Abstract
Purpose
To review the literature on different classifications of T2-weighted (T2W) increased signal intensity (ISI) on preoperative magnetic resonance (MR) images of patients with cervical spondylotic myelopathy (CSM).
Methods
The authors searched the databases of PubMed and Cochrane for studies that used a categorization of T2W ISI to predict the functional outcome after decompressive surgery for CSM. Selected studies were analyzed for the type of ISI classification used, patient selection, methodology and results. The level of evidence provided by each study was determined.
Results
Twenty-two studies fulfilled our search criteria. There were 11 prospective studies and a total of 1,508 patients were studied. The majority of studies classified ISI based on either the longitudinal extent (12 studies) or the qualitative features of the ISI (10 studies). Three studies used both parameters to classify T2W ISI. Other classifications were based on the position of ISI (1 study), presence of snake-eye appearance on axial MR images (1 study) and signal intensity ratio (SIR) (1 study). Poorer functional outcomes correlated with sharp, intense ISI (6 studies) and multisegmental ISI (5 studies) (Class II evidence). Five of ten studies reported that the regression of ISI postoperatively was associated with better neurological outcomes (Class II evidence).
Conclusions
Methodological variations in previous studies made it difficult to compare studies and results. Both multisegmental T2W ISI and sharp, intense T2W ISI are associated with poorer surgical outcome (Class II evidence). The regression of T2W ISI postoperatively correlates with better functional outcomes (Class II). Future studies on the significance of ISI should ensure use of a uniform grading system, standardized outcome measures and multivariate analyses to control for other preoperative variables.
Keywords: Cervical spondylotic myelopathy, Cervical spine surgery, T2-weighted MRI, Intramedullary, Review
Introduction
Magnetic resonance imaging (MRI) of the cervical spine is essential for the preoperative evaluation of patients with cervical spondylotic myelopathy (CSM) [1]. Previous authors have found T2-weighted (T2W) increased signal intensities (ISI) within the cervical cord in 41–97.2 % of patients with CSM [2–13]. The prognostic significance of these radiological findings has been debated in a number of articles with conflicting results [6, 7, 11, 12, 14–16].
Presently, there is more emphasis on classifying these MR changes, since the type of ISI appears to be more important in determining patient outcome than merely its presence or absence. A variety of classifications have been used to categorize T2W ISI on preoperative MR images (Table 1). However, the predictive value of different types of ISI in patients with CSM is still unclear and there is no consensus yet on the best classification or the most important type of ISI for prognostication.
Table 1.
Types of classifications used to study the prognostic significance of T2-weighted increased signal intensity (ISI) on MR images of patients with CSM
| Code | Type of classification and basis of classification | Grade/type | Description |
|---|---|---|---|
| Longitudinal extent of ISI | |||
| L133 | Actual number of segments involved | 0–4 | |
| L22,3,4,8,9,25,26,30,31,37 | One or more segments | 0 | No ISI |
| 1 | Focal/single segment ISI | ||
| 2 | Multisegmental ISI | ||
| L332 | With T1-weighted changes | 1 | 1 disc space + normal T1W image |
| 2 | >1 disc space + normal T1W image | ||
| 3 | T1W hypointensity | ||
| Qualitative | |||
| Q133 | Marginal pattern of ISI | 1 | Localized |
| 2 | Diffuse | ||
| Q236 | ISI delineation | 0 | No signal change |
| 1 | Diffuse signal change | ||
| 2 | Focal signal change | ||
| Q32,5,10,13,34,35 | ISI intensity and border | 0 | No change |
| 1 | Faint, fuzzy border | ||
| 2 | Intense, well-defined border | ||
| Q49,28 | Intensity of ISI | 0 | None |
| 1 | Slight | ||
| 2 | Moderate | ||
| 3 | Intense | ||
| 4 | Very intense | ||
| Other classifications | |||
| O129 | Axial appearance | SEA | Snake-eye appearance |
| NSEA | Non snake-eye appearance | ||
| O227 | Position of ISI | Group A | ISI in gray matter only |
| Group B | ISI in gray and white matter | ||
| O338 | T2W signal intensity ratio | Group 1 | <1.32 |
| Group 2 | ≥1.32 and <1.68 | ||
| Group 3 | ≥1.68 | ||
In this review, we summarized the available literature on the different classifications of ISI used in patients with CSM. We analyzed the data and graded the evidence from different studies to identify which types of ISI have been shown to predict functional outcome after decompressive surgery.
