Abstract
Purpose
A pilot study to examine the impact of cervical myelopathy on corticospinal excitability, using transcranial magnetic stimulation, and to investigate whether motor evoked potential (MEP) and silent period (SP) recruitment curve (RC) parameters can detect changes in corticospinal function pre- and post-surgery.
Methods
We studied six cervical myelopathy patients undergoing surgery and six healthy controls. Clinical and functional scores and neurophysiological parameters were examined prior to and 3 months following the surgery.
Results
MEP latencies for abductor pollicis brevis (APB) and tibialis anterior (TA) muscles and central motor conduction time were prolonged pre- and post-surgery; SP durations were differentially altered. There were significant differences in parameters of RCs for (1) MEP area in APB (max values, S50) and TA (slope) between controls and patients pre- and post-surgery and (2) SP duration in APB (max values) between patients pre-surgery and controls.
Conclusions
The findings of this pilot study suggest an uncoupling of excitatory and inhibitory pathways, which persists at 3 months following cord decompression. RCs for MEP and SP at 3 months provide more information on the functional status of the cord and prompts for a longer term follow-up.
Keywords: Myelopathy, Transcranial magnetic stimulation, Motor evoked potential, Cortical silent period, Recruitment curves
Introduction
Cervical myelopathy is a disease of the spinal cord caused by narrowing of the normal spinal canal diameter. Age-related or chronic degenerative spondylosis of the cervical spine, bulging or herniated disc, degenerated yellow ligaments, congenital narrowing aggravated by acute trauma and bony malformations can all cause compression of the cord [1, 2].
The spinal cord dysfunction following cord compression leads to a spectrum of symptoms, which includes weakness of the upper and lower limbs, paraesthesias, loss of hand dexterity, gait difficulty, bladder and bowel changes and signs of pyramidal tract and posterior column dysfunction. Presently, magnetic resonance imaging (MRI) is the radiological investigation of choice for all patients with suspected myelopathy before surgery is considered. However, the degree and extent of spinal cord compression shown at imaging does not always correlate with the severity of myelopathy [3].
A quantitative assessment of cervical myelopathy can be achieved by the use of electrodiagnostic techniques [4]. Information on the function of the corticospinal (CS) tract can be obtained with transcranial magnetic stimulation (TMS). In cervical myelopathy, this non-invasive technique has been limited to the evaluation of central motor conduction time (CMCT; from the motor cortex to the anterior horn cells), latency and amplitude of the motor evoked potential (MEP) recorded from the upper and lower extremities, and the silent period (SP) duration [4–10]. The aim of this pilot study, therefore, was to examine CS excitability using TMS in cervical myelopathy prior to and following surgery, particularly to look at the impact of the disease on excitatory and inhibitory pathways and to look at cord function below the level of compression. Most importantly, the present study was also designed to test whether MEP and SP recruitment curves offer more insight into the pathophysiology of the disease and are sensitive parameters to detect changes in CS function after surgical intervention on the cord. It was envisaged that the findings of this study would be regarded as groundwork to develop further studies.
Methods
Subjects
Potential study subjects were identified from the surgical waiting list of the participating neurosurgeon and screened for their eligibility to take part in the study. The subjects were included if they had clinical diagnosis of cervical myelopathy and their age was between 18 and 65 years. Exclusion criteria referred to the participant’s clinical features (previous surgery for cervical myelopathy and other co-existing neurological pathologies such as peripheral neuropathy and lumbar spinal stenosis) and contraindications to TMS (history of epilepsy, brain injury, implanted cardiac pacemaker, cranial metal implants and pregnancy).
With local ethical approval and signed informed consent, six patients (all male, mean (±SD) age 52 ± 13 years) were included in this study. They all presented with progressive gait disturbance, paraesthesia in upper and lower limbs, clumsiness of hands; none complained of sphincter disturbance. Neurological examination revealed signs of pyramidal tract involvement and sensory dysfunction in all of them. MRI, performed as part of their routine assessment, showed evidence of cervical cord stenosis associated with intramedullary high signal intensity lesion on T2-weighted MRI. Compression was caused by disc protrusion in three patients and spondylosis in other three patients. The cervical segment was affected at C3/4 in two patients, C4/5 in one patient, C5/6 in three patients; two segments were affected at C5/6 and C6/7 in one patient. They were all scheduled to have surgery for cord decompression. None was taking medication known to affect the parameters measured [11].
