Abstract
Purpose
This study describes a percutaneous technique for C2 transpedicular screw fixation and evaluates its safety and efficacy in the treatment of patients with hangman’s fracture.
Methods
Ten patients with hangman’s fracture were treated by percutaneous C2 transpedicular screw fixation. There are six males and four females, who were, based on the classification of Levine and Edwards, sorted as follows: type I fracture, three cases; type II, five cases; type IIa, two cases. The causes of injury were road traffic accident in six patients and falling injury in four patients. Other associated lesions included rib fractures (7 patients), head injuries (4 patients), and fractures of extremities (6 patients).
Results
The new technique was performed successfully in all cases. The average operation time was 98 min (range 60–130 min) and the estimated blood loss was 25 ml (range 15–40 ml). No complications such as vascular or neural structures injuries were found intraoperatively. Postoperative CT scans demonstrated that 17 (85 %) of 20 screws were placed satisfactorily, and 3 (15 %) screws showed perforations of the pedicle wall (<2 mm). These patients were asymptomatic and no further intervention was required postoperatively. After 8–25 months follow-up (mean 15.3 months), solid fusion was demonstrated by computed tomography. All cases got well-sagittal alignment and no angulation or dislocation was found at the segment of C2–C3. There was no loss of fixation. Clinical examination showed a full range of motion in the neck in all patients.
Conclusions
The fluoroscopically assisted percutaneous C2 transpedicular screw fixation method is a technically feasible and minimally invasive technique for hangman’s fracture.
Keywords: Hangman’s fracture, Transpedicular screw, Percutaneous, Minimal invasive, Cervical spine
Introduction
The term “hangman’s fracture” also known as traumatic spondylolisthesis of the axis was initially introduced by Schneider [1] based on the biomechanics and other similarities of the cervical fractures seen following judicial hanging. It is the second most common fracture of the C2 vertebra and accounts for 4–7 % of all cervical spine fractures and dislocations [2].
Though several surgical technologies were suggested for hangman’s fracture during the past decades, the optimum surgical treatment is still controversial [3–6]. C2 direct transpedicular osteosynthesis for hangman’s fracture was first spoken about by Leconte [7] and further illustrated by Judet et al. [8]. This method is recognized as a “physiologic operation” because it fixates only the fractured bones of C2 and sacrifices no normal motion segments. Since the initial descriptions, several authors have described favorable clinical outcomes in case series [4, 9–13]. Borne et al. [12] reported on direct transpedicular screw fixation in 13 cases of hangman’s fracture, all patients got excellent results. ElMiligui et al. [9] also used this technique in 15 patients and found it to be a simple and safe method of repair, giving consistently good anatomical and functional results. Nevertheless, the traditional surgical technique needs exposure to the posterior aspect of C2 vertebra, and this will increase the access-related morbidity as muscular denervation and atrophy.
New surgical techniques aided by navigation guidance have optimized for the operation. Sugimoto et al. [11] reported a case of percutaneous C2 pedicle screw fixation using a fluoroscopy-assisted navigation system. Tian et al. [14] managed unstable hangman’s fracture with posterior fixation and fusion using intraoperative three-dimensional fluoroscopy-based navigation. However, the operations were not completely percutaneous since an incision was still needed to attach the reference arc to the spinous process of the axis. Moreover, navigation guidance is time consuming and has economical limitations. To our knowledge, there are still no clinical application reports using the percutaneous C2 pedicle screw fixation for the management of hangman’s fracture that is solely fluoroscopically assisted in the literature.
From March 2004 to March 2010, ten patients with hangman’s fracture were treated by percutaneous C2 pedicle screw fixation. All cases achieved satisfactory results. In the present study, we reported initial clinical experience with surgical techniques and preliminary results.
Materials and methods
Between March 2004 and March 2010, ten patients with hangman’s fracture were managed with the percutaneous C2 transpedicular screw fixation. An average age was 45 (range 38–82 years old), six males and four females. Preoperative cervical spine X-rays including lateral and open-mouth views, and computed tomography (CT) were performed in all patients. According to the classification of Levine and Edwards [15], type I fracture has been found in three cases, type II in five cases and type IIa in two cases. The causes of injury were road traffic accidents in six patients and falling injury in four patients. Other associated lesions included rib fractures (7 patients), head injuries (4 patients) and fracture of extremities (6 patients). None of the patients showed neurological deficit. Skull traction was managed to reduce the dislocation preoperatively in five cases. One old patient who associated with right femur and multiple rib fractures could not tolerate skull traction. Other four patients who presented head trauma were not indicated for skull traction; these cases were protected by the soft collar only. The details of each case are shown in Table 1.
