) can influence NMDA receptor function. In the APP double Swedish mutant mouse, Aβ can interfere with cholinergic regulation of NMDA receptors [57]. Aβ can stimulate retraction of synaptic contacts [58] and phosphorylation of Akt via GluN2B subunit-containing NMDA receptors, but phosphorylation of Akt is greatly reduced in older APP transgenic mice [59]. This may be due to the ability of Aβ to induce endocytosis of NMDA receptors in cultured neurons [59–61], with GluN2B subunits slightly more affected than GluN1 [62]. The GluN2B subunit is also decreased in expression in APP(V717I) transgenic mice [60]. This suggests that, although Aβ can influence NMDA receptor-dependent processes, chronic exposure can lead to a reduction in NMDA receptors available to act on.
A deficiency in presenilin 1 impairs NMDA receptor responses and LTP and shows the same pattern of aging of the major NMDA receptor subunits as normal aging in C57BL/6 mice; declines in the GluN2B subunit expression are greater than the GluN1, which is greater than the GluN2A subunits [63]. A conditional double knockout mouse lacking both presenilin 1 and 2 shows declines in NMDA receptor-dependent LTP and NMDA receptor subunit expression in synaptoneurosomes at 2 months of age, but neuro- degeneration is not detectable until 6 months [64]. This could indicate that NMDA receptor hypofunction may contribute to pathology. Three different presenilin mutants, however, show a transient increase in NMDA receptor responses before the onset of decreased NMDA receptor function [63,65,66]. This makes the NMDA receptor suspect for initiating AD pathologies, at least in cases with presenilin mutations.
There is evidence that long-term administration of ketamine, a noncompetitive NMDA receptor antagonist, in imprinting control mice and monkeys can lead to an increase in hyperphosphorylated tau [67], suggesting that hypofunctioning of NMDA receptors can lead to AD-related pathology. One of the current treatments for AD, memantine, may belie this idea, because of its role as an NMDA receptor antagonist [68]. Memantine administered to APP/presenilin 1 transgenic mice enhances object recognition memory [69]. It also reduces amyloid plaque burden in the APP/presenilin 1 and Tg2576 mouse models of AD [70]. This suggests that blocking NMDA receptor function would be more beneficial to preventing AD than restoring the levels found in young mice. However, memantine primarily interferes with abnormal hyperactivity at extrasynaptic NMDA receptors and does little to interfere with normal synaptic activity of NMDA receptors [68,71]. In addition, although there is plenty of evidence that NMDA receptors are involved in excitotoxicity, there is also evidence that normal, physiological levels of NMDA receptor activity can enhance neuronal survival, along with promoting synaptic plasticity [72]. This suggests that it may be neuro protective to repair or prevent the changes in NMDA receptors that occur during aging, but measures would have to be taken to avoid increasing the susceptibility to excitotoxicity, potentially with drugs like memantine.
Results from HD models are more problematic for advocating enhancing NMDA receptor function in aged individuals. Excessive activation of NMDA receptors in the striatum produces many of the characteristic features of HD [73]. The ratio of GluN2B to GluN2A protein subunits is higher in the striatum, the most susceptible region in HD, than in the cerebral cortex or hippocampus of normal animals and transgenic mice that express a mutant form of the protein huntingtin show enhanced NMDA receptor currents, the majority of which appear to be GluN2B subunit-mediated currents [73]. Increased damage from glutamate toxicity is seen in the R6/2 HD transgenic mice and this is associated with decreases in glutamate transporters [74]. There is also evidence in this mouse model of a population of striatal neurons that show an increase in responsiveness to NMDA at an early age, 15–40 days old, prior to overt behavioral changes [75]. An HD model, in which only striatal neurons express mutant huntingtin, also shows an increase in NMDA currents over wild-type mice [76]. These findings suggest that improving NMDA receptor expression in aged individuals may contribute to the pathology associated with HD. The fact that this is an heritable, autosomal dominant disease, which can be identified through genotyping, means that affected individuals could be counseled against interventions that enhance NMDA receptor function. Another possibility would be an intervention that was targeted specifically to the hippocampus or cerebral cortex and not the striatum.
