Abstract
A recent study showed that the angular vestibulo-ocular reflex (VOR) can be better adaptively increased using an incremental retinal image velocity error signal compared with a conventional constant large velocity-gain demand (×2). This finding has important implications for vestibular rehabilitation that seeks to improve the VOR response after injury. However, a large portion of vestibular patients have unilateral vestibular hypofunction, and training that raises their VOR response during rotations to both the ipsilesional and contralesional side is not usually ideal. We sought to determine if the vestibular response to one side could selectively be increased without affecting the contralateral response. We tested nine subjects with normal vestibular function. Using the scleral search coil and head impulse techniques, we measured the active and passive VOR gain (eye velocity / head velocity) before and after unilateral incremental VOR adaptation training, consisting of self-generated (active) head impulses, which lasted ∼15 min. The head impulses consisted of rapid, horizontal head rotations with peak-amplitude 15 o, peak-velocity 150 o/s and peak-acceleration 3,000 o/s2. The VOR gain towards the adapting side increased after training from 0.92 ± 0.18 to 1.11 ± 0.22 (+22.7 ± 20.2 %) during active head impulses and from 0.91 ± 0.15 to 1.01 ± 0.17 (+11.3 ± 7.5 %) during passive head impulses. During active impulses, the VOR gain towards the non-adapting side also increased by ∼8 %, though this increase was ∼70 % less than to the adapting side. A similar increase did not occur during passive impulses. This study shows that unilateral vestibular adaptation is possible in humans with a normal VOR; unilateral incremental VOR adaptation may have a role in vestibular rehabilitation. The increase in passive VOR gain after active head impulse adaptation suggests that the training effect is robust.
Keywords: vestibulo-ocular reflex (VOR), unilateral vestibular adaptation, incremental retinal image velocity error, vestibular rehabilitation
Introduction
The angular vestibulo-ocular reflex (VOR) is evoked by rotations of the head. During head rotations while viewing a far target or scene, an ideal VOR should rotate the eyes in the opposite direction but with equal magnitude to head velocity—to stabilise images on the retina. A debilitating problem after injury to the peripheral vestibular organ is the inability to maintain stable vision during everyday activities in which the head moves, such as walking and driving.
One treatment for loss of vestibular function are rehabilitation exercises that attempt to improve gaze stabilisation (eye orientation with respect to space) during active head movements by increasing the VOR gain (eye velocity / head velocity) and enlisting other oculomotor systems. These exercises are based on the hypothesis that the VOR retains its adaptive capabilities in the presence of unilateral vestibular hypofunction (UVH) and can be enhanced using visual and vestibular stimuli that create retinal image slip (Paige 1994; Szturm et al. 1994; Viirre and Sitarz 2002). Retinal slip that induces a VOR gain change occurs when the head and target velocity are incongruent. A number of human VOR studies have demonstrated a robust capacity for gain adaptation of the normal VOR by coupling head motion with target motion to elicit retinal slip as a velocity error signal (Gauthier and Robinson 1975; Gonshor and Melvill Jones 1976a, b).
Most studies of VOR gain adaptation have delivered a retinal image velocity error signal that seeks a large VOR change ‘all at once’. Typical is to make the target move at the same speed as the head but in the opposite direction (a × 2 stimulus) so that the VOR has to compensate for twice the head velocity—a stimulus that requires a very large adaptation all at once. Non-vestibular motor control studies and auditory perception studies indicate that smaller and incremental error signals in learning tasks drive neural plasticity and learning more effectively than large error signals (Kagerer et al. 1997; Nagarajan et al. 1998, 1999; Kilgard and Merzenich 2002). Recently, Schubert et al. (2008) showed that the VOR can be modified similarly by smaller incremental retinal image velocity slip stimuli during self-generated head rotations (Schubert et al. 2008). A × 1.1 stimulus was used to increase the VOR by 10 % and, after a brief rest, a × 1.2 stimulus took it a further 10 % (20 % total). These increments were repeated until the × 2 stimulus was reached. Typically, the training period lasted for 15 min. For normal subjects, this incremental training technique led to larger VOR gain change compared with the × 2 stimulus alone (mean 17.3 ± 4 vs. 7.1 ± 9 %, P = 0.029). For UVH subjects, there was even greater improvement in VOR gain (18.2 ± 9.2 vs. −6 ± 3.8 %, P = 0.003). These results show that incremental vestibular adaptation is more effective than ‘all at once’ adaptation.
The study of Schubert et al. (2008) used a bilateral stimulus of retinal image velocity slip that induced adaptation so that the VOR gain was driven up for head rotations towards both the healthy and lesioned side. This is not ideal for patients with a unilateral lesion whose VOR is under-compensatory (gain < 1) only for head rotation towards the lesioned side. Only the lesioned side needs increasing—the normal side does not. In fact, increasing the normal side gain is undesirable because it results in over-compensatory eye movements (Schubert et al. 2008). The ideal is a unilateral retinal slip stimulus that incrementally increases the VOR only for rotations towards the lesioned ear.
