Abstract
INTRODUCTION
Angiosarcomas are rare tumours that arise from the vascular endothelium. They can occur anywhere in the body, mostly affecting the head and neck. Their occurrence in the gastrointestinal tract is quite rare with a few reported cases in medical literature.
PRESENTATION OF CASE
A 40-year-old man presented with metastatic sigmoid colon angiosarcoma, for which he was operated due to endoscopically uncontrollable massive tumour bleeding. The patient is presently still alive at 24 months after his first presentation. He is receiving palliative care.
DISCUSSION
This article presents a review of the literature on this rare clinical entity, emphasising the very aggressive behaviour and the poor outcome of this malignancy. We present, briefly, 17 reported cases on primary colonic angiosarcoma since 1949.
CONCLUSION
The role of chemotherapy and radiation is established neither in the adjuvant setting nor in metastatic disease. Surgery is the mainstay to treat localised colorectal angiosarcomas.
Keywords: Angiosarcoma, Colonic angiosarcoma, Colorectal cancer, Bone metastasis, Muscle metastasis, Lower gastrointestinal bleeding
1. Introduction
Gastrointestinal angiosarcomas are very rare malignant tumours that arise from the vascular endothelium, constituting far less than 1% of all gastrointestinal tract malignancies and only 1% of all sarcomas.1,2 In this article, we present a review of the literature on this rare clinical entity, with an emphasis on colorectal angiosarcomas, after reporting the case of a 40-year-old male who presented to our care with worsening lower gastrointestinal bleeding from a sigmoid colonic angiosarcoma that has shown to be metastatic to the bone and muscles upon presentation. The patient underwent a segmental sigmoidectomy to stop rectal bleeding and was referred to the care of the oncologist to receive chemotherapy, radiation and supportive care. Metastases to the bone and muscles have resulted in severe functional disability. Interestingly, the patient is still alive at 24 months after his first presentation despite his metastatic disease.
2. Report of a case
2.1. History
A 40-year-old male presented with a 4-week history of painless, intermittent rectal bleeding and progressively, worsening pain involving the left hip joint and the right thigh, restricting mildly his physical ability. He is an active labourer with no significant past history except for occasional alcohol intake and tobacco smoking. He denied weight loss or any other constitutional symptoms. Physical examination revealed no significant findings except for bright, red blood on rectal examination. Paraclinical studies revealed iron-deficiency anaemia and several areas of translucency involving the right and left femurs and around the left pelvic girdle. The pelvic osteolytic lesions were found on abdominal CT that showed no intra-luminal masses or focal liver lesions. Colonoscopy revealed an ulcerating, friable polyp at 25 cm from the anal verge with the presence of blood and clots (Fig. 1). A biopsy showed the mass to be a glandular epithelioma. CEA and CA19.9 levels were normal. Among others, HIV test was negative. A bone biopsy was free of malignancy contradicting the results of a bone scan that considered the osseous lesions to be highly suggestive of malignancy. The decision to perform a sigmoid colectomy was taken because the patient continued to have severe rectal bleeding that could not be addressed endoscopically.
Fig. 1.
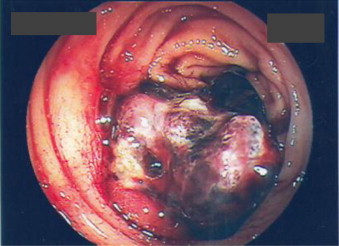
A colonoscopic view of the sigmoid tumour.
2.2. Pathology
A sigmoid colectomy was performed with a smooth early post-operative course. Macroscopically, the reddish, ulcerating polyp was 1.5 cm × 1.3 cm × 0.3 cm, having a sessile morphology. Few diverticula were noted in the specimen. Histopathology revealed a malignant, highly vascularised, poorly differentiated tumour invading the submucosa with a vague aspect (epithelial vs. sarcomatous). Positive immunohistochemical staining for CD31 and von Willebrand factor confirmed the endothelial nature of the mass (Fig. 2), to note; however, that a group of juxtatumoral capillaries stained positive for CD34, while tumour cells stained negative for this marker. Tumour cells stained positive, with variable intensity, to diverse cytokeratines recognised by the following antibodies: CAM 5.2, AE1/AE3 and MNF116. Final pathology revealed an epitheloid angiosarcoma infiltrating both the mucosa and the submucosa. The specimen had normal margins and was free of any lymphadenopathy.
Fig. 2.