A further six (3 males, 3 females, mean [±SEM] age 37.83 ± 11.05 years) healthy subjects were also studied as controls.
Protocol
Patients were studied during two visits: 1 week before and 3 months after the surgery. At each study visit, the protocol included clinical, functional and TMS assessments.
Clinical and functional assessment
Clinical assessment was carried out according to the American Spinal Injury Association (ASIA) impairment scale [12]. Hand dexterity (of the symptomatically most affected side) was assessed using a nine-hole Pegboard test [13]. Gait was assessed using the shuttle walking test [14].
Neurophysiological assessments
Electromyographic (EMG) recordings were obtained from the abductor pollicis brevis (APB) and tibialis anterior (TA) of the symptomatically most affected side, using Ag/AgCl electrodes (self-adhesive, Neurocare, 2 cm diameter, Viasys, USA). The EMG signals were band-pass filtered (100 Hz to 2 kHz) and amplified (×1,000–10,000; Iso-DAM, WPI, UK) before being sampled at 4 kHz by a data acquisition interface (1401 plus and Signal software; Cambridge Electronic Design, UK) connected to a personal computer for subsequent offline analysis.
TMS was applied to the motor cortex using a Magstim 2002 stimulator (The Magstim Company Ltd., Dyfed, UK). To elicit responses from APB, single pulses were delivered using a 9-cm stimulating circular coil centred over the vertex and with the current flowing in the brain optimal for the side being tested. To elicit responses from TA, single pulses were delivered using an angled double cone coil (wing outer diameter 12 cm), which was positioned with its cross-over located over the vertex with the induced current in the brain flowing in a posterior-to-anterior direction.
Subjects first performed three brief maximal voluntary contractions (MVCs) to calibrate a visual feedback lightbox, which displayed rectified EMG signals from the relevant muscle. During the TMS, subjects used this visual display to maintain consistent contractions of 20 % MVC.
Five stimuli were delivered at each stimulator intensity in steps of 5 % maximum stimulator output (MSO), from 20 to 100 % MSO when recording from APB and from 20 to 70 % MSO when recording from TA.
To evoke M-waves and F-waves, electrical stimuli were delivered to the median nerve at the wrist for APB and to the common peroneal nerve at fibular head for TA using an electrical stimulator (DS7A, Digitimer, UK; pulse width 0.5 ms, pulse frequency 0.2 Hz).
Data analysis
Data from clinical and functional tests were averaged for all patients; averaged data were compared for differences pre- and 3 months post-surgery using paired t tests.
EMG signals for measuring MEPs and SPs were rectified and averaged. For measuring M-wave areas and F-wave latencies, individual traces were used.
MEPs were identified visually from averaged rectified EMG traces for each stimulus intensity used; latency (ms), duration (ms) and areas (mVs) were calculated. MEP areas were expressed as a percentage of the maximal M-wave area to electrical stimulation.
SPs were identified visually from averaged rectified EMG traces for each stimulus intensity used and the duration (ms) was calculated. Its onset was defined as the commencement of the MEP and the endpoint coincided with the re-occurrence of EMG activity [15–17].
Recruitment (stimulus/response) curves were then constructed for stimulus intensity against (1) MEP area and (2) SP duration using a Boltzmann sigmoid function (see [18]) which is given by the equation, using MEP area (MEP area) at a given stimulus intensity (S) as an example:
MEP area (S) = MEP area max/(1 + exp [S50−S/k]). This has three parameters: maximum value (or plateau)–MEP area max; stimulus intensity required to obtain a response at 50 % maximum (S50) and the slope parameter (k), which is proportional to the maximum slope. These parameters were compared for differences using one-way ANOVA.
M-waves and F-waves were identified visually, and maximal M-wave area (mVs) and minimal F-wave latency (ms) were calculated. CMCT was calculated using the F-wave method (MEP latency − (F latency + M latency − 1)/2; see [19]). These parameters were compared for differences using one-way ANOVA.
