Abstract
INTRODUCTION
Crohn's involvement of duodenum is a rare event and may be associated to proteiform symptoms and uncommon pathological aspects which make diagnosis and treatment complex.
PRESENTATION OF CASE
The peculiar aspect of this case was a suspected duodeno-biliary fistula. The patient (female, 22 years old) was affected by duodenal Crohn's disease. Magnetic resonance imaging showed a dilated common bile duct, whose final part linked to a formation containing fluid, and characterized by filling of the contrast medium in the excretory phase. Abdominal ultrasound showed intra-hepatic and intra-gallbladder aerobilia. At surgery, the duodenum was mobilized showing an inflammatory stricture and a slight dilatation of the common bile duct, with no signs of fistulas. The opened duodenum was anastomized side to side to a transmesocolic loop of the jejunum. After surgery, the general condition of the patient improved.
DISCUSSION
Only two cases of fistula between a narrow duodenal bulb and the common bile duct have been described in literature and the Authors were not be able to verify the occurrence of a duodenal biliary fistula at surgery. The association between duodenal Crohn's disease and Sphincter of Oddi incontinence is a very rare finding with different etiology: chronic intestinal pseudo-obstruction, common bile duct stones, progressive systemic sclerosis.
CONCLUSION
The treatment to resolve Sphincter of Oddi incontinence for primary duodenal Crohn's disease is not clear. Strictureplasty could be the treatment of choice, because, resolving the stricture, the duodenal pressure is likely to decrease and the reflux through the incontinent sphincter can be avoided.
Keywords: Duodenal Crohn's disease, Sphincter of Oddi incontinence, Strictureplasty
Abbreviations: CD, Crohn's disease; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; UGI, upper gastro intestinal; MRI, magnetic resonance imaging; CKK, cholecystokinin; SO, Sphincter of Oddi
1. Introduction
Crohn's disease (CD) can affect the entire gastrointestinal tract, but gastroduodenal involvement is rarely observed. First described in 1937 by Gottlieb and Alpert, duodenal localization occurs in 0.5% to 4% of patients with CD.1 In nearly all cases epigastric pain or dyspepsia are the predominant symptoms, which mimic peptic ulcer or non-ulcer dyspepsia. In more advanced phases of the disease, most patients have symptoms due to obstructive lesions, such as epigastric distress, anorexia, nausea, vomiting and weight loss. Only a few have haematemesis or maelena.1 Every part of the duodenum can be involved, but the second part is most commonly affected. Different types of ulcer, such as aphthous, longitudinal, transverse, and deep, may occur. Abnormal folds, stenosis and ulceration are the main radiographical features.2 Obstruction is the most common complication of duodenal CD, but fistulae arising from the duodenum are extremely rare. It is commonly believed that the duodenal fistulae originate only from other primary localizations of diseased small or large bowel.3 Submitting patients with duodenal CD to X-ray examination, filling of pancreatic or biliary ducts may occur. This may be due to either fistula formation or reflux through a damaged ampulla of Vater.4 Consequences of this reflux can be an obstruction of the duodenal portion of the bile duct and pancreatitis.5,6
The most frequent indication for surgery is gastroduodenal obstruction, whereas major hemorrage, extensive fistula formation or suspicion of malignancy may warrant resection. Dilatation of strictures and strictureplasty may be viable treatment options in selected patients with stenosing duodenal CD.
2. Presentation of case
Female, 22 years old, with duodenal CD, onset at age of 8, with diffuse abdominal pain and malabsorption syndrome. At the age of 11, she was hospitalized for persistent abdominal pain associated with dyspepsia, constipation, fatigue and 7-kg weight loss in 3 months, and a BMI of 15.9. Blood tests showed an increased ERS, CRP 15 mg/dL, Hb 12.1 g/dL and normal WBC, glucose, coagulation, serum protein, amylase. Upper gastro intestinal (UGI) endoscopy was performed with difficulty to overcome the superior duodenal knee, showing a “granulomatous and easily bleeding duodenal mucosa, with multiple ulcers covered with fibrin.” The biopsy confirmed the suspected diagnosis of duodenal CD. Colonoscopy was negative. From the age of 11–16, the patient was treated with azathioprine and corticosteroid therapy, and reported a relative good condition with some exacerbation episodes. At the age of 19, the patient began Thalidomide therapy because of poor response to previous drugs. In 2009, due to exacerbation of symptoms, feeding difficulties and weight loss, an MRI was performed and showed a dilated common bile duct (9 mm) with its final part communicating with a formation (about 1 cm in diameter) containing fluid, and characterized by filling of the contrast medium in the excretory phase. This situation was attributable to a biliary inflammatory stricture. A new UGI endoscopy was performed: the duodenal bulb and the second duodenal portion appeared deformed and stenotic with hyperemic and ulcerated mucosa. A pneumatic dilatation was performed. At the age of 21, the patient suspended treatment with Thalidomide and began Infliximab with poor results. In 2011 another exacerbation of symptoms occurred, namely: diffused abdominal pain, dyspepsia, weight loss, diarrhea, and fever. Abdominal ultrasound showed intrahepatic and intragallbladder aerobilia (Fig. 1). Gastro-duodenal follow-through contrast X-ray documented a rigid, retracted and shortened duodenum, and slight opacification of the main biliary duct and of some intrahepatic branches (Fig. 2). Considering duodenal stricture, the failure of previous medical therapy and the suspicious of a biliary-digestive fistula, we decided to perform surgery.