Conclusion
There is ample evidence for changes in NMDA receptor complex expression and functions during the aging process in multiple species. With the help of the C57BL/6 mouse, we have been able to characterize more fully the hetero geneity in the effects of aging on subunits and splice variants at both the transcript and protein levels, which suggests that the subunit compositions of the NMDA receptors in the aged individual are not the same as they are in the young. In addition, there is evidence from both behavioral and electro-physiological studies that the role of NMDA receptors in plasticity changes with increased age. It is not clear exactly when this happens. The new form of LTP in the hippocampus is established by 12–18 months of age in C57BL/6 mice [36]. The switch in the hippocampus from high expression of NMDA receptor subunits being associated with good memory to being in poor performers occurs sometime between 11 and 26 months of age in the same strain [30]. Studies on aging interventions suggest that enhancing NMDA receptor expression in older animals is beneficial to memory in normal aging. Several mouse models of neurodegenerative diseases, which develop cognitive declines during aging, show a period of enhanced NMDA receptor function in an early stage of the disease, prior to overt signs, and mouse models of HD show heightened sensitivity to glutamate toxicities. This, along with the prevalence of stroke in the aged population, indicates that caution must be exercised to design interventions that do not lead to activations of NMDA receptor populations that are excitotoxic. The use of treatments like memantine, which reduce the toxic effects of NMDA receptor activation without interfering with normal transmission, in concert with interventions to restore or alter specific subunits, however, may help to reduce forgetfulness in aged individuals. It may also help to delay the onset of debilitating cognitive declines in AD.
Future perspective
One obvious target to explore is the enhancement of the expression of the GluN2B subunit of the NMDA receptor. Some of the decline in expression of this subunit during aging is likely to be due to inhibition of transcription. Exploration of the epigenetic changes that occur during the developmental decreases in this subunit and/or changes to proteins that interact with the promoter region could provide new drug targets. The evidence that there may be an additional change during aging in the synaptic membranes suggests that trafficking and localization of GluN2B subunit-containing NMDA receptors may be altered. This is worth pursuing further. If there is an increase in the extrasynaptic localization of GluN2B subunit-containing receptors, it could cause an increased susceptibility to excitotoxicity, which may be amenable to treatments like memantine. The GluN1 splice variants that lack the N-terminal cassette appear to enhance memory performance and those containing the C1 cassette appear to be associated with memory deficits in the aged mice. Enhancing deletion of the N1 and/or C1 cassettes within old individuals could be beneficial to memory, but it would also be important to try to understand why these relationships develop during the aging process. More effort should be made to determine the age at which the switch in the relationship of the NMDA receptors to memory occurs and to explore the changes that occur prior to and during this switch in order to identify a cause. It would also be interesting to determine whether the transient increases in NMDA receptor function in the mouse models produce similar subunit and splice variant declines to normal aging and whether there is any indication of a similar transient enhancement occurring during normal aging. Ultimately, any interventions that enhance NMDA receptor expression and/or function in aged individuals should be tested to make sure that it does not also enhance excitotoxicity or accelerate the onset of pathologies in the mouse models of neurodegenerative disease. It appears from what we have learned from mice and other species that it may be possible to enhance memory in older individuals by restoring optimal functioning of the NMDA receptor, but it will be important to achieve the right balance between too little and too much receptor activity.
Executive summary.
NMDA receptors & aging
■ NMDA receptors are more susceptible to declines in binding density during aging than other glutamate receptors and these declines show a relationship to memory declines.
GluN2B subunit & aging
■ The GluN2B subunit of the NMDA receptor is more affected by aging than the other major cortical and hippocampal subunits, GluN1 and GluN2A.
■ Some of the effects of aging are on GluN2B mRNA expression, but in the frontal lobe there is an additional effect of aging on expression in the synaptic membrane.
■ High levels of GluN2B and GluN1 subunit expression in the synaptic membrane of the hippocampus in middle-aged mice show a stronger association with good spatial reference memory performance than in young mice. In the aged mice, this relationship is reversed, with high expressers showing the worst spatial learning.
■ There are multiple examples of maintenance of higher levels of function of the GluN2B subunit-containing NMDA receptors in older mice being associated with memory improvements.