Results
The surgical procedure performed was anterior cervical discectomy and fusion in five patients, and posterior decompressive laminectomy in one patient. There were no post-operative complications and all patients reported symptomatic improvement.
Clinical and functional scores
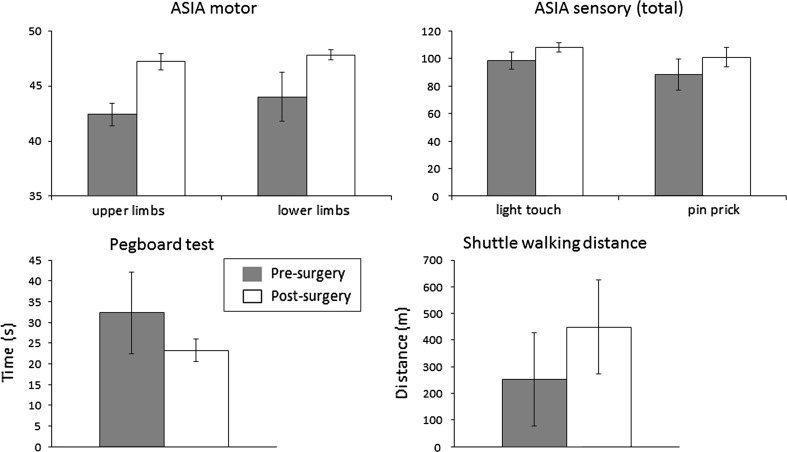
Motor and sensory scores, hand dexterity and walking ability were not statistically different post-surgery compared to pre-surgery (see Fig. 1 and Table 1).
Fig. 1.
Mean (±SEM) ASIA motor (upper left panel) and sensory (upper right panel) scores and Pegboard test time (lower left panel) and shuttle walking distance (lower right panel) in the patients prior to and following surgery
Table 1.
Clinical and functional scores
| ASIA motor (upper limbs) | ASIA motor (lower limbs) | ASIA sensory (light touch) | ASIA sensory (pin prick) | Pegboard test (s) | Shuttle walking (m) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| 42.40 ± 1.03 | 47.20 ± 0.73 | 44.00 ± 2.24 | 47.80 ± 0.45 | 98.40 ± 6.29 | 107.80 ± 3.25 | 88.20 ± 11.10 | 100.80 ± 6.92 | 32.32 ± 9.80 | 23.22 ± 2.75 | 252.50 ± 175.39 | 449.00 ± 175.43 |
Neurophysiology
MEP latencies
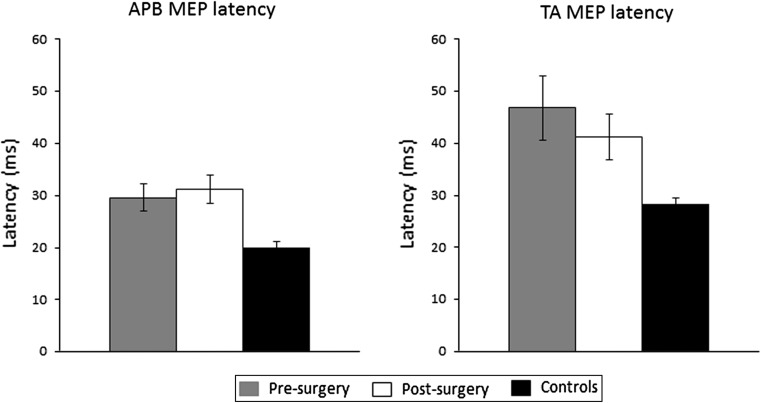
The mean (±SEM) latencies (at 100 %MSO) of MEPs from APB for the pre-, post-surgical and control groups were 29.58 ± 2.57, 31.18 ± 2.73 and 20.11 ± 0.80 ms, respectively. ANOVA revealed that there was a significant (p = 0.004) difference between the latencies of the three groups (Fig. 2). Post hoc tests revealed that there were differences between the controls and both pre- (p = 0.006) and post-surgery (p = 0.002) groups.
Fig. 2.