The only study to examine unilateral VOR adaptation was in normal and vestibular lesioned monkeys. Normal monkeys were rapidly rotated (passive whole body) only to the left side while wearing × 1.7 magnifying glasses for 3 h (Ushio et al. 2011). Post-adaptation, the leftward VOR gain measured in darkness was ∼20 % greater than pre-adaptation. The rightward VOR gain was unchanged. Similarly, monkeys with complete unilateral vestibular lesions rotated only towards their lesioned ear, showed significant ipsilesional unilateral VOR adaptation. This finding shows that unilateral vestibular adaptation is possible in (a species of) primates even after a complete unilateral lesion. Our study investigated whether unilateral VOR adaptation in humans is possible.
Methods
Subjects
We studied nine normal subjects (mean age, 36 years; range, 21–58 years) during one session each. None of these subjects had a history or clinical signs of vestibular disease. Normal vestibular function was confirmed via observation of a negative head impulse test in yaw. Participation in this study was voluntary, and informed consent was obtained as approved by the University of New South Wales Human Ethics Committee.
Recording system
The movements of the left eye were recorded in three dimensions using a dual-axis scleral search coil embedded in a silicon annulus. The instrumentation and technique using a similar system and setup have been described in detail elsewhere (Straumann et al. 1995; Migliaccio et al. 2004). A search coil embedded in a bite block was used to measure head rotation. Eye and head angular position signals were filtered with a single-pole, low-pass analogue filter that had a 3-dB bandwidth of 100 Hz. They were then sampled at 1,000 Hz at 16-bit resolution and digitally filtered with a 50-tap zero-phase low-pass FIR filter with a bandwidth of 50 Hz.
Each subject was tested while seated upright with the head centred within a uniform magnetic field with the interpupillary line and Frankfort line (from the top of the external acoustic meatus to the infraorbital foramen) in the Earth-horizontal plane, i.e. horizontal canals were approximately Earth-horizontal. The magnetic frame was a 62-cm cube. There is a 10-cm3 region of linearity at the centre of this cube within which there is no effect of translation of the search coil on its orientation within the magnetic field.
Visual fixation target laser system
We used a laser and real-time dual-axis/dual-mirror galvanometer system (Model 6210HSM60 with 673XX dual-axis servo driver amplifier, Cambridge Technology, USA) for display of a visual target (a 2-mm diameter red laser dot) onto a matte black-painted wall 111 cm directly in front of the subject along the naso-occipital axis. The laser projection unit was placed behind and above the subject. Horizontal and vertical laser position was recorded in Fick coordinates with respect to the subject. The laser position update rate was 1,000 Hz with settling time <1 ms. Laser position was controlled using real-time yaw and pitch head position signals from the ‘head’ scleral search coil. Apart from the laser target, subjects were tested in a darkened room.
Passive and active head impulses
A head impulse consists of a head rotation with peak-amplitude ∼15 °, peak-velocity ∼150 °/s and peak-acceleration ∼3,000 °/s2 (Halmagyi and Curthoys 1988). Passive head impulses are unpredictable and delivered manually, whereas active head impulses are generated by the subject. Before the start of each active or passive head impulse, the subject’s head was centred straight ahead within a ±2 o (yaw and pitch) window. When the head was within the ±2 o window, a flashing red laser target appeared straight ahead (10 Hz at 3 % duty cycle)—this was the cue to commence each head impulse. Passive head impulses were delivered manually in the horizontal canal plane, i.e. leftward and rightward. Subjects were trained to perform active head impulses similar in profile to the passive head impulses. We measured the effect of unilateral incremental VOR adaptation training by comparing the active and passive VOR gains before and after training.
Unilateral incremental VOR adaptation training
Subjects were asked to make active (self-generated) head impulses from a neutral, neck-centred starting position alternating to the left and right. Once each head impulse was complete, subjects were instructed to pause and slowly return to centre before performing an impulse to the opposite side.
During the head impulse, the subject’s task was to either fixate the visible laser target or fixate the last remembered location of the laser target before it turned off. Vertical laser position moved in the same direction and magnitude as the head for pitch head movements < ± 2 o, but the laser turned off for angles outside this range. Horizontal laser position was controlled by horizontal head position, head direction (left or right) and the adaptation gain (see below).