Table 1.
Patients’ data of this group
| No. | Age (years) | Sex | Etiology | Other lesions | Classification (Levine and Edwards) | Operation time (min) | Total blood loss (ml) | Follow-up time (months) | Malplacement of screw |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | M | Traffic accident | Right femur neck and rib fracture | Type I | 120 | 20 | 25 | One screw reached the medial pedicle wall (<2 mm) |
| 2 | 36 | F | Fall from a height | Rib and left humerus fracture | Type II | 130 | 40 | 13 | None |
| 3 | 45 | F | Fall from a height | Head injury | Type IIa | 110 | 25 | 19 | One screw reached the lateral pedicle wall (<2 mm) |
| 4 | 52 | M | Traffic accident | Right femur and rib fracture | Type II | 100 | 25 | 20 | None |
| 5 | 28 | M | Traffic accident | Head injury, rib fracture | Type I | 95 | 30 | 18 | None |
| 6 | 39 | F | Fall from a height | Rib fracture | Type II | 115 | 30 | 9 | None |
| 7 | 50 | M | Fall from a height | Head injury, rib fracture | Type IIa | 100 | 25 | 14 | None |
| 8 | 72 | F | Traffic accident | Head injury, right tibial fracture | Type I | 80 | 20 | 15 | None |
| 9 | 44 | M | Traffic accident | Left radius and tibial fracture | Type II | 60 | 20 | 12 | None |
| 10 | 63 | M | Traffic accident | Rib and left tibial fracture | Type II | 70 | 15 | 8 | One screw reached the medial pedicle wall (<2 mm) |
Preoperative radiological measurement