Mean (±SEM) MEP latency of responses recorded from APB (left panel) and TA (right panel) following TMS to the motor cortex at the maximum stimulus intensity used (100 % MSO for APB and 70 %MSO for TA)
The mean (±SEM) latencies (at 70 %MSO) of MEPs from TA for the pre-, post-surgical and control groups were 46.81 ± 6.17, 41.23 ± 4.32 and 28.43 ± 1.20 ms, respectively. ANOVA revealed that there was a significant (p = 0.012) difference between the latencies of the three groups (Fig. 2). Post hoc tests revealed that the differences were between the controls and the pre-surgery groups (p = 0.005).
CMCT
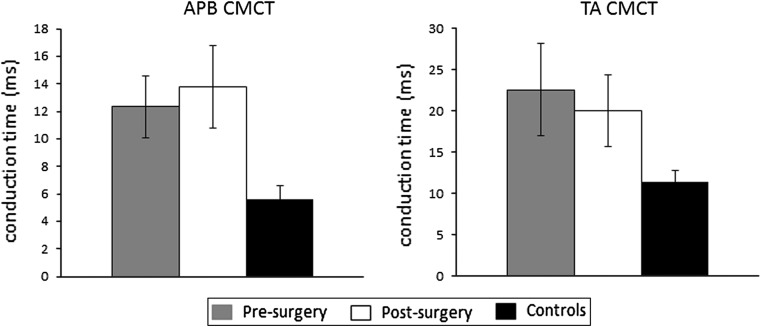
The mean (±SEM) CMCT (at 100 %MSO) of MEPs from APB for the pre-, post-surgical and control groups were 12.34 ± 2.26, 13.78 ± 2.97 and 5.58 ± 0.77 ms, respectively. ANOVA revealed that there was a significant (p = 0.033) difference between the CMCT of the three groups (see Fig. 3). Post hoc tests revealed that there were differences between the controls and the post-surgery groups (p = 0.017).
Fig. 3.
Mean (±SEM) CMCT calculated from responses recorded from APB (left panel) and TA (right panel) following TMS to the motor cortex at the maximum stimulus intensity used (100 % MSO for APB and 70 %MSO for TA)
The mean (±SEM) CMCT (at 70 %MSO) of MEPs from TA for the pre-, post-surgical and control groups were 22.61 ± 5.58, 20.03 ± 4.37 and 11.48 ± 1.40 ms, respectively. ANOVA revealed that there was no significant (p = 0.127) difference between the CMCT of the three groups (see Fig. 3).
SP duration
The mean (±SEM) SP duration (at 100 %MSO) from APB for the pre-, post-surgical and control groups were 215.71 ± 23.67, 233.09 ± 12.18 and 250.14 ± 37.49 ms. ANOVA revealed that there was no significant (p = 0.695) difference between the SP duration of the three groups.
The mean (±SEM) SP duration (at 70 %MSO) from TA for the pre-, post-surgical and control groups were 283.80 ± 40.70, 251.98 ± 15.15 and 195.59 ± 50.95 ms. ANOVA revealed that there was no significant (p = 0.369) difference between the SP duration of the three groups.
Recruitment curves
Parameters obtained from Boltzmann function are shown in Table 2.
Table 2.