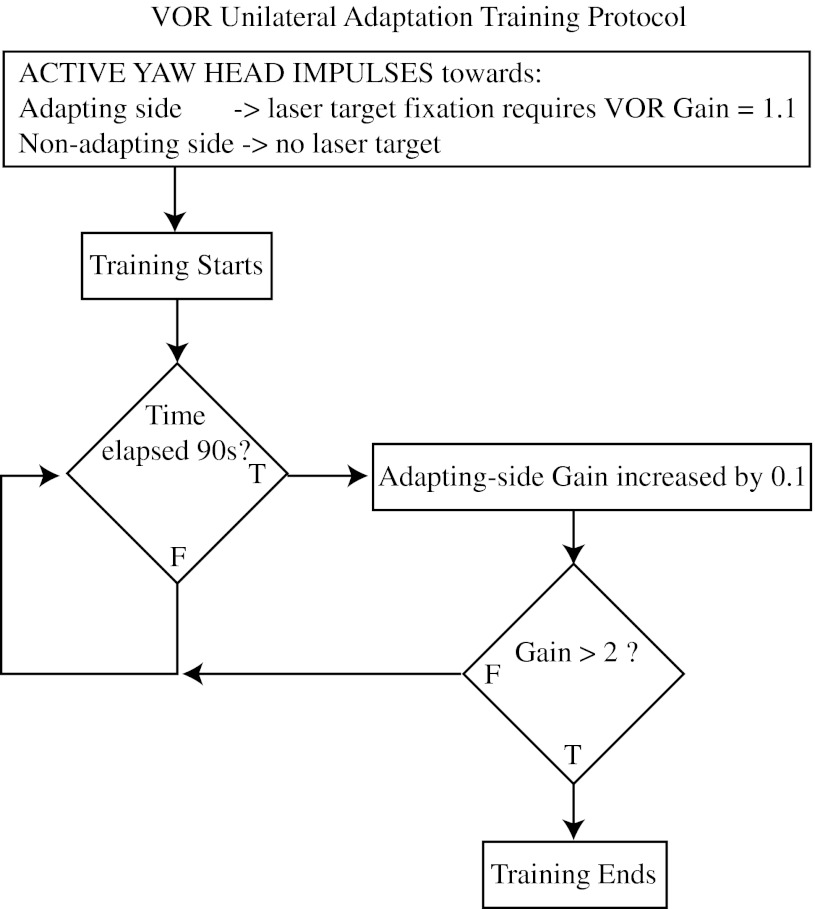
All subjects performed about 300 active head impulses, divided into 10 epochs of 30 head impulses (15 to each side) with 30- to 60-s rest periods between epochs. For example, during epoch 1, the subject made 30 active head impulses—alternating from right to left (n = 15 per side). During the 15 active head impulses towards the adapting side, the laser target moved in the opposite direction of the head rotation but with 10 % of the magnitude of head velocity. During the 15 active head impulses to the non-adapting side, the laser target was turned off during the head impulse. Thus, epoch 1 asked for a 10 % VOR gain increase for rotations towards the adapting side only. The target velocity for epoch 2 was manually increased by another 10 % (now 20 % of the head velocity) and this continued until the final epoch (10), which asked for a 100 % gain increase, i.e. adaptation gain equals 2, towards the adapting side (see Fig. 1). We randomised the adapting side (rightwards for five subjects and leftwards for four subjects). The adaptation training typically took 15 min.
FIG. 1.
Unilateral VOR adaptation protocol flow diagram. For active head rotations towards the adapting side, the gain required for image stabilisation was initially set to 1.1. For active head rotations towards the non-adapting side, the target was extinguished. After 90 s of training, the adapting side gain was increased by 0.1 and the training was repeated. This cycle continued until the adapting side gain was greater than 2.
Data analysis
Eye and head angular positions were represented by rotation vectors with roll, pitch and yaw coordinates (Haslwanter 1995; Migliaccio and Todd 1999). The orientation of each eye relative to the head was also determined as rotation vectors. The velocity vectors of head-in-space, eye-in-space and eye-in-head were calculated from the corresponding rotation vectors (Hepp 1990). Head velocity was calculated with reference to a head-fixed coordinate frame using the methods of Aw et al. (1996), so that eye and head velocities were expressed with reference to exactly the same coordinate frame.
The onset of each head impulse was calculated from the magnitude of the head-in-space velocity vector by fitting a polynomial curve to the head-in-space velocity versus time. The point where the magnitude of the fitted curve was greater than 2 % of the curve’s peak magnitude (typically this threshold was 4 o/s) was defined as the time of onset. The horizontal VOR gain was calculated by dividing inverted horizontal eye velocity by horizontal head velocity during the 30 ms period prior to peak head velocity.
Statistical analysis
Statistical analysis was performed using Matlab 2008a (Mathworks, USA) and Excel 2003 (Microsoft, USA) software. We used a multi-way analysis of variance (ANOVA) with two-factor interactions to analyse the data (Diggle et al. 1994). Independent variables included: impulse (‘active’, ‘passive’), time (‘pre’, ‘post’, ‘training’), head rotation direction (‘left’, ‘right’) and training trial number (1, 2, 3 …10). The only dependent variable was gain. All variables were included in the ANOVA initially and those found insignificant were subsequently removed. Paired t tests were performed on the pre- and post-training VOR gains. A general linear model (GLM) was used to analyse the training data. Only the interaction effects found to be significant are included in the results. Pooled data are described as mean ± 1 SD, whereas pooled means are described as mean ± 1 SE.