Radiological measurements were obtained from preoperative CT scans in each patient (Fig. 1). The parameters include (1) angle between the C2 pedicle axis and midsagittal line; (2) distance from the entry point of pedicle screw to midsagittal line; (3) distance from the skin entry point (the intersection between pedicle axis and skin) to midsagittal line; and (4) pedicle length: the distance between the most anterior and most posterior point of the pedicle axis excluding the fracture gap.
Fig. 1.
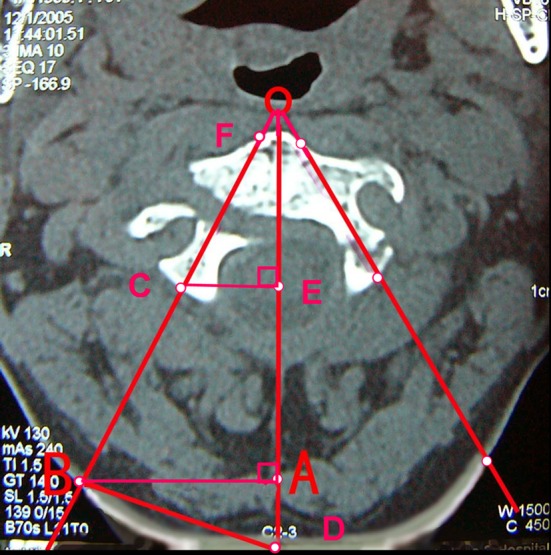
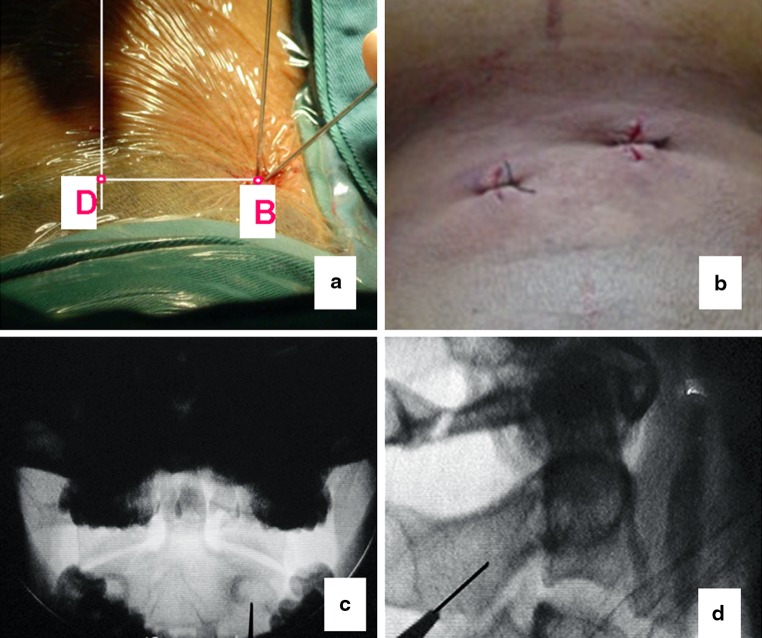
Preoperative morphometric measurements on the CT scans. OB The axis of C2 pedicle; OD midline of C2 vertebral body; B The skin entry point of C2 pedicle screw; ∠BOD screw insertion angle from the mid-sagittal plane; BD distance between midline (spinal process) to the skin entry point; CF pedicle screw length (excluding the fracture gap)
Operative technique
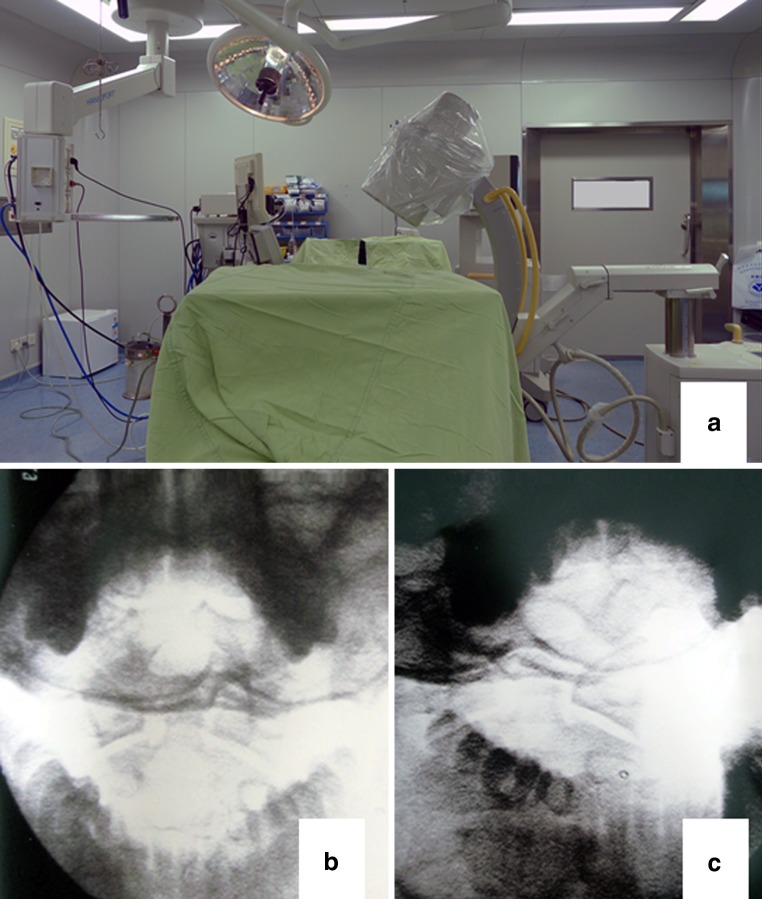
The patient was placed in supine position on a radiolucent table. A nasal intubation was performed for local anesthesia while the patient was still awake. Then general anesthesia was given and a radiolucent circular plastic tube was placed in the mouth to facilitate the open-mouth view. Thereafter, the patient was position-prone immobilized in a hard collar with the head fixed in a carbon Mayfield clamp. The open-mouth and lateral fluoroscopic views were obtained to identify anatomic locations preoperatively (Fig. 2). Somatosensory-evoked potential monitoring was set up to electrophysiological activities of the spinal cord during surgery.