Fig. 1.
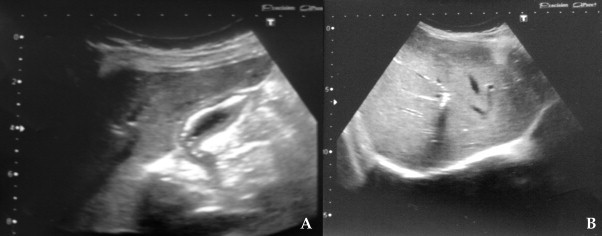
Abdominal ultrasound: intragallbladder and intrahepatic aerobilia (A and B).
Fig. 2.
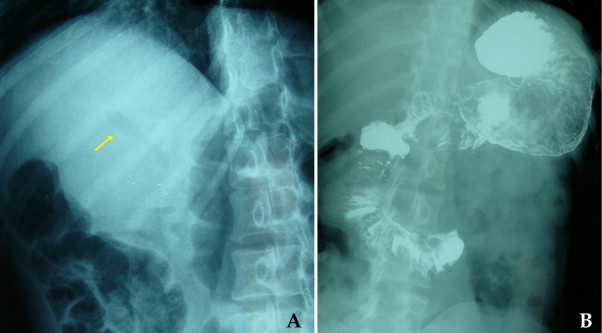
Opacification of main biliary duct and intrahepatic branches (A) and duodenal strictures (B) are visible at gastro-duodenal follow-through contrast X-ray.
Surgery was carried out in July 2011. The duodenum was completely mobilized showing an inflammatory stricture, starting near the pylorus until the III portion, many gallbladder adhesions, and a slight dilatation of the common bile duct, without signs of fistulas. The duodenum was opened longitudinally for a length of about 8 cm confirming the widespread inflammatory involvement of the duodenal mucosa and wall thickening, up to 8 mm. The papilla was found within the inflammatory context and was probed for some centimeters, penetrating easily both into the Wirsung and the biliary duct, from which clear mucus and bile flowed out (Fig. 3). The opened duodenum was anastomized side to side to a transmesocolic loop of the jejunum, taken about 20 cm from Treitz.
Fig. 3.
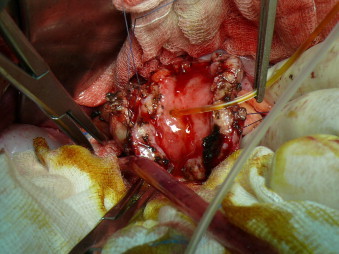
At a longitudinal duodenal opening, duodenal mucosa was inflamed, the duodenal wall was thickened and the papilla was probed for some centimeters, penetrating both into the Wirsung and the biliary duct, from which clear mucus and bile were flowing out.
About 25 cm from the Roux anastomosis there were three stenosis, that were treated with 12 cm Finney strictureplasty (Fig. 4). Below this, there were other 2 short jejunal strictures which were treated with Heineke-Mikulicz strictureplasties. After surgery, the general condition of the patient improved. Post-operative therapy was performed with 5-ASA. Twelve months after the operation there was a complete remission of symptoms with no difficulties in intaking food nor signs of occlusion. At abdominal ultrasound no signs of intrahepatic and intragallbladder aerobilia were found.
Fig. 4.
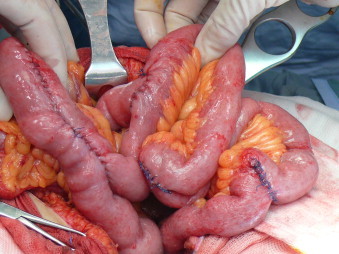
The outcome of the operation consisted in the Roux-en-Y duodenal jejunal anastomosis, jejunal Finney strictureplasty and jejunal H-M strictureplasty.
