Abstract
INTRODUCTION
Pancreatic neuroendocrine tumors (PNET) have an incidence of one per 100,000 individuals per year. They represent about 1–2% of all pancreatic tumors. PNETs are a heterogeneous group with various clinical presentations and lineage. Non functional PNET (NF-PNET) are incidentally discovered in most cases. This article presents a review of NF-PNET and the case of a patient with this disease, in addition to its diagnosis, clinical presentation, and treatment.
PRESENTATION OF CASE
The patient is a 37-year-old asymptomatic man who was sent from his reference unit because of a pancreatic tumor that was visualized incidentally during a laparotomy performed three months before due to an acute abdomen secondary to blunt abdominal trauma. A CT scan was requested that showed a retroperitoneal mass 7.5 cm × 6.6 cm × 7 cm with infiltration of the duodenal wall. Endoscopy was performed, which reported a duodenal ulcer with nonconclusive histological findings. A percutaneous biopsy was obtained out and a diagnosis of a neuroendocrine tumor was made. Chemotherapy was started because infiltration of the portomesenteric axis was suspected. The patient presented signs of toxicity during his third cycle and therefore was scheduled for exploratory laparotomy. Pancreatoduodenectomy was performed with a histologic diagnosis of a pancreatic neuroendocrine tumor.
DISCUSSION
The presentation of a NF-PNET is nonspecific. They continue to be tumors with a low incidence and few studies directed toward early detection and management have been carried out. Currently, CT scans are the studies most used for detection.
CONCLUSION
Surgical treatment is preferred in patients without evidence of unresectability with longer survival. The characteristics of NF-PNETs make their detection difficult and new strategies are needed for early detection and management. New studies in early stages with new cytotoxics or analogs are promising.
Keywords: Neuroendocrine tumor, Pancreatic tumors, Pancreatoduodenectomy
1. Introduction
Neuroendocrine tumors comprise a rare group of neoplasms that arise from the neuroendocrine system of the intestine. They have an incidence of 1 in 100,0001 and it is estimated that at least 3% of primary neoplasms are derived from the pancreas and more than 10% are associated with genetic syndromes such as MEN1.2 By definition all PNET express neuroendocrine markers such as synaptophysin, neuron-specific enolase and chromogranin A (present in 88–100% of patients with PNET). At least 14 different types of cells have been implicated to date in their pathogenesis. This heterogeneity describes the multiple syndromes of overproduction and hypersecretion of hormones, thus PNET are classified as functional or nonfunctional based on the presence or absence, respectively, of a particular clinical syndrome associated with hormone hypersecretion.3,4 Recent series have stated that NF-PNET occupy 20–60% of all neuroendocrine tumors.5 NF-PNET are often found by chance and patients usually present with advanced disease (>60% have liver metastases). Symptoms can be caused by tumor growth. Among these is abdominal pain (40–60%), weight loss (25–50%) and jaundice (30–40%). In recent years there has been an increase in the incidental diagnosis of NF-PNET with imaging studies because of their nonspecific symptoms (over 35% of patients in some series). Screening asymptomatic individuals result in lower rates of metastasis, increased resectability, and improved survival.6
We present a rare case in which a NF-PNET was found incidentally in a patient with a history of exploratory laparotomy for blunt abdominal trauma.
2. Case report
The patient is a 37-year-old man first seen in the unit in October 2011 because of a tumor of probable pancreatic origin that was found incidentally after exploratory laparotomy for an acute abdomen secondary to blunt abdominal trauma in October 2010. Prior to the event and even afterwards he never complained of pain or the presence of an abdominal mass or any other associated symptoms, which is why he was referred to our unit for further management.
On admission, an abdominal CT scan was requested, which showed a retroperitoneal neoplastic lesion in the right upper quadrant with densities of 30–50 Hounsfield units and a size of 7.5 cm × 6.6 cm × 7 cm with enhancement after administration of contrast media and infiltration of the duodenal wall with compression of the large vessels (Fig. 1). An endoscopy was requested and the finding of an infiltrating duodenal ulcer of probable pancreatic origin was reported. A biopsy was performed with a histopathological report of mild chronic inflammation and edema. It was also reported that chromogranin, Ca 19-9, CK 7 and 20 were negative.