Results
Passive and active pre- and post-adaptation VOR gains
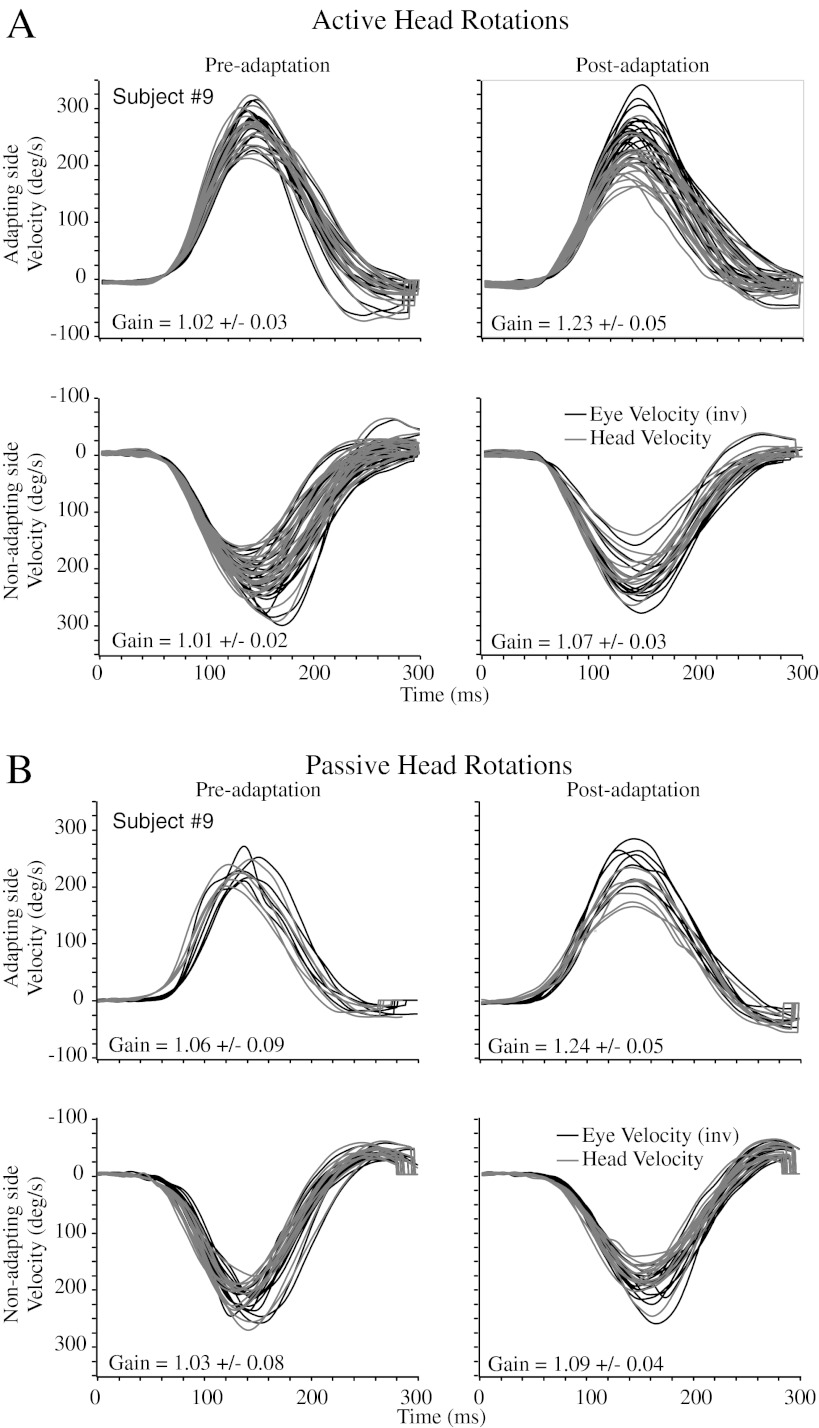
Figure 2 shows both the active (panel A) and passive (panel B) horizontal VOR responses before (left column) and after (right column) adaptation training in one subject (#9). The pre-adaptation gains for both active and passive head rotations are all close to unity. For this subject, the post-adaptation gains are significantly different from the pre-adaptation gains in three out of four conditions (active and passive, adapting and non-adapting, i.e. 2 × 2 conditions). For active head impulses, the VOR gain during rotations towards the adapting side increased by ∼21 % and towards the non-adapting side by ∼7 %. Similarly, for passive head impulses, the VOR gain during rotations towards the adapting side increased by ∼17 %. However, for impulses towards the non-adapting side, the increase was not significant (t test: T(25) = −1.84, P = 0.0777).
FIG. 2.
(A) Active head rotations in subject #9. The first row (head impulses towards adapting side) shows the vestibular stimulus (head velocity—grey) and the inverted vestibular response (eye velocity—black) pre-adaptation (left panel) and post-adaptation (right panel) training. The active VOR gain increased by ∼21 % in this subject. The second row (head impulses towards non-adapting side) shows that the VOR increased by ∼7 %. (B) Passive head rotations in subject #9. The passive VOR gain increased by ∼17 % in this subject. In contrast to the active VOR, there was no significant VOR increase during head impulses towards the non-adapting side.