Fig. 2.
a The picture of C arm setup; b the open-mouth fluoroscopy; c the C2 pedicle oblique fluoroscopy
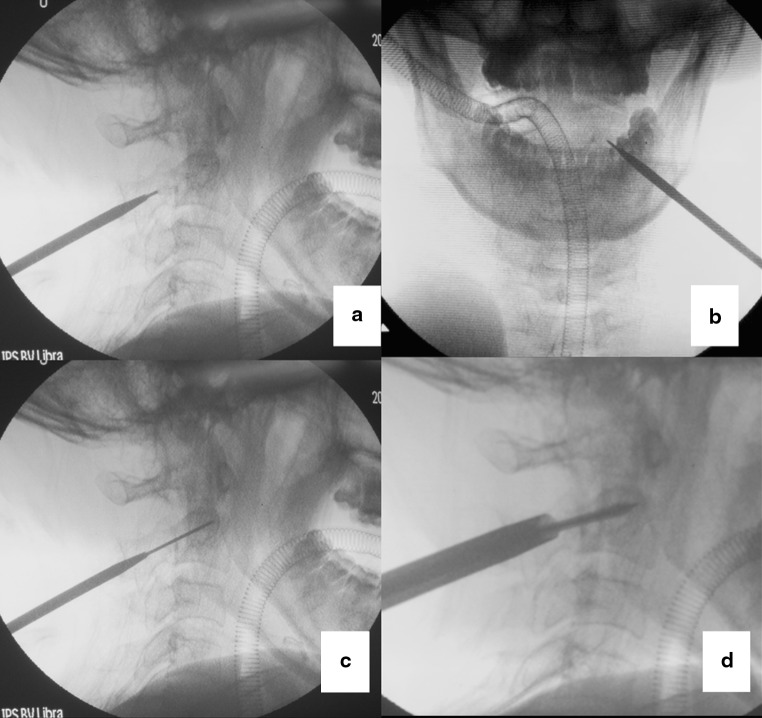
The cranio-caudal position of skin entry point is at the lower margin of C2 spinous process. The distance from skin entry point to posterior middle line is in accordance with preoperative measurements. A stab incision was initially made, and a guide tube containing a sharp tipped K-wire was placed through the incision aiming the pedicle under the guidance of fluoroscopy. The inclination of K-wire from the midsagittal plane was determined in accordance with the angle measured preoperatively. The open-mouth and pedicle axis fluoroscopy were used to determine the entry point of the screw, making sure the K-wire was positioned in the center of the pedicle [16, 17] (Fig. 3). The screw angle from the coronal plane and screw length can be controlled on lateral fluoroscopy. When the position was determined, a K-wire was inserted into the pedicle by a power drill. Then, an initial cannulated soft tissue dilator was inserted over the K-wire, and the next bigger size dilators were sequentially placed over the initial dilator. At last, the final dilator remained in place to serve as a protective sheath and a drill was used to complete the screw canal. Thereafter, a 3.5-mm self-tapping cannulated screw threaded at the extremity was placed along the K-wire, making sure all threads were passing through the fracture line (Fig. 4). Once the threads passed through the fracture line, separated bones can be compressed. All procedures were carried out under an imaging intensifier. Both of the screws were placed using the same technique. Usually, we place the screw one side first, and then deal with the other side. In order to avoid anterior tissue lesions (posterior wall of throat), the screws were not allowed to penetrate the anterior cortex of C2 vertebral body. When proceeding with the drill, care must be taken to avoid advancing the K-wire further.
Fig. 3.
a A stab incision was made at the entry point B which was determined according to the preoperative measurements. Then a guide tube containing a sharp tipped K-wire was placed through the incision aiming the pedicle of C2; b postoperative skin incision after percutaneous C2 transpedicular screw fixation; c the screw entry point on the open-mouth view; d the screw entry point on the lateral radiograph
Fig. 4.
a, b The guide tube containing a sharp tipped K-wire was placed at the desired entry point; c the guide K-wire was advanced into the pedicle; d the screw was inserted through a protection tube
Postoperatively, a soft neck collar was worn for 8–12 weeks. Patients were permitted to ambulate the day after surgery, unless contraindicated by their general condition. Computed tomography was performed in all cases. Radiographic and clinical evaluation was obtained at 1 week, 2, 6 and 12 months, postoperatively.