3. Discussion
CD of the duodenum may be associated to proteiform symptoms and uncommon pathological aspects which make diagnosis and treatment rather complex.7 The above mentioned patient had a long history of abdominal pain and weight loss before diagnosis. The peculiar aspect of this case was a suspected duodeno-biliary fistula. Only two cases of fistula between a narrow duodenal bulb and the common bile duct have previously been described in a clinical radiological report, some years ago.8 However, the Authors were not be able to verify the occurrence of a duodenal biliary fistula at surgery. In fact, the comment of the radiological finding of one of the cases was: “filling of the common bile duct and the cystic duct from the duodenum, probably through a duodenobiliary fistula.”8 The radiological picture could be due to a biliary filling of duodenal contrast medium through an incontinent Sphincter of Oddi (SO). The association between duodenal CD and SO incontinence is a very rare finding with different etiology, such as chronic intestinal pseudo-obstruction,9 common bile duct stones or progressive systemic sclerosis.
Oddi's Sphincter plays a major role in controlling bile and pancreatic juice flowing into the duodenum and in preventing the reflux of duodenal contents into the biliary and pancreatic ducts. These functions are regulated by the SO motility. Local reflexes involving the SO have been demonstrated between the duodenum,10,11 gallbladder and bile ducts.12–14 Sphincter of Oddi motility is controlled by cholecystokinin (CCK), which is released into the bloodstream by the duodenal mucosa, as a response to duodenal luminal acid and nutrients. Oddi's Sphincter plays an important role in decreasing the basal pressure and the amplitude of phasic waves. The cause of SO incontinence is uncertain, and one potential mechanism may be a defect of the neural connections that coordinate the interaction between the duodenum, the biliary tract and the SO. There is evidence that SO dysfunction may be a part of a generalized motor disorder of the gastrointestinal tract, such as small intestinal dysmotility.15 In one study, patients with irritable bowel syndrome and SO dysfunction demonstrated paradoxical responses to CCK more often than patients with SO dysfunction alone.16 Among the causes, there is the assumption that increased duodenal pressure overcomes the baseline pressure of SO. Reflux of the contrast medium into the pancreaticobiliary tree was noticed in the upper gastrointestinal series, in other cases of duodenal CD, with filling of either the pancreatic and bile ducts or of the bile duct alone.4,17 In these cases, one patient suffered from recurrent pancreatitis which was explained by periductal fibrosis induced by the persistently open, patulous, ampulla of Vater.18 In another case, there was evidence of cholangitis caused by the reflux of the duodenal content into the biliary tract.19
4. Conclusion
The treatment to resolve SO incontinence for primary duodenal CD is not clear yet; there is not sufficient literature on this topic. Surgery for duodenal CD includes several surgical options such as duodenal or gastro-duodenal resection, gastro-enteric or duodenal enteric by-pass or strictureplasty. The procedure most frequently employed for duodenal CD is gastro-jejunal bypass although, in a considerable percentage of cases, the procedure is followed by major complications in the postoperative condition.20 Strictureplasty could be the treatment of choice, because, resolving the stricture, the duodenal pressure is likely to decrease and the reflux through the incontinent SO can be avoided. Furthermore, since strictureplasty involves a progressive reduction of local inflammation, the pathogenetic mechanism that induces SO incontinence will also be interrupted.
Conflict of interest
Authors certify that there is no actual or potential conflict of interest in relation to this article and they state that there are no financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated—including pertinent commercial or other sources of funding for the individual author(s) or for the associated department(s) or organization(s), personal relationships, or direct academic competition.
Funding
Authors state that there are no financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated—including pertinent commercial or other sources of funding for the individual author(s) or for the associated department(s) or organization(s), personal relationships, or direct academic competition
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
All authors contributed equally to this work: Giovanni Alemanno, Francesco Bellucci and Alessandro Sturiale collected the data, Giovanni Alemanno and Alessandro Sturiale analyzed data, Giovanni Alemanno, Alessandro Sturiale, Francesco Giudici, Francesco Bellucci and Francesco Tonelli wrote the manuscript, Giovanni Alemanno and Francesco Tonelli supervised all the manuscript.
Acknowledgement
Prof. Maria Rosaria Buri, Professional Translator/Aiic Conference Interpreter, University of Salento for the English language editing.