Fig. 1.
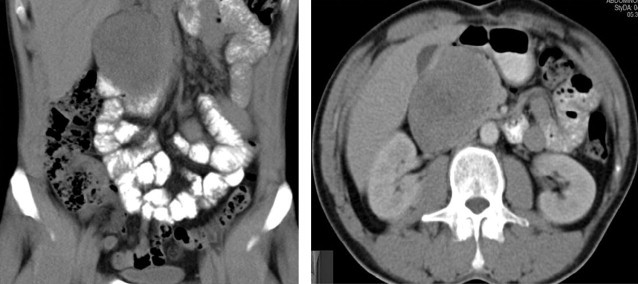
CT scan showing retroperitoneal lesion at the level of the pancreas with duodenal and large vessel compression.
With the endoscopy result a percutaneous biopsy was carried out, which was reported as a neuroendocrine tumor with intense positivity with immunohistochemistry. Neoadjuvant chemotherapy was started on suspicion of infiltration of the portomesenteric axis with dacarbazine, epirubicin, and 5FU, only for 3 cycles since the patient had toxicity during the last cycle conducted in July 2011. He was then subjected to programmed exploratory laparotomy where a pancreatoduodenectomy (Whipple procedure) was performed (Fig. 2) with findings of a tumor of the head of the pancreas about 7 cm × 4 cm × 7 cm in diameter, well defined, free in relation to large vessels, indurated, and involving the duodenum on its posterior and medial wall (Fig. 3). The definite histopathological report was that of a neuroendocrine tumor of the head of the pancreas, with a mitotic index of 1 mitosis per 10 HPF, and lesion-free borders (Fig. 4). The patient had an uneventful clinical course without complications and is currently in clinical control and follow-up with improvement.
Fig. 2.
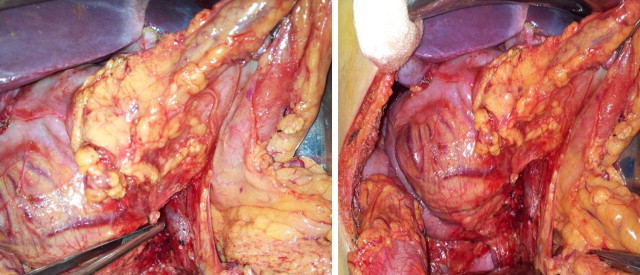
Surgical images that show the neuroendocrine lesion that involves the head of the pancreas with infiltration and compression of the second and third portion of the duodenum; (a) arrow indicating the portal vein; (b) arrow showing the lesion.
Fig. 3.
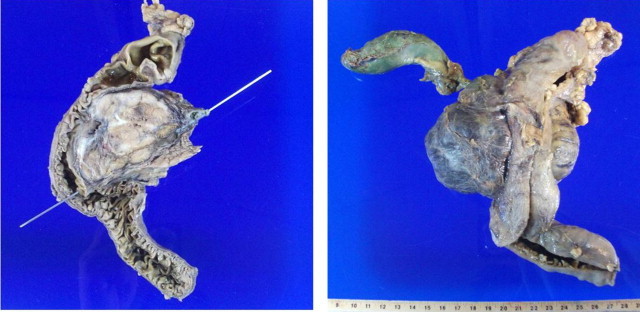
Fixed macroscopic specimen of pancreatic neuroendocrine tumor: (a) sagittal view; (b) posterior view where portions of the duodenum, the gall bladder and the biliary canal are seen.
Fig. 4.
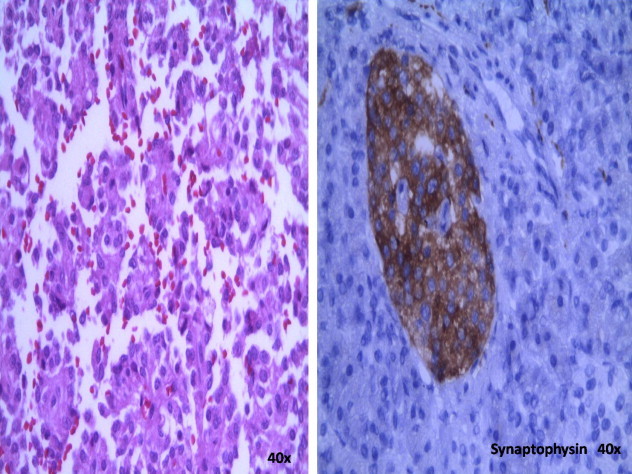
Positive immunohistochemistry reaction: (a) chromogranin positive; (b) synaptophysin positive. Both are diagnostic of pancreatic neuroendocrine tumors (400×).