Parameters obtained from Boltzmann function
| Muscle | Parameter | ||
|---|---|---|---|
| Plateau | Slope | S50 | |
| APB | |||
| MEP area (%Mmax) | |||
| Pre | 34.02 ± 0.95 | 6.57 ± 1.09 | 43.03 ± 1.76 |
| Post | 22.52 ± 0.64 | 3.95 ± 0.80 | 50.39 ± 0.91 |
| Controls | 67.82 ± 2.31 | 8.29 ± 1.73 | 39.29 ± 1.60 |
| SP (ms) | |||
| Pre | 217.67 ± 4.78 | 12.03 ± 0.94 | 49.08 ± 0.98 |
| Post | 242.21 ± 9.44 | 16.12 ± 2.00 | 51.26 ± 1.62 |
| Controls | 270.08 ± 17.84 | 15.03 ± 2.69 | 56.58 ± 2.74 |
| TA | |||
| MEP area (%Mmax) | |||
| Pre | 37.42 ± 1.77 | 6.53 ± 1.04 | 44.70 ± 1.11 |
| Post | 24.31 ± 2.62 | 6.54 ± 2.49 | 46.27 ± 2.31 |
| Controls | 169.15 ± 54.30 | 19.66 ± 2.85 | 72.79 ± 11.76 |
| SP (ms) | |||
| Pre | 303.31 ± 16.37 | 6.33 ± 1.25 | 43.81 ± 1.28 |
| Post | 318.99 ± 93.24 | 14.67 ± 6.33 | 51.12 ± 9.50 |
| Controls | 268.27 ± 65.72 | 14.10 ± 3.42 | 55.68 ± 7.86 |
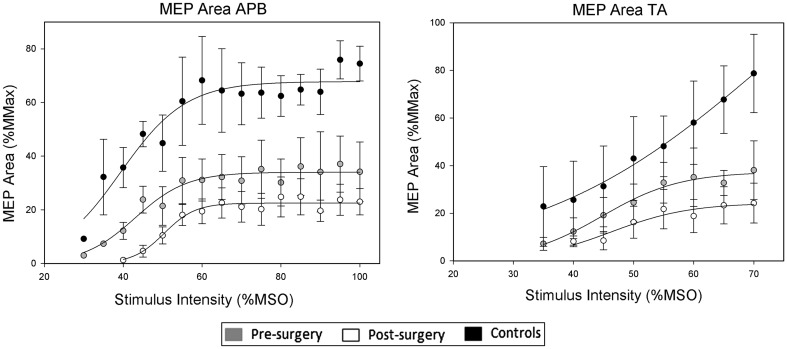
Recruitment curves for MEP area of APB showed that there were significant differences between pre- versus post-surgical (p < 0.001), pre-surgical versus controls (p < 0.001) and post-surgical versus controls (p < 0.001) maximum values.
There were no significant differences between pre- versus post-surgical, pre-surgical versus controls and post-surgical versus controls slope parameters (overall p = 0.126).
There were significant differences between pre- versus post-surgical (p < 0.008) and post-surgical versus controls (p < 0.001) S50 values (Fig. 4).
Fig. 4.
Stimulus response curves showing mean (±SEM) MEP area recorded from APB (left panel) and TA (right panel) following TMS to the motor cortex at differing stimulus intensities. The lines are applied to the mean data for each group using the Boltzmann function (see text for details)
Recruitment curves for MEP area of TA showed that there was a significant difference between post-surgical and controls (p < 0.014) slope parameters.
There were no significant differences between pre- versus post-surgical, pre-surgical versus controls and post-surgical versus controls maximum values (overall p = 0.165) or S50 (overall p = 0.208) values (Fig. 4).
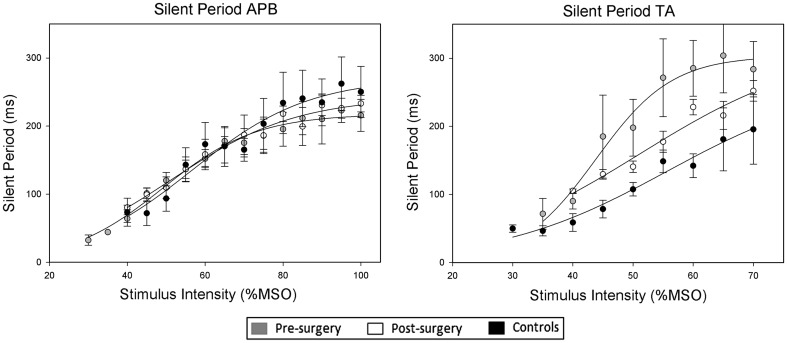
Recruitment curves for SP of APB showed that there was a significant difference between pre-surgical versus controls (p = 0.013) maximum values (Fig. 5).
Fig. 5.
Stimulus response curves showing mean (±SEM) silent period duration in the electromyogram recorded from APB (left panel) and TA (right panel) following TMS to the motor cortex at differing stimulus intensities. The lines are applied to the mean data for each group using the Boltzmann function (see text for details)
There were no significant differences between pre- versus post-surgical, pre-surgical versus controls and post-surgical versus controls slope parameters (overall p = 0.418) or S50 (p = 0.056) values (Fig. 5).