GluN1 subunit splice variants & aging
■ A behavioral experience can enhance the expression of the GluN10XX (GluN1-a) splice variants in aged mice only and these splice variants are associated with good spatial reference and working memory.
■ This behavioral experience exacerbated the effects of aging on the GluN1X10 (GluN1-3) splice variants.
■ There is heterogeneity in the effects of aging on GluN1 splice variants; GluN1X11 (GluN1-1) and GluN1X10 (GluN1-3) splice variants are susceptible to aging changes, but the GluN11XX (GluN1-b), GluNX01 (GluN1-2), and GluN1X00 (GluN1-4) are not.
■ Both the C2 and C2′ protein cassettes of the GluN1 subunit show declines during aging, but the proteins may still be present, but in a modified form.
■ Expression of GluN1 subunits with the C1 cassette in aged mice may contribute to deficits in cognitive flexibility and accuracy.
NMDA receptor function & aging
■ A new form of long-term potentiation, involving low-frequency stimulation, arises during middle age.
■ Chronic stress can enhance NMDA receptor subunit expression in middle age, but this is associated with an imbalance in excitatory and inhibitory transmission and deficits in memory and long-term potentiation.
■ Recently studied interventions, such as caloric restriction, environmental enrichment, ginseng and green tea catechins, can enhance NMDA receptor expression and function in aged animals, and this is associated with memory improvements.
Mouse models
■ There is evidence for a period of enhanced NMDA receptor function prior to the onset of NMDA receptor and behavioral deficits or morphological changes in senescence-accelerated mice, mouse models of Alzheimer’s disease expressing mutant presenilin and a striatal-specific model of Huntington’s disease.
■ Memantine, a current therapy for Alzheimer’s disease, acts primarily at extrasynaptic NMDA receptors that are excessively activated and spares receptors involved in normal transmission and synaptic plasticity.
■ Glutamate toxicity appears to play a role in Huntington’s disease and mice expressing the mutant protein huntingtin show enhanced NMDA receptor responsiveness.
Acknowledgments
This work was supported by RO1 grant AG016322 to KR Magnusson from National Institute on Aging at the National Institutes of Health.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Singh-Manoux A, Kivimaki M, Glymour M, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:1–8. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Small BJ, Gagnon E, Robinson B. Early identification of cognitive deficits: preclinical Alzheimer’s disease and mild cognitive impairment. Geriatrics. 2007;62(4):19–23. [PubMed] [Google Scholar]
- 3.Van Rossum IA, Vos S, Handels R, Visser PJ. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer-type dementia: implications for trial design. J. Alzheimer’s Dis. 2010;20(3):881–891. doi: 10.3233/JAD-2010-091606. [DOI] [PubMed] [Google Scholar]
- 4.Albert MS. Changes in cognition. Neurobiol. Aging. 2011;32(Suppl. 1):S58–S63. doi: 10.1016/j.neurobiolaging.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotman CW, Bridges RJ, Taube JS, Clark AS, Geddes JW, Monaghan DT. The role of the NMDA receptor in central nervous system plasticity and pathology. J. NIH Res. 1989;1:65–74. [Google Scholar]
- 6.Morris RGM, Davis M. The role of NMDA receptors in learning and memory. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. Oxford University Press; Oxford: 1994. pp. 340–375. [Google Scholar]
- 7.Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Front. Aging Neurosci. 2010;2(11) doi: 10.3389/fnagi.2010.00011. ■In-depth review of studies on the effects of aging on the NMDA receptor and subunits across different species and decades.