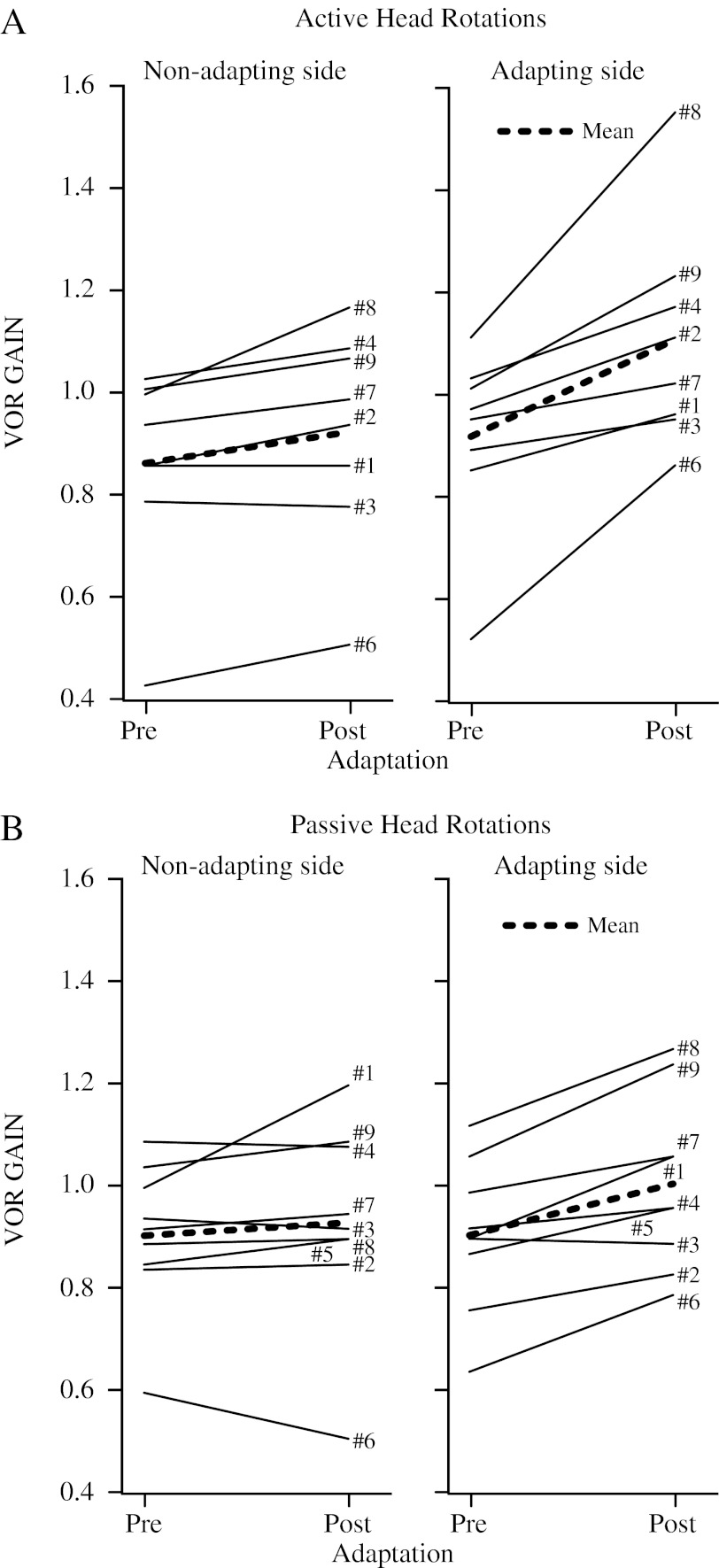
Figure 3 shows the pre- and post-adaptation gains for all subjects (n = 9). The pre- and post-adaptation gains are significantly different (ANOVA: time variable, F(2,63) = 3.50, P < 0.0362). The thick dashed line represents the mean change. At first glance, it seems that the pre- and post-adaptation gains for passive and active head impulses towards the non-adapting side are similar, i.e. there is minimal change. In contrast, the change between pre- and post-adaptation gains for passive and active head impulses towards the adapting side is large, ∼20 % during active impulses and ∼10 % during passive impulses. For active head impulses, seven out of eight subjects had a significant (i.e. P < 0.05) gain increase towards the adapting side and five out of eight subjects had a, albeit less, significant increase towards the non-adapting side. For passive head impulses, eight out of nine subjects had a significant gain increase towards the adapting side, whereas only three out of nine subjects had a significant increase towards the non-adapting side.
FIG. 3.
(A) Comparison of the pre- and post-adaptation active VOR gain for all subjects (n = 9) (mean across subjects—thick dashed black line). SD ranged 0.02–0.08. Although there is variation between subjects, there is a consistent unilateral increase in the active VOR gain due to adaptation training. (B) Comparison of the pre- and post-adaptation passive VOR gain for all subjects (n = 9). SD ranged from 0.03 to 0.12. Albeit different to the active head impulse training context, there is a consistent unilateral increase in the passive VOR gain due to training. This increase is ∼50 % of that observed in the active VOR gain.
For active head impulses towards the adapting side, the average pre-adaptation gain was 0.92 ± 0.18 and the average post-adaptation gain was 1.11 ± 0.22 (an increase of 22.7 ± 20.2 %). This 0.19 ± 0.18 increase was statistically significant (paired t test: T(7) = 3.96, P = 0.00546). Similarly, for active head impulses towards the non-adapting side, the average pre-adaptation gain was 0.87 ± 0.20 and the average post-adaptation gain was 0.93 ± 0.21 (7.6 ± 7.2 %). This 0.06 ± 0.06 increase was ∼70 % smaller than the increase towards the adapting side; however, it was significant (paired t test: T(7) = 3.13, P = 0.0167). The active non-adapting side gain increase was 30.5 ± 28.8 % (paired t test: T(7) = 3.52, P = 0.00965) of the adapting side gain increase.
For passive head impulses towards the adapting side, the average pre-adaptation gain was 0.91 ± 0.15 and the average post-adaptation gain was 1.01 ± 0.17 (11.3 ± 7.5 %). This 0.10 ± 0.06 increase was significant (paired t test: T(8) = 4.70, P = 0.00154). In contrast, for passive head impulses towards the non-adapting side, the average pre-adaptation gain was 0.91 ± 0.14 and the average post-adaptation gain was 0.93 ± 0.20 (2.0 ± 9.1 %). This 0.03 ± 0.08 increase was not significant (paired t test: T(8) = 0.981, P =0.355). The passive non-adapting side gain increase was 43.0 ± 78.4 % (paired t test: T(8) = 1.63, P = 0.143) of the adapting side gain increase.