Results
The operation was successfully performed in a completely percutaneous invasive procedure in all cases (Fig. 5). The average operation time was 98 min (range 60–130 min) and the estimated blood loss was 25 ml (range 15–40 ml). No complications such as vascular or neural structures injuries were found intraoperatively. Postoperative CT scans demonstrated that 17 (85 %) of 20 screws were placed satisfactorily, and 3 (15 %) screws showed perforations of the pedicle wall (<2 mm). Of these three screws, two breached the medial pedicle wall and one breached the lateral wall (Fig. 6). These patients were asymptomatic and no further intervention was required postoperatively. Radiographs of lateral view and open-mouth view were obtained postoperatively and repeated at a 2, 6, and 12-month intervals to assess bone healing and alignment. After an 8–25 month follow-up (mean 15.3 months), solid fusion was demonstrated by computed tomography in all cases. All cases in our series got well-sagittal alignment and no angulation or dislocation was found at the segment of C2–C3. There was no loss of fixation. Clinical examination showed a full range of motion in the neck in all patients.
Fig. 5.
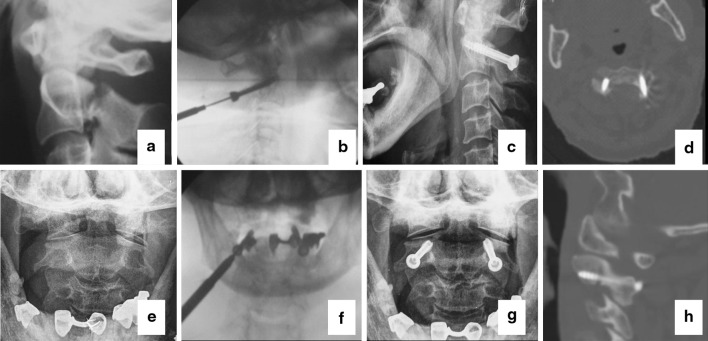
A 72 years old female patient associated hangman’s fracture, head injury and tibial fracture. a The lateral and e the open-mouth X-plain preoperative; b, f the screw was inserted under the guidance of K-wire; c, g the anteroposterior and lateral X-ray plain of the patient postoperative; d, h postoperative CT showing appropriate screw position in the same patient
Fig. 6.
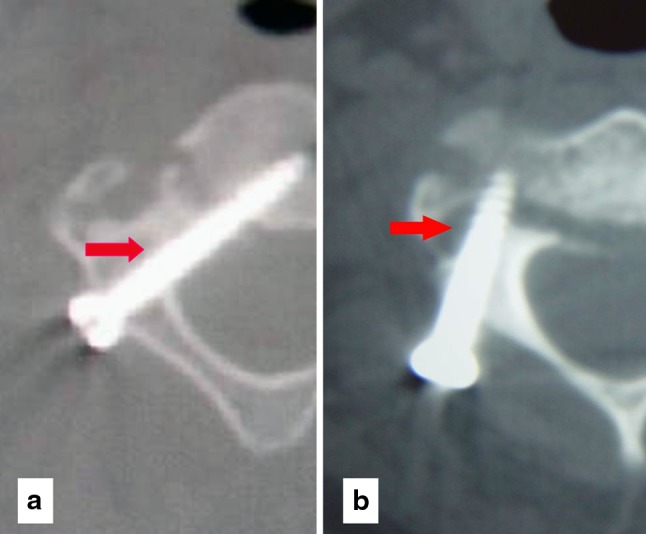
a The postoperative CT scan shows a perforation of the pedicle wall medial; b a perforation of the pedicle wall lateral
Discussion
Hangman’s fracture was named descriptively: initially due to the similarities with the fractures that occurred on the cervical spine of hanged individuals. It is usually caused by traffic accidents or falling injuries, and often associating with a high incidence of head and chest injury. The stability of hangman’s fracture is usually evaluated by the signs of angulation, translation, displacement or diastasis of the fracture on initial lateral and dynamic (flexion/extension) radiograph. Meanwhile, the quality of the C2/3 disc can be appreciated on the cervical MRI. Though the evaluation methods are definitive, the criterion of stability or instability reported in the literature is still uncertain. In general, Levine–Edwards type I and type II hangman’s fracture were considered to be stable [3, 4].