3. Discussion
NF-PNETs are still considered rare entities, with 10 cases per million inhabitants. Most are functional tumors and only 15–30% are NF-PNET.7 They are a heterogeneous group of neoplasms that are characterized by nonspecific symptoms, leading to a delay of several years in their diagnosis; most present liver metastases.8 Most of the cases reported in the literature have been as a palpable abdominal mass with duodenal and gall bladder compression. Imaging techniques such as computed tomography, ultrasound, and endoscopic or intraoperative ultrasound have been useful for locating most PNETs greater than 2 cm in diameter, but imaging techniques have difficulty visualizing PNETs less than 5 mm,9 with endoscopic ultrasound having the highest sensitivity and specificity, 82% and 95%, respectively. Endoscopic ultrasound helps us to identify tumors in the duodenal wall and peripancreatic nodules with a sensitivity of 58%, similar to that of scintigraphy.10 Rodney et al. reported a sensitivity of 78% in the localization of lesions with computed tomography. Histopathologically, NF-PNET cannot be distinguished from functional tumors by immunohistochemistry. In general, positive staining with chromogranin A and synaptophysin confirms the diagnosis and these are elevated in 60–100% of NF-PNETs, although it is important to point out that a negative chromogranin result does not completely rule out an endocrine tumor since those that usually originate in the colon and the appendix are chromogranin subtype A negative, but chromogranin subtype B positive. Somatostatin receptor scintigraphy, based on the presence of somatostatin receptors present in 80–90% of all PNETs, is a tool for patients with this disease.11–13
Initial management of a PNET includes surgical resection of the tumor. Haynes et al. in a case series of more than 139 patients concluded that those with incidental NF-TNPs should undergo tumor resection and careful postoperative surveillance even if pathological findings are suggestive of benign disease.14 A considerable amount of patients with NF-PNETs are diagnosed incidentally. In 2010, in a PNET database, the Lee Moffitt Cancer Center concluded that 26% of tumors were discovered incidentally and 55% were in stage I according to AJCC stratification. Moreover, median patient survival was 103 months in those who already had symptoms versus 84 in those who did not.15
Medical treatment with drugs such as somatostatin analogs are used in patients who do not benefit with surgery alone16,17 and a chemotherapy regimen with doxorubicin, 5-FU and streptozocin has significant activity in patients with locally advanced and metastatic pancreatic endocrine carcinomas.18 In 2011 two therapies, sunitinib, and everolimus, were approved by the FDA for use in the control of unresectable PNET. Although everolimus has no benefits on survival, it does if disease progression is present, as well as being effective in patients who have been treated with somatostatin analogs and/or cytotoxic therapy.19 In February 2012, Raymond et al. in a randomized, double-blind, multinational study concluded that daily administration of 37.5 mg of sunitinib improved progression-free survival, overall survival, and the objective response rate in comparison with a placebo group in patients with advanced PNET.20 New cytotoxic therapies such as temozolomide and capecitabine are in phase II studies, but these have not shown greater effectiveness than the use of streptozocin.
Further studies are still in process, and this latest evidence shows the need for new management guidelines for patients with these characteristics that should be used to make good decisions. The characteristics of this pathology and its presentation remind us to continue the search for new strategies for early diagnosis and treatment.
Conflict of interest statement
None.
Funding
None.
Ethical approval
I have signed informed consent by the patient where self riza usage information as well as photos.
Author contributions
Co-authors participated in the realization of the surgeries and taking photos.