- 8.Qiu S, Li XY, Zhuo M. Post-translational modification of NMDA receptor GluN2B subunit and its roles in chronic pain and memory. Semin. Cell Dev. Biol. 2011;22(5):521–529. doi: 10.1016/j.semcdb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Sweatt JD. Mechanisms of Memory. Elsevier; NY, USA: 2010. The NMDA receptor; pp. 191–207. [Google Scholar]
- 10.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 11.Seeburg PH. The molecular biology of mammalian glutamate receptor channels. Trends Pharmacol. Sci. 1993;14:297–302. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- 12.Zukin RS, Bennett MVL. Alternatively spliced isoforms of the NMDAR1 receptor subunit. Trends Neurosci. 1995;18(7):306–311. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- 13.Anantharam V, Panchal RG, Wilson A, Kolchine VV, Treistman SN, Bayley H. Combinatorial RNA splicing alters the surface charge on the NMDA receptor. FEBS Lett. 1992;305(1):27–30. doi: 10.1016/0014-5793(92)80648-z. [DOI] [PubMed] [Google Scholar]
- 14.Durand GM, Gregor P, Zheng X, Bennett MV, Uhl GR, Zukin RS. Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc. Natl Acad. Sci. USA. 1992;89(19):9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollmann M, Boulter J, Maron C, et al. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- 16.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem. Biophys. Res. Comm. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M. Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett. 1992;300(1):39–45. doi: 10.1016/0014-5793(92)80160-i. [DOI] [PubMed] [Google Scholar]
- 18.Das SR, Magnusson KR. Relationship between mRNA expression of splice forms of the zeta1 subunit of the N-methyl-D-aspartate receptor and spatial memory in aged mice. Brain Res. 2008;1207:142–154. doi: 10.1016/j.brainres.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J. Neurosci. 1994;14(5):3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J. Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. ■First description of the susceptibility of the GluN2B subunit to declines during aging.
- 21.Magnusson KR, Scruggs B, Zhao X, Hammersmark R. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 2007;8(43) doi: 10.1186/1471-2202-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii T, Moriyoshi K, Sugihara H, et al. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J. Biol. Chem. 1993;268(4):2836–2843. [PubMed] [Google Scholar]
- 23.Kutsuwada T, Kashiwabuchi N, Mori H, et al. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 24.Monyer H, Sprengel R, Schoepfer R, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 25.Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J. Neurosci. 2004;24(40):8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 27.Barnes CA. Long-term potentiation and the ageing brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358(1432):765–772. doi: 10.1098/rstb.2002.1244. ■■Excellent review on the effects of aging on behavior and NMDA receptor-dependent synaptic plasticity.
- 28.Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca(2) channels in senescent synaptic plasticity. Prog. Neurobiol. 2012;96(3):283–303. doi: 10.1016/j.pneurobio.2012.01.007. ■■In-depth assessment of the sensitivity of the water maze for detecting age-related declines in memory and discussion of the electrophysiological changes that occur in NMDA receptor function during aging.
- 29.Ontl T, Xing Y, Bai L, et al. Development and aging of N-methyl-D-aspartate receptor expression in the prefrontal/frontal cortex of mice. Neuroscience. 2004;123:467–479. doi: 10.1016/j.neuroscience.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Rosenke R, Kronemann D, et al. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162(4):933–945. doi: 10.1016/j.neuroscience.2009.05.018. ■Describes the switch in relationship between NMDA receptor expression and spatial reference memory between middle and old age.
- 31.Cao X, Cui Z, Feng R, et al. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur. J. Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. ■Transgenic mice overexpressing the GluN2B subunit still show superior memory to wild-type mice at 18 months of age.
- 32.Magnusson KR, Bai L, Zhao X. The effects of aging on different C-terminal splice forms of the zeta1(NR1) subunit of the N-methyl-d-aspartate receptor in mice. Brain Res. Mol. Brain Res. 2005;135(1–2):141–149. doi: 10.1016/j.molbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Das SR, Jensen R, Kelsay R, et al. Reducing expression of GluN1(0XX) subunit splice variants of the NMDA receptor interferes with spatial reference memory. Behav. Brain Res. 2012;230(2):317–324. doi: 10.1016/j.bbr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das SR, Magnusson KR. Changes in expression of splice cassettes of NMDA receptor GluN1 subunits within the frontal lobe and memory in mice during aging. Behav. Brain Res. 2011;222(1):122–133. doi: 10.1016/j.bbr.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chernova T, Nicotera P, Smith AG. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Mol. Pharmacol. 2006;69(3):697–705. doi: 10.1124/mol.105.016675. [DOI] [PubMed] [Google Scholar]
- 36.Huang YY, Kandel ER. Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus. Learn. Memory. 2006;13(3):298–306. doi: 10.1101/lm.166906. ■Description of a new form of long-term potentiation that develops during aging.