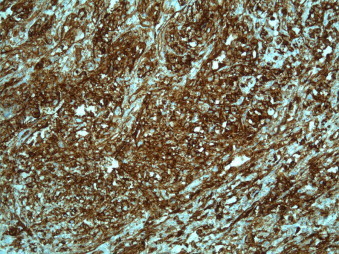
A photomicrograph illustrating positive CD31 immunohistochemical staining in the specimen (×5).
2.3. Outcome
The early post-operative course was unremarkable. The patient was referred to the oncologist and was started on a taxane-based chemotherapy protocol. Three months later and during chemotherapy, the patient presented with abdominal pain that showed to be secondary to a contained pelvic abscess related to a colonic perforation that was treated supportively (antibiotics and total parenteral nutrition) with good resolution and restoration of oral feeding. Eight weeks later, chemotherapy was resumed. The patient is presently, at 24 months after his presentation, on supportive care being almost continuously bed-ridden secondary to bone and muscle metastases that severely restrict his mobility. He is receiving palliative radiotherapy sessions for his bone metastases.
3. Discussion
Angiosarcomas are a subtype of soft tissue sarcomas and are aggressive, malignant endothelial-cell tumours of vascular or lymphatic origin.4 They account for less than 1% of all soft tissue sarcomas.3 About 60% of these lesions occur in the skin and superficial soft tissues of the head and neck.5 They rarely occur in the gastrointestinal tract and most of these occur in the stomach and small bowel.6,7 Colorectal angiosarcomas are rare constituting less than 0.001% of all colorectal cancers,8 with only a few cases reported in medical literature (Table 1). The first description of a colonic angiosarcoma was made by Steiner and Palmer.9
Table 1.
A summary of 17 reported cases on primary colonic and rectal angiosarcoma since 1949.
| Author | Patient Age, Gender | Tumour size (max. diameter in cm.) | Tumour location | Clinical presentation | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Steiner and Palmer9 | 46, Female | 3.5 | Sigmoid | Abdominal pain, palpable mass and obstruction | Sigmoidectomy | Alive 21 months postoperatively with uterine recurrence |
| Ormos and Sin22 | 34, Female | 7 | Cecum | Abdominal pain, palpable mass, weight loss | Right hemicolectomy with adjuvant radiotherapy | Died 23 months postoperatively with liver metastases |
| Saito et al.23 | 72, Male | 10 | Descending colon | Abdominal pain, rectal bleeding | Left colectomy | Death from disseminated disease 1 month postoperatively |
| Taxy and Battifora24 | 57, Female | 6 | Cecum | N/A | Right hemicolectomy | Death from disseminated disease 6 months postoperatively |
| Lo et al.25 | 37, Male | 2 | Rectum | Rectal bleeding | Abdomino-perineal resection | Alive 18 months postoperatively with no recurrence |
| Smith et al.26 | 16, Female | 5.5 | Sigmoid | Abdominal pain, palpable mass, rectal bleeding | Sigmoidectomy | Alive and disease-free 36 months postoperatively |
| Deleaval et al.27 | 74, Female | 3 | Cecum, transverse colon and rectum | Melaena | Palliative care | Died at about 1 month after diagnosis |
| Hofman et al.28 | 66, Male | 6 | Sigmoid | Abdominal pain, rectal bleeding | Sigmoidectomy | Death from disseminated disease 5 months postoperatively |
| Solheim et al.29 | 75, Female | NA | Cecum | Systemic upset, weight loss | Right hemicolectomy | Death from disseminated disease 4 months postoperatively |
| Ben-Izhak et al.12 | 70, Female | 5 | Rectum | Rectal bleeding | Surgical resection and adjuvant radiotherapy | Death from persistent haemorrhage 2 months postoperatively |
| Watanabe et al.30 | 70, Female | 1.7 | Sigmoid | Rectal bleeding | Sigmoidectomy | Alive at 2 years follow-up |
| Rozario and Ravi31 | 55, Male | 2.5 | Cecum | Abdominal pain, palpable abdominal mass | Right hemicolectomy | Alive 13 months postoperatively with no recurrence |
| Brown et al.3 | 77, Male | 4.5 | Sigmoid | Rectal bleeding, abdominal mass, weight loss | Sigmoid colectomy | Died 6 months postoperatively with liver metastases |
| Komorowski et al.32 | 17, Male | 12 | Sigmoid | Rectal bleeding, constipation | Sigmoid colectomy | Alive and well 19 months postoperatively |
| El Chaar and McQuary11 | 60, Female | N/A | Sigmoid | Abdominal pain | Segmental resection | Died 4 months postoperatively with metastases |
| Pascual et al.21 | 85, Female | 2 | Cecum | Lower gastrointestinal bleeding, haemorrhagic shock | Right hemicolectomy | Alive 24 months postoperatively with no evidence of recurrence |
| Lo et al.10 | 21, Female | 9 | Sigmoid | Abdominal pain and intraperitoneal bleeding | Sigmoid colectomy | Alive 36 months postoperatively with no evidence of recurrence |
Angiosarcomas have a similar distribution between both sexes. They can develop at any age, being commoner in older patients.4
The usual presentation of colorectal angiosarcomas is abdominal pain associated with a mass and rectal bleeding. This context is usually suggestive of colonic adenocarcinoma in spite of the unusual haemorrhagic endoscopic appearance. Unusual presentations have also been reported including free intraperitoneal bleeding,10,11 intestinal obstruction9,32 and chief complaints related to metastatic disease.