Adaptation training VOR gain
The VOR gain during the training period depended on the whether the head impulse was towards the adapting or non-adapting side (ANOVA: direction variable, F(1,97) = 21.02, P < 0.0001).
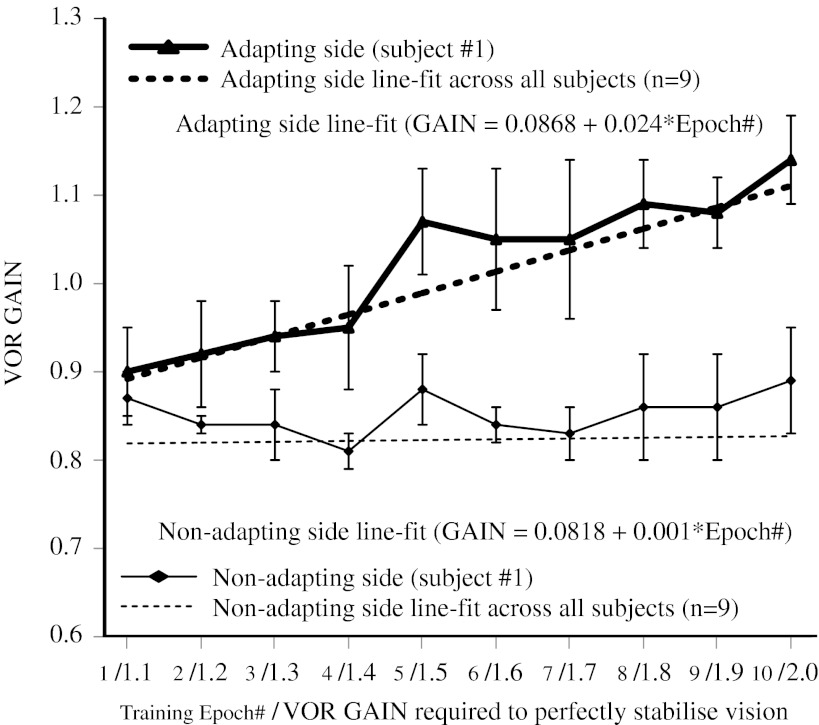
Figure 4 shows the VOR gain measured during each training epoch (1–10) in one subject (#1). The dashed lines represent the best line fits across all subjects (n = 9). The VOR gain during impulses towards the adapting side steadily increased up to 20 % (epoch 10) from baseline (epoch 1), and the line-fit sensitivity coefficient (0.024) was significant (GLM: T(57) = 16.64, P < 0.0001). In contrast, the VOR gain during impulses towards the non-adapting side did not increase (epoch 10) from baseline (epoch 1) and the line-fit sensitivity coefficient (0.001) was not significant (GLM: T(56) = 0.0980, P = 0.922).
FIG. 4.
The active VOR measured during each training epoch for subject #1. The active VOR gain for head impulses towards the adapting side steadily increased from 0.9 to 1.14 during unilateral incremental training. In contrast, the gain towards the non-adapting side did not vary during the training. Best line fits (adapting side—thick dashed black; non-adapting side—thin dashed black) for the training data across all subjects (n = 9) show that there was a significant relationship between gain and training epoch number only for head impulses towards the adapting side.
Discussion
Unilateral incremental adaptation
Our study shows that unilateral incremental VOR adaptation can be induced in normal subjects with 15 min of training. The VOR gain towards the adapting side was increased by ∼22 % during active and ∼11 % during passive head impulses. The fact that unilateral adaptation training in normal subjects consisting only active head impulses caused a significant increase in the VOR gain during passive head impulses (i.e. different from the training context), suggests that the training is not only causing changes in the predictive eye movement mechanisms that receive efferent copy signals of internal motor commands but also in the modifiable pathways of the VOR.
During active impulses, the VOR gain towards the non-adapting side also increased by ∼8 %, an increase ∼70 % less than the adapting side. This result might be due to changes in the signals carried by the inhibitory commissural vestibular pathways from the adapting side, which contribute to the non-adapting side response (McCrea et al. 1981; Shimazu and Precht 1966). If the sensitivity of Type I neurons on the adapting side (medial vestibular nucleus (MVN); for horizontal VOR) is increased due to adaptation training, then their firing rate will be greater than normal during rotations to the adapting side, and presumably less than normal during rotations to the non-adapting side. Consequently, during rotations to the non-adapting side, Type II inhibitory neurons (on the non-adapting side) that receive commissural input from Type I neurons (on the adapting side) will be less inhibitory, which could explain the increase in VOR gain.