- 37.Mathur P, Graybeal C, Feyder M, Davis MI, Holmes A. Fear memory impairing effects of systemic treatment with the NMDA NR2B subunit antagonist, Ro 25–6981, in mice: attenuation with ageing. Pharmacol. Biochem. Behav. 2009;91(3):453–460. doi: 10.1016/j.pbb.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterlemann V, Rammes G, Wolf M, et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20(4):540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- 39.Adjan V, Centers A, Jennes L. Expression and activation of N-methyl-D-aspartate receptor subunit-1 receptor subunits in gonadotrophin-releasing hormone neurones of young and middle-aged mice during the luteinising hormone surge. J. Neuroendocrinol. 2008;20(10):1147–1154. doi: 10.1111/j.1365-2826.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 40.Lalo U, Palygin O, North RA, Verkhratsky A, Pankratov Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell. 2011;10(3):392–402. doi: 10.1111/j.1474-9726.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 41.Qi C, Zou H, Zhang R, Zhao G, Jin M, Yu L. Age-related differential sensitivity to MK-801-induced locomotion and stereotypy in C57BL/6 mice. Eur. J. Pharmacol. 2008;580(1–2):161–168. doi: 10.1016/j.ejphar.2007.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niimi K, Takahashi E, Itakura C. Improved short-term memory and increased expression of NR2B observed in senescence-accelerated mouse (SAM) P6. Exp. Gerontol. 2008;43(9):847–852. doi: 10.1016/j.exger.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Matsukawa N, Yuasa H, et al. Differential expression of HCNP-related antigens in hippocampus in senescence-accelerated mice. Brain Res. 2007;1158:169–175. doi: 10.1016/j.brainres.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Qiao H, Wen L, Zhou W, Zhang Y. D-serine enhances impaired long-term potentiation in CA1 subfield of hippocampal slices from aged senescence-accelerated mouse prone/8. Neurosci. Lett. 2005;379(1):7–12. doi: 10.1016/j.neulet.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc. Natl Acad. Sci. USA. 1997;94(24):13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol. Behav. 2004;80(5):589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Magnusson KR, Das SR, Kronemann D, Bartke A, Patrylo PR. The effects of aging and genotype on NMDA receptor expression in growth hormone receptor knockout (GHRKO) mice. J. Gerontol. Series A. Biol. Sci. Med. Sci. 2011;66(6):607–619. doi: 10.1093/gerona/glr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J. Neurosci. 2007;27(38):10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freret T, Billard JM, Schumann-Bard P, et al. Rescue of cognitive aging by long-lasting environmental enrichment exposure initiated before median lifespan. Neurobiol. Aging. 2012;33(5):1005.e1–10. doi: 10.1016/j.neurobiolaging.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H, Li Q, Pei X, et al. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav. Brain Res. 2009;201(2):311–317. doi: 10.1016/j.bbr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Li Q, Zhao H, Zhao M, Zhang Z, Li Y. Chronic green tea catechins administration prevents oxidative stress-related brain aging in C57BL/6J mice. Brain Res. 2010;1353:28–35. doi: 10.1016/j.brainres.2010.07.074. [DOI] [PubMed] [Google Scholar]
- 52.Doehner J, Knuesel I. Reelin-mediated signaling during normal and pathological forms of aging. Aging Dis. 2010;1(1):12–29. [PMC free article] [PubMed] [Google Scholar]
- 53.Dubroqua S, Singer P, Boison D, Feldon J, Mohler H, Yee BK. Impacts of forebrain neuronal glycine transporter 1 disruption in the senescent brain: evidence for age-dependent phenotypes in Pavlovian learning. Behav. Neurosci. 2010;124(6):839–850. doi: 10.1037/a0021556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kochlamazashvili G, Bukalo O, Senkov O, et al. Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors. J. Neurosci. 2012;32(7):2263–2275. doi: 10.1523/JNEUROSCI.5103-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blalock EM, Chen KC, Sharrow K, et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 2003;23(9):3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. ■■Microarray analysis of the hippocampus, which identifies new genes and pathways that are affected in cognitive aging.