Most reported colorectal angiosarcomas have been localised to the sigmoid colon, which is the case of the patient presented herein.
Several predisposing factors for colorectal angiosarcomas have been suggested including radiation, chronic lymphoedema and foreign body implants11,12 yet no final associations can be made. However, arsenic, thorium dioxide (Thorotrast) and polyvinyl chloride have been associated with hepatic angiosarcomas.13,14 Most angiosarcomas arise spontaneously, yet there are few reports of malignant transformation within pre-existing benign vascular lesions.4,15
Histopathologically, these tumours are highly vascularised with abundance of endothelial cells and areas of solid and spindled cell tumour with an infiltrative and destructive growth pattern typical of angiosarcoma.3 With better immunohistochemical studies, some atypical sarcomas would now be correctly classified as angiosarcomas.10 These tumours typically express endothelial markers including von Willebrand factor, CD34, CD31, Ulex europaeus agglutinin 1 and vascular endothelial growth factor (VEGF). Immunohistochemistry is; therefore, a mainstay in confirming the diagnosis. In poorly differentiated cases, von Willebrand factor and CD 31 are the most useful markers. Kaposi's sarcoma, which has a similar immunohistochemical staining pattern to conventional angiosarcoma, can be distinguished by its tendency to be multifocal in the intestine and its usual association with HIV infection or other forms of immunosuppression.3,16
Tumour size and age at presentation have been suggested as prognostic factors affecting the course after treatment and survival in angiosarcomas. Longer survival has been associated with tumours less than 5 cm; size has shown to be an independent prognostic factor in angiosarcomas.14 Furthermore, younger age at presentation is associated with better survival, with patients younger than 50 years of age have a significantly better 2-year survival rate compared to older patients.3,13 However, data regarding better survival in younger patients is sometimes conflicting.17,21,30 Other obvious factors associated with a poorer outcome include metastatic disease at presentation, poor patient performance status4,18 and involved margins (R1 or R2) upon resection.
Concerning tumour staging, the International Union Against Cancer and the American Joint Committee on Cancer (UICC/AJCC) staging systems apply. This is based on the TNM (tumour-node-metastasis) staging system with an additional notation for histological grade.19 By definition, angiosarcomas are high-grade tumours. The tumour size defines the T entity in the TNM system, with tumours less than or equal to 5 cm being T1, larger tumours being T2.
As for treatment, radical surgery with complete (R0) resection is the treatment of choice. Wide margins are recommended because of the invasive and often multifocal nature of angiosarcoma,4 but this is often difficult to achieve. The role of adjuvant therapy in angiosarcoma is unclear, but there generally seems to be limited, if any, survival benefit with adjuvant chemotherapy in the treatment of sarcomas.3,13 Limited experience in angiosarcomas has shown disappointing results.13
Cytotoxic chemotherapy is the primary treatment option for metastatic angiosarcoma, although the evidence for this is also limited. Anthracyclines, ifosfamide and more recently taxanes are the mainstay of chemotherapy.4 Furthermore, biological therapies, in particular antiangiogenic therapies, may have a significant role in the treatment of angiosarcomas, either in the adjuvant setting or as a primary option in metastatic disease.20
4. Conclusion
Angiosarcoma of the colon and rectum is a rare malignancy. The case presented in this article and the review of literature suggest that colorectal angiosarcomas behave aggressively resulting in poor outcomes. No clear-cut guidelines concerning prognostic factors and treatment options can be made. However, complete (R0) surgical resection is the only treatment that has shown to result in long-term survival. Younger age at presentation and tumours less than 5 cm may be associated with better outcomes, yet the overall survival is generally poor. The role of adjuvant therapy and chemotherapy/radiation in the context of metastatic disease is unclear reflecting the need for further prospective studies to clarify treatment strategies.