References
- 1.Nugent F.W., Roy M.A. Duodenal Crohn's disease: an analysis of 89 cases. American Journal of Gastroenterology. 1989;84(3):249–254. [PubMed] [Google Scholar]
- 2.Miller E.M., Moss A.A., Kressel H.Y. Duodenal involvement with Crohn's disease: a spectrum of radiographic abnormality. American Journal of Gastroenterology. 1979;71(1):107–116. [PubMed] [Google Scholar]
- 3.Jacobson I.M., Schapiro R.H., Warshaw A.L. Gastric and duodenal fistulas in Crohn's disease. Gastroenterology. 1985;89(6):1347–1352. doi: 10.1016/0016-5085(85)90654-7. [DOI] [PubMed] [Google Scholar]
- 4.Legge D.A., Carlson H.C., Hoffman H.N. A roentgenologic sign of regional enteritis of the duodenum. Radiology. 1971;100(1):37–39. doi: 10.1148/100.1.37. [DOI] [PubMed] [Google Scholar]
- 5.Foutch P.G., Ferguson D.R. Duodenal Crohn's disease complicated by common bile duct obstruction: report of a case and review of the literature. American Journal of Gastroenterology. 1984;79(7):520–524. [PubMed] [Google Scholar]
- 6.Altman H.S., Phillips G., Bank S., Klotz H. Pancreatitis associated with duodenal Crohn's disease. American Journal of Gastroenterology. 1983;78(3):174–177. [PubMed] [Google Scholar]
- 7.Grabig A., Veltzke-Schlieker W., Sturm A. Acute pancreatitis and duodenobiliary fistula—a rare complication of Crohn's disease. Deutsche Medizinische Wochenschrift. 2007;132(23):1264–1267. doi: 10.1055/s-2007-982024. [DOI] [PubMed] [Google Scholar]
- 8.Rutgeerts P., Onette E., Vantrappen G., Geboes K., Broeckaert L., Talloen L. Crohn's disease of the stomach and duodenum: a clinical study with emphasis on the value of endoscopy and endoscopic biopsies. Endoscopy. 1980;12(6):288–294. doi: 10.1055/s-2007-1021762. [DOI] [PubMed] [Google Scholar]
- 9.Allescher H.D., Safrany L., Neuhaus H., Feussner H., Classen M. Aerobilia and hypomotility of the sphincter of Oddi in a patient with chronic intestinal pseudo-obstruction. Gastroenterology. 1992;102(5):1782–1787. doi: 10.1016/0016-5085(92)91744-o. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt A.P. The relationship of the sphincter of Oddi to the stomach, duodenum and gall-bladder. The Journal of Physiology. 1967;193(2):225–243. doi: 10.1113/jphysiol.1967.sp008354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saccone G.T., Harvey J.R., Baker R.A., Toouli J. Intramural neural pathways between the duodenum and sphincter of Oddi in the Australian brush-tailed possum in vivo. The Journal of Physiology. 1994;481(Pt 2):447–456. doi: 10.1113/jphysiol.1994.sp020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thune A., Thornell E., Svanvik J. Reflex regulation of flow resistance in the feline sphincter of Oddi by hydrostatic pressure in the biliary tract. Gastroenterology. 1986;91(6):1364–1369. doi: 10.1016/0016-5085(86)90188-5. [DOI] [PubMed] [Google Scholar]
- 13.Thune A., Saccone G.T., Scicchitano J.P., Toouli J. Distension of the gall bladder inhibits sphincter of Oddi motility in humans. Gut. 1991;32(6):690–693. doi: 10.1136/gut.32.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller E.L., Lewinski M.A., Pitt H.A. The cholecysto-sphincter of Oddi reflex. Journal of Surgical Research. 1984;36(4):377–383. doi: 10.1016/0022-4804(84)90114-8. [DOI] [PubMed] [Google Scholar]
- 15.Soffer E.E., Johlin F.C. Intestinal dysmotility in patients with sphincter of Oddi dysfunction. A reason for failed response to sphincterotomy. Digestive Diseases and Sciences. 1994;39(9):1942–1946. doi: 10.1007/BF02088129. [DOI] [PubMed] [Google Scholar]
- 16.Evans P.R., Dowsett J.F., Bak Y.T., Chan Y.K., Kellow J.E. Abnormal sphincter of Oddi response to cholecystokinin in postcholecystectomy syndrome patients with irritable bowel syndrome. The irritable sphincter. Digestive Diseases and Sciences. 1995;40(5):1149–1156. doi: 10.1007/BF02064214. [DOI] [PubMed] [Google Scholar]
- 17.Legge D.A., Hoffman H.N., Carlson H.C. Pancreatitis as a complication of regional enteritis of the duodenum. Gastroenterology. 1971;61(6):834–837. [PubMed] [Google Scholar]
- 18.Barthelemy C.R. Crohn's disease of the duodenum with spontaneous reflux into the pancreatic duct. Gastrointestinal Radiology. 1983;8(4):319–320. doi: 10.1007/BF01948142. [DOI] [PubMed] [Google Scholar]
- 19.Kato M., Ninomiya M., Sugiura J., Kato T. A case of duodenal Crohn's disease associated with ampullar insufficiency and cholangitis. Digestive Endoscopy. 1992;4:159–164. [Google Scholar]
- 20.Yamamoto T., Bain I.M., Connolly A.B., Allan R.N., Keighley M.R. Outcome of strictureplasty for duodenal Crohn's disease. British Journal of Surgery. 1999;86(2):259–262. doi: 10.1046/j.1365-2168.1999.01022.x. [DOI] [PubMed] [Google Scholar]