References
- 1.Baltogiannis G., Katsios C., Roukos D. New target therapies for patients with neuroendocrine tumors of the pancreas. Expert Review of Gastroenterology and Hepatology. 2011;5(5):563–566. doi: 10.1586/egh.11.55. [DOI] [PubMed] [Google Scholar]
- 2.Matthew H.K., Johanna B., Larry K., Joel P., Rodney P., James Y. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. Journal of Hematology and Oncology. 2011;4(29):1–8. doi: 10.1186/1756-8722-4-29. http://www.jhoonline.org/content/4/1/29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florian E., Hans D., Saeger C. Neuroendocrine tumors of the pancreas. The Oncologist. 2009;14:456–467. doi: 10.1634/theoncologist.2008-0259. [DOI] [PubMed] [Google Scholar]
- 4.Ramon S., Carlos V., Joan F. Tumores Neuroendocrinos gastrointestinales y pancreaticos. Medicine Clinica Barcelona. 2006;127(6):227–231. doi: 10.1157/13091016. [DOI] [PubMed] [Google Scholar]
- 5.Halfdanarson T.R., Rabe K.G., Rubin J., Petersen G.M. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annals of Oncology. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David C., Robert T. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135(November (5)):1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodney H.R. Focus on: neuro-endocrine tumours. CT/MRI of neuroendocrine tumours. Cancer Imaging. 2006;6(6):S163–S177. doi: 10.1102/1470-7330.2006.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvin M., Steven F., Daniel C. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. Journal of the National Cancer Institute. 2008;100:1282–1289. doi: 10.1093/jnci/djn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazuhiro H. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World Journal of Gastroenterology. 2010;16(36):4519–4525. doi: 10.3748/wjg.v16.i36.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binnaz D., Likay T., Barbro E. A patient with nonfunctional pancreatic neuroendocrine tumor and incidental metachronos colon carcinoma detected by positrón emission tomography, case report. The Turkish Journal of Gastroenterology. 2009;20(3):214–219. doi: 10.4318/tjg.2009.0010. [DOI] [PubMed] [Google Scholar]
- 11.Strosberg J., Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World Journal of Gastroenterology. 2010;16(June (24)):2963–2970. doi: 10.3748/wjg.v16.i24.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appetecchia M., Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. Journal of Experimental & Clinical Cancer Research. 2010;29:19. doi: 10.1186/1756-9966-29-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes A., Deshpande V., Ingkakul T. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Archives of Surgery. 2011;146(5):534–538. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheema A, Jill W, Strosberg R. Incidental deteccion of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Annals of Surgical Oncology 2010; http://dx.doi.org/10.1245/s10434.012.2285.7. [DOI] [PubMed]
- 15.Faiss S., Pape U., Böhmig M., Dörffel Y., Mansmann U., Golder W. Prospective, randomized, multicenter trial on the antiprolifertive effect of lanreotide, interferon alfa and their combination for therapy of metastasic neuroendocrine gastroenteropancreatic tumors. The International Lanreotide and Interferon Alfa Study Group. Journal of Clinical Oncology. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 16.Detlef K., Volker F., Langer P. Outcome of Duodenopancreatic reseccions in patients with multiple endocrine neoplasia type 1. Annals of Surgery. 2005;242(December (6)) doi: 10.1097/01.sla.0000189549.51913.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouvaraki A., Ajani J., Hoff P., Wolff R., Evans D., Lozano R. Fluorouracil, doxorubicin and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. Journal of Clinical Oncology. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Oberstein P. Safety and efficacy of everolimus in adult patients with neuroendocrine tumors. Clinical Medicine Insights: Oncology. 2012;6:757–761. doi: 10.4137/CMO.S7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond E., Laetitia D., Raoul J. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. The Nex England Journal of Medicine. 2012;364:501–513. doi: 10.1056/NEJMoa1003825. [6 Feb 10] [DOI] [PubMed] [Google Scholar]
- 20.Miljković M.D., Girotra M., Abraham R.R., Erlich R.B. Novel medical therapies of recurrent and metastatic gastroenteropancreatic neuroendocrine tumors. Digestive Diseases and Sciences. 2012;57(January (1)):9–18. doi: 10.1007/s10620-011-1854-0. [DOI] [PubMed] [Google Scholar]