Conflict of interest
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Dr. Ahmad Tabech, the attending general surgeon, he has operated on the patient and has put the design of the paper and analysed data and approved the final version to be submitted.
Dr. Roberto Algaba, general surgeon and admitted patient for treating his acute bout of sigmoid diverticulitis. He revised the article.
Dr. Christophe Firket, general surgeon and shared in decision-making concerning patient's treatment and overall management. He revised the article.
Dr. Daniel Brenez, the attending medical oncologist, he treated the patient and has put the chemotherapy and palliative care regimens. He revised the article.
Dr. Osama Al Beteddini, general surgery fellow, he has written the article and collected relevant literature.
Acknowledgements
We would like to thank Dr. Bernard Van Den Heule for his help and guidance concerning histopathology reporting and for providing the pathological slide. The contribution of Dr. Sophie Ghewy is highly acknowledged as well.
References
- 1.Shaver T.R., Lee Y.T. Nonosseous sarcomas in a military hospital. Journal of Surgical Oncology. 1987;36:284–289. doi: 10.1002/jso.2930360414. [DOI] [PubMed] [Google Scholar]
- 2.Bardwil J.M., Mocega E.E., Butler J.J., Russin D.J. Angiosarcomas of the head and neck region. American Journal of Surgery. 1968;116:548–553. doi: 10.1016/0002-9610(68)90391-7. [DOI] [PubMed] [Google Scholar]
- 3.Brown C.J., Falck V.G., MacLean A. Angiosarcoma of the colon and rectum: report of a case and review of the literature. Diseases of the Colon and Rectum. 2004;47(12):2202–2207. doi: 10.1007/s10350-004-0698-5. [DOI] [PubMed] [Google Scholar]
- 4.Young R.J., Brown N.J., Reed M.W., Hughes D., Woll P.J. Angiosarcoma. Lancet Oncology. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 5.Mark R.J., Poen J.C., Tran L.M., Fu Y.S., Juillard G.F. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77:2400–2406. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Gentry R.W., Dockerty M.B., Clagett O.T. Vascular malformations and vascular tumors of the gastrointestinal tract. International Abstracts of Surgery. 1949;88:281–323. [PubMed] [Google Scholar]
- 7.Chami T.N., Ratner L.E., Henneberry J., Smith D.P., Hill G., Katz P.O. Angiosarcoma of the small intestine: a case report and literature review. American Journal of Gastroenterology. 1994;89:797–800. [PubMed] [Google Scholar]
- 8.Cerilli L.A., Wick M.R. Immunohistology of soft tissue and osseous neoplasms. In: Dabbs D.J., editor. Diagnostic immunohistochemistry. Churchill Livingstone; Philadelphia: 2002. pp. 59–102. [Google Scholar]
- 9.Steiner C.A., Palmer L.H. Angiosarcoma of the colon with case report. Annals of Surgery. 1949;129:538–542. doi: 10.1097/00000658-194904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo T.H., Tsai M.S., Chen T.A. Angiosarcoma of sigmoid colon with intraperitoneal bleeding: case report and literature review. Annals of The Royal College of Surgeons of England. 2011;93(6):e91–e93. doi: 10.1308/147870811X591017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Chaar M., McQuary N., Jr. Sigmoid colon angiosarcoma with intrapeirtoneal bleeding and early metastasis. Journal of Surgical Education. 2007;64(1):54–56. doi: 10.1016/j.cursur.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Izhak O., Kerner H., Brenner B., Lichtig C. Angiosarcoma of the colon developing in a capsule of a foreign body. Report of a case with associated hemorrhagic diasthesis. American Journal of Clinical Pathology. 1992;97:416–420. doi: 10.1093/ajcp/97.3.416. [DOI] [PubMed] [Google Scholar]
- 13.Naka N., Ohsawa M., Tomito Y. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. Journal of Surgical Oncology. 1996;61:170–176. doi: 10.1002/(SICI)1096-9098(199603)61:3<170::AID-JSO2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Creech J.L., Jr., Johnson M.N. Angiosarcoma of the liver in the manufacture of polyvinyl chloride. Journal of Occupational Medicine. 1974;16:150–151. [PubMed] [Google Scholar]