Mechanism of unilateral adaptation
Models of the VOR in the alert monkey have suggested the existence of a highly modifiable phasic component of the reflex. This phasic component is probably mediated by irregularly discharging vestibular afferents because regularly discharging afferents have relatively linear response dynamics between 2 and 20 Hz (Hullar and Minor 1999). This phasic component has high sensitivity, so it is large during excitation, but can rapidly go into cut-off (negative saturation) during inhibition. The likely reason that the head impulse test is a form of unilateral stimulation (Halmagyi et al. 1990) is because this component dominates the afferent signal coming from the excited canal, but is in cut-off when coming from the inhibited (contralateral paired) canal. In contrast, the tonic pathway provides linear encoding of head velocity with little susceptibility to inhibitory cut-off. A recent VOR adaptation study in monkeys with unilateral vestibular lesions showed robust unilateral adaptation using × 1.7 spectacles for rotations towards the lesioned ear (Ushio et al. 2011). The peripheral vestibular signal could only be coming from the contralesional ear (i.e. the unilateral lesion was a complete labyrinthectomy) and clearly inhibitory cut-off was not occurring. This finding strongly suggests that the tonic component of the VOR is responsible for most of the unilateral adaptation, especially after a complete unilateral lesion and that the modifiable VOR pathways receive significant input from regular firing afferents (Lisberger and Pavelko 1986).
The flocculus of the vestibulocerebellum plays an important role in partially restoring the amplitude and symmetry of the VOR gain after a large unilateral labyrinthine deficit (e.g. Babalian and Vidal 2000). The flocculus appears to have a more prominent role in adjusting the passive gaze stabilising VOR, rather than the active VOR (Belton and McCrea 2004). The Purkinje cells in the flocculus participate in adjusting the VOR during viewing contexts that require fast changes in gain, e.g. during near viewing (Lisberger and Fuchs 1978; Snyder and King 1996). The floccular target neurons (FTN) receive input from both the flocculus and primary vestibular afferents and are thought to be important for VOR adaptation (Lisberger 1994; Blazquez et al. 2006). The modulation changes of FTN neurons have corresponded with changes in the VOR gain induced by training with visual-vestibular conflict (Lisberger et al. 1994a, b; Galiana and Green 1998), so they seem to be a likely main contributor to unilateral VOR adaptation. However, other neuron types in the MVN, e.g. position-vestibular-pause, eye–head, vestibular-only and burst-position neurons have also shown sensitivity changes depending on the particular combination of vestibular and visual stimuli including: VOR cancellation, eccentric rotation and active versus passive head rotation (McConville et al. 1996; McCrea and Luan 2003; Cullen 2004). Clearly, further studies are needed to determine the precise mechanisms behind unilateral VOR adaptation.
Rehabilitation potential
Classic studies of VOR adaptation using magnifying glasses have shown that the normal VOR gain can be increased (Gauthier and Robinson 1975), decreased (Gonshor and Melvill Jones 1976a) and reversed (Gonshor and Melvill Jones 1976b). In the reversal study, the adaptation was retained overnight and with subsequent sessions, the VOR response was reversed by day 27. De-adaptation occurred over a similar time period to adaptation, i.e. 2–3 weeks. These results indicate that VOR adaptation can potentially be retained in vestibular patients.
While the experiments above were critical to establish a stout plasticity within the VOR pathways, they are not useful in a rehabilitation context in part because the adaptation was most evident when testing the VOR in darkness. In light, the adapted response significantly decreased (∼70 %). In contrast, the VOR gains measured in our study were measured in a darkened environment (not complete dark) while the subject viewed a laser target that disappeared only after head impulse onset. This suggests that incremental adaptation can improve the VOR response in light—a critical component to rehabilitation programmes.
Our study is an important step towards developing a practical rehabilitation technique that can be implemented via a portable head-mounted device, for patients with vestibular hypofunction. First, we induced significant unilateral VOR adaptation after only 15 min of training, i.e. versus 3 h (i.e. the method of Ushio et al. 2011). Second, our training consisted of self-generated active head impulses, i.e. versus passive head impulses that require the use of a rotary chair or manual operator. Third, our VOR gains were measured in a darkened (dimly lit) environment, i.e. versus strict darkness. The next step is to determine whether patients with vestibular hypofunction show similar ipsilesional adaptation. We believe studying unilateral VOR adaptation in patients with complete unilateral hypofunction in particular will help determine the basic mechanisms behind unilateral adaptation. Other important steps include: determining the retention period after unilateral adaptation, which is relevant for maintenance training, and comparing short- and long-term outcomes in patients performing regular training versus current best practise. These outcomes could include: VOR gain, dynamic visual acuity score, balance and gait measures, fall risk assessment and an activities of daily living score.
Conclusion
Our study shows that unilateral VOR gain adaptation is possible in normal humans. This is achieved by exposing subjects to 15 min of incremental velocity error signals during self-generated head rotations at velocities relevant to daily function. This unilateral adaptation is robust enough to encourage contextual transfer to passive head rotation and the contralateral side (albeit less so).
Acknowledgments
This study was supported by a National Health and Medical Research Council (Australia) Biomedical Career Development Award to A.A. Migliaccio.