As treatment, external immobilization in a hard collar or halo device was suggested for most Levine–Edwards type I and minimal displaced type II fractures [3, 18, 19]. Nevertheless, sometimes these methods are poorly tolerated in polytrauma or elderly patients. Patients with skull fractures or scalp lesion usually cannot be submitted to treatment with external immobilization. In these cases, surgical treatment can be a good option. Boullosa et al. [20], reviewing ten cases, reported the use of direct C2 pedicle screw fixation in two polytrauma patients of Effendi type I hangman’s fracture with good clinical results. A similar good outcome was also reported by Sugimoto et al. [11]. In our series, three patients of Levine–Edwards type I hangman’s fractures were treated by surgical stabilization. All patients got good clinical results. Patients benefit from surgical treatment since it allows for early rehabilitation and reduced nursing care.
Surgical stabilization is recommended in Levine–Edwards type IIa and type III fractures with significant dislocation [3]. Several techniques have been described using anterior or posterior approaches for surgical treatments [3, 9, 20–23]. The anterior approaches include anterolateral and transoral C2–3 discectomy, fixing with bony graft and plating (ACDF). Though many authors reported good clinical results using ACDF, the access way demands great surgical knowledge with the local anatomy [20, 21]. Moreover, the anterior approach cannot address the detached posterior arch of C2 and is usually followed by high level of morbidity including vital structures injury (the facial and hypoglossal nerves, branches of the external carotid artery and the superior laryngeal nerve), throat pain and dysphagia [20, 21, 23]. Roy-Camille et al. [22] described the technique of posterior C2–C3 screw fixation by connecting screws inserted into the fractured C2 pedicles and C3 lateral masses. It is another effective and commonly used way in the repair of C2. The posterior approach has a relative simple exposure with no major vascular and visceral structures. Nevertheless, this technique poses a particular challenge because more screw instrumentation will increase the morbidity of the spinal cord and vertebral arteries injury. Furthermore, both the ACDF and posterior C2–C3 screw fixation will loss the mobility of C2–C3 segment.
In this series, all cases were treated by C2 direct transpedicular screw fixation. Though the direct bilateral transpedicular osteosynthesis for hangman’s fracture is recognized as a “physiologic operation”, it does not appropriate for all kinds of hangman’s fracture. The selection of the patients should be based on careful consideration. Biomechanical research identified that direct C2 transpedicular osteosynthesis after a hangman’s fracture was ineffective against flexion and extension if there was excessive disc and ligamentous system damage [24, 25]. Therefore, the Levine–Edwards type III hangman’s fracture is not advised to manage with direct C2 transpedicular screw fixation. We performed C2–3 stabilization in such cases, although Verheggen et al. [4] have described the use of direct bilateral transpedicular osteosynthesis for Levine–Edwards type III hangman’s fracture with good clinical result. In addition, direct transpedicular screw fixation is not indicated in cases of a traumatic disk herniation compromising the spinal cord. In this instance, we advocate an anterior C2–3 discectomy and fusion with optional plating.
The traditional surgical technique of C2 transpedicular screw fixation needs a big incision and exposure to the posterior aspect of C2 vertebra. These procedures are big trauma to patients. Recently, with the development of technology, fluoroscopy-assisted navigation system has been widely used in the placement of pedicle screw. This technique not only improved the accuracy of screw fixation obviously, but also made the operation less invasive. Though the navigation system has great advantages compared to the traditional technique, it is still not completely percutaneous and is time consuming and holds economical limitations.
In our series, all screws were placed completely percutaneous which is solely fluoroscopically assisted. Compared to the traditional surgical treatments, it can offer significant benefits: (1) the main advantage of the technique is minimally invasive. The percutaneous procedures reduce muscular trauma subsequent to the surgical approach, intraoperative blood loss and postoperative pain. (2) It preserves the mobility of C2–3 segment. (3) It reduces nursing care and hospital stay, and allows for early rehabilitation and shortening of the treatment period with better quality of life.
In the present study, 3 (15 %) screws perforated the pedicle wall. Though none of the patients have clinical symptom, the critical screw placements should be kept in mind. Previous anatomical and radiographic studies have established a recommended entry point and screw angle to avoid local structures [26–28]. Nevertheless, to optimize the surgical techniques, adequate preoperative imaging studies should be made. Ebraheim et al. [26] demonstrated that using individual anatomy as a trajectory guide was safer than adhering to a predetermined trajectory of 30° medial to the sagittal plane and 20° cephalad to the transverse plane. In this series, the skin entry points and optimal trajectory of pedicle screw were determined preoperatively by radiological measurements. When placing the screw, all procedures should be checked under the fluoroscopy. Biplanar imaging intensifier monitoring is very critical to maintain the correct direction, angulation and length. Preoperative radiographic studies combined with the knowledge of the regional anatomy and C-arm orientation create a reproducible method for C2 transpedicular screw placement that is low in morbidity.