- 15.Rossi S., Fletcher C.D. Angiosarcoma arising in hemangioma/vascular malformation: report of four cases and review of the literature. American Journal of Surgical Pathology. 2002 Oct;26:1319–1329. doi: 10.1097/00000478-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Friedman S.L., Wright T.L., Altman D.F. Gastrointestinal Kaposi's sarcoma in patients with acquired immunodeficiency syndrome. Endoscopic and autopsy findings. Gastroenterology. 1985;89:102–108. doi: 10.1016/0016-5085(85)90750-4. [DOI] [PubMed] [Google Scholar]
- 17.Lezama-del valle P., Gerald W.L., Tsai J., Meyers P., La Qualglia M.P. Malignant vascular tumors in young patients. Cancer. 1998;83:1634–1639. doi: 10.1002/(sici)1097-0142(19981015)83:8<1634::aid-cncr20>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Lahat G., Dhuka A.R., Lahat S., Smith K.D., Pollock R.E., Hunt K.K. Outocome of locally recurrent and metastatic angiosarcoma. Annals of Surgical Oncology. 2009;16:2502–2559. doi: 10.1245/s10434-009-0569-3. [DOI] [PubMed] [Google Scholar]
- 19.Weiss S.W., Goldblum J.R. 5th ed. Mosby; St. Louis, MO: 2008. Enzinger and Weiss's soft tissue tumors. [Google Scholar]
- 20.Verschraegen C.F., Quinn R., Rabinowitz I., Arias-Pulido H., Muller C. Phase I/II study of docetaxel (D), gemcitabine (G), and bevacizumab (B) in patients with advanced or recurrent soft tissue sarcoma (STS) Proceedings of the American Society of Clinical Oncology. 2009;27 doi: 10.1093/annonc/mdr299. abstr 10522. [DOI] [PubMed] [Google Scholar]
- 21.Pascual M., Juanpere N., Pera M. Primary colonic angiosarcoma as a cause of massive lower gastrointestinal bleeding. Revista Espanola de Enfermedades Digestivas. 2010;102(4):282–291. [PubMed] [Google Scholar]
- 22.Ormos J., Sin l. Uber das Hamangiosarkom des Darmes. Zentralblatt fur Allgemeine Pathologie und Pathologische Anatomie. 1959;99:352–357. [PubMed] [Google Scholar]
- 23.Saito R., Bedetti C.D., Caines M.J., Kramer K. Malignantepithelioid hemangioendothelioma of the colon: report of a case. Diseases of the Colon and Rectum. 1987;30:707–711. doi: 10.1007/BF02561694. [DOI] [PubMed] [Google Scholar]
- 24.Taxy J.B., Battifora H. Angiosarcoma of the gastrointestinal tract. A report of three cases. Cancer. 1988;62:210–216. doi: 10.1002/1097-0142(19880701)62:1<210::aid-cncr2820620132>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Lo Y.M., Gillett M.B., Vina M., Collin J., Fleming K.A. Hemangiosarcoma of the rectum after chronic anorectal ulceration. Journal of Clinical Gastroenterology. 1989;11:77–81. doi: 10.1097/00004836-198902000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Smith J.A., Bhathal P.S., Cuthbertson A.M. Angiosarcoma of the colon: report of a case with long-term survival. Diseases of the Colon and Rectum. 1990;33:330–333. doi: 10.1007/BF02055479. [DOI] [PubMed] [Google Scholar]
- 27.Deleaval J.P., Peter M.Y., Laurencet F., Fontolliet C. Multicentric intestinal angiosarcoma: report on a case. Annales de Pathologie. 1991;11:342–344. [PubMed] [Google Scholar]
- 28.Hofman P., Bernard J.L., Michielis J.F., Saint Paul M.C., Rampal A., Benchimol D. Primary angiosarcoma of the colon. Anatomo-clinical study of a case. Annales de Pathologie. 1991;11:25–30. (in French) [PubMed] [Google Scholar]
- 29.Solheim K., Dammen I., Roald B.B., Carlsen E. Angiosarcoma of the colon. Tidsskrift for Den Norske Laegeforening. 1991;111:3068–3069. [PubMed] [Google Scholar]
- 30.Watanabe K., Hoshi N., Suzuki T., Suzuki T. Epithelioid angiosarcoma of the intestinal tract with endothelin-1-like immunoreactivity. Virchows Archiv A: Pathological Anatomy and Histopathology. 1993;423:309–314. doi: 10.1007/BF01606896. [DOI] [PubMed] [Google Scholar]
- 31.Rozario A., Ravi H.R. Angiosarcoma of cecum: unusual presentation with intussusception. Indian Journal of Gastroenterology. 1995;14:31–32. [PubMed] [Google Scholar]
- 32.Komorowski A.L., Darasz Z., Kołodziejski L.S., Gruchała A. Acta Chirurgica Belgica. 2004;204:465–467. [PubMed] [Google Scholar]