Recruitment curves for SP of TA showed that there were no significant differences between pre- versus post-surgical, pre-surgical versus controls and post-surgical versus controls maximum value (p = 0.865), slope parameter (overall p = 0.339) or S50 (overall p = 0.522) values (Fig. 5).
Discussion
This pilot study quantitatively examined corticospinal excitability using TMS in cervical myelopathy prior to and following surgery and looked at the impact of the disease on excitatory and inhibitory pathways. In accordance with previous studies, we found that preoperative values for MEP latency, area and CMCT were abnormal reflecting damage to CS fibres leading to conduction slowing and axonal loss. However, values for SP were similar to controls, suggesting that the disease has less impact upon the inhibitory control pathways.
These differing effects have been reported previously [20], where a divergence between descending motor tract function (within normal limits) and cortical inhibitory activity (prolonged SP) was observed in patients with hemiparetic stroke due to cerebral hemispheric lesion below the primary motor cortex. In our patients, the lesion to the motor fibres is at the cervical cord level and our results suggest that myelopathy affects descending corticospinal fibres function (abnormal CMCT and MEP) but partially spares the inhibitory control (normal SP) of both upper and lower limbs.
Our pilot study focused on the assessment of cord function below the level of compression and showed that all parameters affected pre-surgery remain statistically unchanged when assessed 3 months post-surgery. This is in keeping with previous observations that after a surgical intervention, even if the spinal canal diameter is restored, complete neurological recovery does not always occur [21]. We can speculate that in our patients the extent of damage to the CS tract was such that at 3 months post-surgery functional recovery, as assessed by TMS, was not achieved.
To the best of our knowledge, this is the first study to test the value of MEP and SP recruitment curves as sensitive parameters to assess cord function in cervical myelopathy before and after surgical intervention. The recruitment curves for both MEP and SP probe the excitability of both excitatory and inhibitory pathways, respectively, over a range of stimulus intensities and different mechanisms are thought to be responsible for the increase in both MEP size and silent period duration with increasing stimulus intensity. The size of the MEP reflects both motor cortex and spinal motor neuron excitability [18], whereas the silent period is a result of processes predominantly within the motor cortex [22–24].
A study of TMS in multiple sclerosis and hereditary spastic paraplegia [25] showed CMCT changes and reduced MEP amplitude, reflecting demyelination and axonal degeneration; however, recruitment curves for MEP were identical between the two patient groups and between patients and healthy controls. Similarly, we found prolonged CMCT and reduced MEP areas but preserved responsiveness of MEP to increased stimuli. However, we explored the use of other additional recruitment curve parameters such as maximum value, slope parameter and stimulus intensity required to obtain a response at 50 % maximum value; we found significant differences in some of these parameters calculated for MEP areas.
Of note is that while SP duration was similar between controls and patients (both pre- and post-surgery), the recruitment curves showed a significant difference between maximum values in controls versus patients pre-surgery, revealing that using this parameter an abnormality of the inhibitory control can be found. There was no significant difference between maximum values in controls versus patients post-surgery indicating a recovery of the partially affected inhibitory function.
Our findings suggest that objective improvement of cord function measured with TMS parameters, including the “more sensitive” recruitment curves for MEP and SP at 3 months, provides a real objective view of the functional status of the cord and prompts for a closer and more frequent patient follow-up to avoid further worsening. Long-term recovery of corticospinal function could become apparent when assessed using TMS in a sequence of visits for at least 18 months following the surgery. It remains that the techniques used in this study are not able to reveal the mechanisms underlying the subjective improvement reported by our patients and cannot be used to monitor surgery outcome. This study served as a pilot study to test the value of certain techniques; further investigations are needed to test whether corticospinal excitability changes occur in the longer term.
Conflict of interest
None.