- 56.Penner M, Barnes CA. Memory changes with age: neurobiological correlates. In: Kesner RP, Martinez J, editors. Neurobiology of Learning and Memory. Academic Press; NY, USA: 2007. pp. 483–518. [Google Scholar]
- 57.Chen G, Chen P, Tan H, et al. Regulation of the NMDA receptor-mediated synaptic response by acetylcholinesterase inhibitors and its impairment in an animal model of Alzheimer’s disease. Neurobiol. Aging. 2008;29(12):1795–1804. doi: 10.1016/j.neurobiolaging.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronicke R, Mikhaylova M, Ronicke S, et al. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol. Aging. 2011;32(12):2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Abbott JJ, Howlett DR, Francis PT, Williams RJ. Aβ(1–42) modulation of Akt phosphorylation via α7 nAChR and NMDA receptors. Neurobiol. Aging. 2008;29(7):992–1001. doi: 10.1016/j.neurobiolaging.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Dewachter I, Filipkowski RK, Priller C, et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol. Aging. 2009;30(2):241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Lacor PN, Buniel MC, Furlow PW, et al. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 63.Dewachter I, Ris L, Croes S, et al. Modulation of synaptic plasticity and tau phosphorylation by wild-type and mutant presenilin1. Neurobiol. Aging. 2008;29(5):639–652. doi: 10.1016/j.neurobiolaging.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 64.Saura CA, Choi SY, Beglopoulos V, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42(1):23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 65.Auffret A, Gautheron V, Mattson MP, Mariani J, Rovira C. Progressive age-related impairment of the late long-term potentiation in Alzheimer’s disease presenilin-1 mutant knock-in mice. J. Alzheimer’s Dis. 2010;19(3):1021–1033. doi: 10.3233/JAD-2010-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Auffret A, Gautheron V, Repici M, et al. Age-dependent impairment of spine morphology and synaptic plasticity in hippocampal CA1 neurons of a presenilin 1 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2009;29(32):10144–10152. doi: 10.1523/JNEUROSCI.1856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeung LY, Wai MS, Fan M, et al. Hyperphosphorylated tau in the brains of mice and monkeys with long-term administration of ketamine. Toxicol. Lett. 2010;193(2):189–193. doi: 10.1016/j.toxlet.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 2007;8(10):803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 69.Scholtzova H, Wadghiri YZ, Douadi M, et al. Memantine leads to behavioral improvement and amyloid reduction in Alzheimer’s-disease-model transgenic mice shown as by micromagnetic resonance imaging. J. Neurosci. Res. 2008;86(12):2784–2791. doi: 10.1002/jnr.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong H, Yuede CM, Coughlan C, Lewis B. Csernansky JG. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2008;33(13):3226–3236. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug. Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 72.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13(6):572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Fan M, Icton CD, et al. Role of NR2B-type NMDA receptors in selective neurodegeneration in Huntington disease. Neurobiol. Aging. 2003;24(8):1113–1121. doi: 10.1016/j.neurobiolaging.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Estrada-Sanchez AM, Montiel T, Segovia J, Massieu L. Glutamate toxicity in the striatum of the R6/2 Huntington’s disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol Dis. 2009;34(1):78–86. doi: 10.1016/j.nbd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 75.Starling AJ, Andre VM, Cepeda C, de Lima M, Chandler SH, Levine MS. Alterations in N-methyl-D-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington’s disease. J. Neurosci. Res. 2005;82(3):377–386. doi: 10.1002/jnr.20651. [DOI] [PubMed] [Google Scholar]
- 76.Gu X, Andre VM, Cepeda C, et al. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington’s disease. Mol. Neurodegener. 2007;2(8) doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Das SR. PhD Dissertation. Vol. 135. Oregon State Univeristy; OR, USA: 2010. Influence of aging and behavioral experience on expression of GluN1 splice variants of the NMDA receptor in prefrontal cortex of mice brain. [Google Scholar]
Website
- 101.Administration on aging: aging statistics. 2012 www.aoa.gov/AoARoot/Aging_Statistics/index.aspx.