Conclusion
The fluoroscopically assisted percutaneous C2 transpedicular screw fixation method is a technically feasible and minimally invasive technique for hangman’s fracture. The preliminary result of the study is satisfactory, however, experience with greater number of patients and long-term follow-up is still necessary to further evaluate this technique.
Acknowledgments
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Conflict of interest
Authors have no conflicts of interest to declare.
Footnotes
Y.-S. Wu and Y. Lin contributed equally to this work and should be considered co-first authors.
Contributor Information
Yan Lin, Phone: +86-577-88879158, FAX: +86-577-88879123, Email: wys9453@sina.com.
Zhi-jun Pan, Phone: +86-571-87767023, FAX: +86-571-87022776, Email: Zepzj@163.com.
References
- 1.Schneider RC, Livingston KE, Cave AJ, Hamilton G. “Hangman’s fracture” of the cervical spine. J Neurosurg. 1965;22:141–154. doi: 10.3171/jns.1965.22.2.0141. [DOI] [PubMed] [Google Scholar]
- 2.Hadley MN, Dickman CA, Browner CM, Sonntag VK. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71:642–647. doi: 10.3171/jns.1989.71.5.0642. [DOI] [PubMed] [Google Scholar]
- 3.Li XF, Dai LY, Lu H, Chen XD. A systematic review of the management of hangman’s fractures. Eur Spine J. 2006;15:257–269. doi: 10.1007/s00586-005-0918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verheggen R, Jansen J (1998) Hangman’s fracture: arguments in favor of surgical therapy for type II and III according to Edwards and Levine. Surg Neurol 49:253–261 (discussion 261–252). pii:S0090-3019(97)00300-5 [DOI] [PubMed]
- 5.Fuentes S, Metellus P, Dufour H, Do L, Fesselet J, Grisoli F. Traumatic spondylolisthesis of the axis: arguments in favor of surgical management after analysis of 8 patients. Neurochirurgie. 2003;49:25–30. [PubMed] [Google Scholar]
- 6.Cornish BL. Traumatic spondylolisthesis of the axis. J Bone Joint Surg Br. 1968;50:31–43. [PubMed] [Google Scholar]
- 7.Leconte P (1964) Fracture et luxation des deux premieres vertebrescervicales. In: Judet R (ed) Luxation congenitale de la hanche. Fractures du cou-de-pied rachis cervical. Actualites de chirurgie orthopedique de l’hopital Raymond-poincare, vol 3. Masson et Cie, Paris, pp 147–166
- 8.Judet R, Roy-Camille R, Saillant G. Actualités de chirurgie orthopédique de l’Hospital Raymond-Poin caré. VIII. Fractures du rachis cervical. Paris: Masson; 1970. pp. 174–195. [Google Scholar]
- 9.ElMiligui Y, Koptan W, Emran I. Transpedicular screw fixation for type II Hangman’s fracture: a motion preserving procedure. Eur Spine J. 2010;19:1299–1305. doi: 10.1007/s00586-010-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajasekaran S, Vidyadhara S, Shetty AP. Iso-C3D fluoroscopy-based navigation in direct pedicle screw fixation of Hangman fracture: a case report. J Spinal Disord Tech. 2007;20:616–619. doi: 10.1097/BSD.0b013e318074f978. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto Y, Ito Y, Shimokawa T, Shiozaki Y, Mazaki T. Percutaneous screw fixation for traumatic spondylolisthesis of the axis using iso-C3D fluoroscopy-assisted navigation (case report) Minim Invasive Neurosurg. 2010;53:83–85. doi: 10.1055/s-0030-1247503. [DOI] [PubMed] [Google Scholar]
- 12.Borne GM, Bedou GL, Pinaudeau M. Treatment of pedicular fractures of the axis. A clinical study and screw fixation technique. J Neurosurg. 1984;60:88–93. doi: 10.3171/jns.1984.60.1.0088. [DOI] [PubMed] [Google Scholar]