References
- 1.Cusick JF. Pathophysiology and treatment of cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:661–681. [PubMed] [Google Scholar]
- 2.White AA, III, Panjabi MM. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856–860. doi: 10.1097/00007632-198807000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Wada E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine. 1995;20:2226–2232. doi: 10.1097/00007632-199510001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak J, Sutter M, Herdmann J. Cervical myelopathy: clinical and neurophysiological evaluation. Eur Spine J. 2003;12(Suppl 2):S181–S187. doi: 10.1007/s00586-003-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rosler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Nakamae T, Tanaka N, Nakanishi K, Fujimoto Y, Sasaki H, Kamei N, Hamasaki T, Yamada K, Yamamoto R, Izumi B, Ochi M. Quantitative assessment of myelopathy patients using motor evoked potentials produced by transcranial magnetic stimulation. Eur Spine J. 2010;19:685–690. doi: 10.1007/s00586-009-1246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu T, Hino T, Komori T, Hirai S. Loss of the muscle silent period evoked by transcranial magnetic stimulation of the motor cortex in patients with cervical cord lesions. Neurosci Lett. 2000;286:199–202. doi: 10.1016/S0304-3940(00)01125-3. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka N, Nakanishi K, Fujimoto Y, Sasaki H, Kamei N, Hamasaki T, Yamada K, Yamamoto R, Nakamae T, Ochi M. Clinical results of cervical myelopathy in patients older than 80 years of age: evaluation of spinal function with motor evoked potentials. J Neurosurg Spine. 2009;11:421–426. doi: 10.3171/2009.4.SPINE08584. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi K, Tanaka N, Sasaki H, Kamei N, Hamasaki T, Yamada K, Yamamoto R, Nakamae T, Ochi M. Assessment of central motor conduction time in the diagnosis of compressive thoracic myelopathy. Spine (Phila Pa 1976) 2010;35:E1593–E1598. doi: 10.1097/BRS.0b013e3181d9e7a4. [DOI] [PubMed] [Google Scholar]
- 10.Bednarik J, Kadanka Z, Vohanka S, Novotny O, Surelova D, Filipovicova D, Prokes B. The value of somatosensory and motor evoked evoked potentials in pre-clinical spondylotic cervical cord compression. Eur Spine J. 1998;7:493–500. doi: 10.1007/s005860050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 13.Gallus J, Mathiowetz V. Test-retest reliability of the Purdue Pegboard for persons with multiple sclerosis. Am J Occup Ther. 2003;57:108–111. doi: 10.5014/ajot.57.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Taylor S, Frost H, Taylor A, Barker K. Reliability and responsiveness of the shuttle walking test in patients with chronic low back pain. Physiother Res Int. 2001;6:170–178. doi: 10.1002/pri.225. [DOI] [PubMed] [Google Scholar]
- 15.Byrnes ML, Thickbroom GW, Phillips BA, Wilson SA, Mastaglia FL. Physiological studies of the corticomotor projection to the hand after subcortical stroke. Clin Neurophysiol. 1999;110:487–498. doi: 10.1016/S1388-2457(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 16.Mills KR. Magnetic brain stimulation: a review after 10 years experience. Electroencephalogr Clin Neurophysiol Suppl. 1999;49:239–244. [PubMed] [Google Scholar]
- 17.Pearce AJ, Kidgell DJ. Corticomotor excitability during precision motor tasks. J Sci Med Sport. 2009;12:280–283. doi: 10.1016/j.jsams.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/PL00005641. [DOI] [PubMed] [Google Scholar]
- 19.Mills KR, Nithi KA. Peripheral and central motor conduction in amyotrophic lateral sclerosis. J Neurol Sci. 1998;159:82–87. doi: 10.1016/S0022-510X(98)00148-8. [DOI] [PubMed] [Google Scholar]
- 20.Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120(Pt 4):605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- 21.Kohno K, Kumon Y, Oka Y, Matsui S, Ohue S, Sakaki S. Evaluation of prognostic factors following expansive laminoplasty for cervical spinal stenotic myelopathy. Surg Neurol. 1997;48:237–245. doi: 10.1016/S0090-3019(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 22.Classen J, Benecke R. Inhibitory phenomena in individual motor units induced by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1995;97:264–274. doi: 10.1016/0924-980X(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 23.Davey NJ, Romaiguere P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. J Physiol. 1994;477(Pt 2):223–235. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen LM, Nielsen JE, Ravnborg M. MEP recruitment curves in multiple sclerosis and hereditary spastic paraplegia. J Neurol Sci. 2005;237:25–29. doi: 10.1016/j.jns.2005.05.002. [DOI] [PubMed] [Google Scholar]