References
- Aw ST, Haslwanter T, Halmagyi GM, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol. 1996;76:4009–4020. doi: 10.1152/jn.1996.76.6.4009. [DOI] [PubMed] [Google Scholar]
- Babalian AL, Vidal PP. Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: an in vitro whole brain study. J Neurophysiol. 2000;84:2514–2528. doi: 10.1152/jn.2000.84.5.2514. [DOI] [PubMed] [Google Scholar]
- Belton T, McCrea RA. Context contingent signal processing in the cerebellar flocculus and ventral paraflocculus during gaze saccades. J Neurophysiol. 2004;92:797–807. doi: 10.1152/jn.00218.2004. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Hirata Y, Highstein SM. Chronic changes in inputs to dorsal Y neurons accompany VOR motor learning. J Neurophysiol. 2006;95:1812–1825. doi: 10.1152/jn.01061.2005. [DOI] [PubMed] [Google Scholar]
- Cullen KE. Sensory signals during active versus passive movement. Curr Opin Neurobiol. 2004;14:698–706. doi: 10.1016/j.conb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 1994. [Google Scholar]
- Galiana HL, Green AM. Vestibular adaptation: how models can affect data interpretations. Otolaryngol Head Neck Surg. 1998;119:231–243. doi: 10.1016/S0194-5998(98)70058-0. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Robinson DA. Adaptation of human’s vestibulo-ocular reflex to magnifying glasses. Brain Res. 1975;92:331–335. doi: 10.1016/0006-8993(75)90279-6. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Melvill Jones G (1976a) Short-term adaptive changes in the human vestibulo-ocular reflex arc. J Physiol 256:361–379 [DOI] [PMC free article] [PubMed]
- Gonshor A, Melvill Jones G (1976b) Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol 256:381–414 [DOI] [PMC free article] [PubMed]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, Todd MJ, Staples MJ, D'Cruz DM. The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res. 1990;81:479–490. doi: 10.1007/BF02423496. [DOI] [PubMed] [Google Scholar]
- Haslwanter T (1995) Mathematics of three-dimensional eye rotations. Vis Res 35:1727–1739 [DOI] [PubMed]
- Hepp K. On Listing’s law. Commun Math Phys. 1990;132:285–295. doi: 10.1007/BF02278012. [DOI] [Google Scholar]
- Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J Neurophysiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuomotor distortions. Exp Brain Res. 1997;115:557–561. doi: 10.1007/PL00005727. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA. Vestibular signals carried by pathways subserving plasticity of the vestibulo-ocular reflex in monkeys. J Neurosci. 1986;6:346–354. doi: 10.1523/JNEUROSCI.06-02-00346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol. 1994;72:928–953. doi: 10.1152/jn.1994.72.2.928. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Neural basis for motor learning in the vestibulo-ocular reflex of primates. III Computational and behavioral analysis of the sites of learning. J Neurophysiol. 1994;72:974–999. doi: 10.1152/jn.1994.72.2.974. [DOI] [PubMed] [Google Scholar]
- McConville KM, Tomlinson RD, NA EQ. Behavior of eye-movement-related cells in the vestibular nuclei during combined rotational and translational stimuli. J Neurophysiol. 1996;76:3136–3148. doi: 10.1152/jn.1996.76.5.3136. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Yoshida K, Evinger C, Berthoz A. The location, axonal arborization and termination sites of eye-movement-related secondary vestibular neurons demonstrated by intra-axonal HRP injection in the alert cat. In: Fuchs A, Becker W, editors. Progress in oculomotor research. Amsterdam: Elsevier/North Holland; 1981. pp. 379–386. [Google Scholar]
- McCrea RA, Luan H. Signal processing of semicircular canal and otolith signals in the vestibular nuclei during passive and active head movements. Ann N Y Acad Sci. 2003;1004:169–182. doi: 10.1196/annals.1303.015. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Todd MJ. Real-time rotation vectors. Australas Phys Eng Sci Med. 1999;22:73–80. [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human horizontal vestibulo-ocular reflex is eliminated by a partial peripheral gentamicin lesion. Exp Brain Res. 2004;159:92–98. doi: 10.1007/s00221-004-1974-2. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Cortical auditory signal processing in poor readers. Proc Natl Acad Sci USA. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan SS, Blake DT, Wright BA, Byl N, Merzenich MM. Practice-related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality. J Neurosci. 1998;18:1559–1570. doi: 10.1523/JNEUROSCI.18-04-01559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige GD. Senescence of human visual-vestibular interactions: smooth pursuit, optokinetic, and vestibular control of eye movements with aging. Exp Brain Res. 1994;98:355–372. doi: 10.1007/BF00228423. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966;29:467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, King WM. Behavior and physiology of the macaque vestibulo-ocular reflex response to sudden off-axis rotation: computing eye translation. Brain Res Bull. 1996;40:293–301. doi: 10.1016/0361-9230(96)00118-9. [DOI] [PubMed] [Google Scholar]
- Straumann D, Zee DS, Solomon D, Lasker AG, Roberts DC. Transient torsion during and after saccades. Vision Res. 1995;35:3321–3334. doi: 10.1016/0042-6989(95)00091-R. [DOI] [PubMed] [Google Scholar]
- Szturm T, Ireland DJ, Lessing-Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. J Vestib Res. 1994;4:461–479. [PubMed] [Google Scholar]
- Ushio M, Minor LB, Della Santina CC, Lasker DM. Unidirectional rotations produce asymmetric changes in horizontal VOR gain before and after unilateral labyrinthectomy in macaques. Exp Brain Res. 2011;210:651–660. doi: 10.1007/s00221-011-2622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viirre E, Sitarz R. Vestibular rehabilitation using visual displays: preliminary study. Laryngoscope. 2002;112:500–503. doi: 10.1097/00005537-200203000-00017. [DOI] [PubMed] [Google Scholar]