- 13.Taller S, Suchomel P, Lukas R, Beran J. CT-guided internal fixation of a hangman’s fracture. Eur Spine J. 2000;9:393–397. doi: 10.1007/s005860000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian W, Weng C, Liu B, Li Q, Hu L, Li ZY, Liu YJ, Sun YZ. Posterior fixation and fusion of unstable Hangman’s fracture by using intraoperative three-dimensional fluoroscopy-based navigation. Eur Spine J. 2012;21:863–871. doi: 10.1007/s00586-011-2085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67:217–226. [PubMed] [Google Scholar]
- 16.Schaefer C, Begemann P, Fuhrhop I, Schroeder M, Viezens L, Wiesner L, Hansen-Algenstaedt N. Percutaneous instrumentation of the cervical and cervico-thoracic spine using pedicle screws: preliminary clinical results and analysis of accuracy. Eur Spine J. 2011;20:977–985. doi: 10.1007/s00586-011-1775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Nakashima H, Machino M. Placement and complications of cervical pedicle screws in 144 cervical trauma patients using pedicle axis view techniques by fluoroscope. Eur Spine J. 2009;18:1293–1299. doi: 10.1007/s00586-009-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo UG, Denaro L, Campi S, Maffulli N, Denaro V. Upper cervical spine injuries: indications and limits of the conservative management in Halo vest. A systematic review of efficacy and safety. Injury. 2010;41:1127–1135. doi: 10.1016/j.injury.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Vaccaro AR, Madigan L, Bauerle WB, Blescia A, Cotler JM. Early halo immobilization of displaced traumatic spondylolisthesis of the axis. Spine (Phila Pa 1976) 2002;27:2229–2233. doi: 10.1097/00007632-200210150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Boullosa JL, Colli BO, Carlotti CG, Jr, Tanaka K, dos Santos MB. Surgical management of axis’ traumatic spondylolisthesis (Hangman’s fracture) Arq Neuropsiquiatr. 2004;62:821–826. doi: 10.1590/S0004-282X2004000500015. [DOI] [PubMed] [Google Scholar]
- 21.Wilson AJ, Marshall RW, Ewart M (1999) Transoral fusion with internal fixation in a displaced hangman’s fracture. Spine (Phila Pa 1976) 24:295–298 [DOI] [PubMed]
- 22.Roy-Camille R, Saillant G, Mazel C (1989) Internal fixation of the unstable cervical spine by a posterior osteosynthesis with plats and screws. In: The Cervical Spine Research Society (ed) The cervical spine, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 390–403
- 23.Tuite GF, Papadopoulos SM, Sonntag VK (1992) Caspar plate fixation for the treatment of complex hangman’s fractures. Neurosurgery 30:761–764 (discussion 764–765) [PubMed]
- 24.Arand M, Neller S, Kinzl L, Claes L, Wilke HJ (2002) The traumatic spondylolisthesis of the axis. A biomechanical in vitro evaluation of an instability model and clinical relevant constructs for stabilization. Clin Biomech (Bristol, Avon) 17:432–438. pii:S0268003302000372 [DOI] [PubMed]
- 25.Duggal N, Chamberlain RH, Perez-Garza LE, Espinoza-Larios A, Sonntag VK, Crawford NR. Hangman’s fracture: a biomechanical comparison of stabilization techniques. Spine (Phila Pa 1976) 2007;32:182–187. doi: 10.1097/01.brs.0000251917.83529.0b. [DOI] [PubMed] [Google Scholar]
- 26.Ebraheim N, Rollins JR, Jr., Xu R, Jackson WT (1996) Anatomic consideration of C2 pedicle screw placement. Spine (Phila Pa 1976) 21:691–695 [DOI] [PubMed]
- 27.Smith ZA, Bistazzoni S, Onibokun A, Chen NF, Sassi M, Khoo LT. Anatomical considerations for subaxial (C2) pedicle screw placement: a radiographic study with computed tomography in 93 patients. J Spinal Disord Tech. 2010;23:176–179. doi: 10.1097/BSD.0b013e3181b40234. [DOI] [PubMed] [Google Scholar]
- 28.Howington JU, Kruse JJ, Awasthi D. Surgical anatomy of the C-2 pedicle. J Neurosurg. 2001;95:88–92. doi: 10.3171/spi.2001.95.1.0088. [DOI] [PubMed] [Google Scholar]