Methods
We searched the databases of PubMed and Cochrane for articles published (electronically or in print) until October 2011 with the following keywords—‘magnetic resonance imaging and cervical spondylotic myelopathy’ (283 results) and ‘magnetic resonance imaging and cervical spine surgery’ (3,030 results). All English language articles, which used a classification of T2W ISI in CSM patients to predict outcome after decompressive surgery, were selected for review. If additional references were found within selected articles, these were also reviewed. Case series that included patients with ossified posterior longitudinal ligament (OPLL) were also reviewed, provided the majority of the patients in the series had CSM. We excluded descriptive articles and case reports that did not analyze the effect of the type of ISI on functional outcome. We also excluded studies that focused on a combination of T1W and T2W intramedullary changes only and did not classify the T2W changes.
Guidelines to grade the evidence in therapeutic trials have limited application when applied to prognostic studies [17, 18]. In 2003, the Journal of Bone and Joint Surgery Am adopted a system to grade the level of evidence based on a modification of Sackett’s grading system [19]. This system has been used in a number of spine-related reviews [20–23] and can grade different types of studies [24]. In the present review, we used the criteria for prognostic studies to grade the level of evidence (Table 2).
Table 2.
Levels of evidence for prognostic studies (adapted from Wupperman et al. [24])
| Level of evidence | Study characteristics |
|---|---|
| I | High-quality prospective studya (all patients were enrolled at the same point in their disease with ≥80 % follow-up of enrolled patients |
| Systematic reviewb of level I studies | |
| II | Retrospectivec study |
| Untreated controls from an RCT | |
| Lesser quality prospective study (e.g., patients enrolled at different points in their disease or <80 % follow-up) | |
| Systematic review of level II studies | |
| III | Case control studyd |
| IV | Case series |
| V | Expert opinion |
aStudy was started before the first patient enrolled
bA combination of results from two or more prior studies
cStudy was started after the first patient enrolled
dPatients identified for the study based on their outcome, called ‘cases’ are compared to those who did not have that outcome, called ‘controls’
Results
Twenty-two articles fulfilled our search criteria (Table 3)
Table 3.
Summary of 22 publications that have studied the impact of types of T2-weighted increased signal intensity (ISI) on surgical outcome in patients with cervical spondylotic myelopathy
| References | Level of evidencea | Nature of studyb | Number of patients | Mean age (years) | T2W ISI classificationc | Surgeryd | Outcome variablese | Mean follow-up duration (months) | Statistical analysisf | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al. [33] | II | R | 39 | 62.4 | Q1, L1 | P | JOA RR | 19.1 | Correlation | Number of ISI segments, focal ISI and rostral location of ISI were associated with poor prognosis |
| Arvin et al. [36] | II | Pr | 52 | 56.2 | Q2 | C | JOA RR, Nurick grade, SF36, NDI | 12.0 | Multivariate regression | Absence of focal ISI and regression of ISI after surgery were associated with good recovery |
| Avadhani et al. [2] | II | R | 35 | 57.8 | Q3, L2 | P | Nurick grade | 51.3 | Linear regression | T2W ISI alone had no prognostic significance |
| Chatley et al. [4] | II | Pr | 64 | 47.1 | L2 | A | JOA RR | 47.1 | Linear regression | Multisegmental ISI predicted poor outcome |
| Chen et al.g [5] | II | Pr | 64 | 56.6 | Q3 | P | JOA RR | 6.0 | ANCOVA | Type 1 ISI had better surgical outcome compared to type 2 |
| Fernandez de Rota et al. [8] | II | Pr | 67 | 59.5 | L2 | C | JOA RR | 39.0 | Linear regression | Multisegmental T2W ISI had poorer prognosis |
| Kohno et al. [37] | II | Pr | 22 | 60.5 | L2 | P | JOA RR | 61.2 | Mann–Whitney U test | Multisegmental T2W ISI was associated with poorer outcomes |
| Mastronardi et al. [9] | II | Pr | 47 | 54.0 | Q4, L2 | A | JOA, Nurick grade | 40.2 | Correlation | Best results were produced in the absence of T2W ISI. Better outcomes were seen when T2W ISI regressed after surgery |
| Mehalic et al. [28] | IV | R | 19 | 33–85 | Q4 | C | NS | NS | NS | Decreased intensity of T2W ISI in postoperative MR images was associated with improved clinical outcome |
| Mizuno et al. [29] | II | R | 144 | 56.5 | O1 | A | JOARR | NS | NS | Snake-eye appearance of ISI associated with worse functional outcome |
| Papadopoulos et al. [26] | II | Pr | 42 | 57.5 | L2 | C | JOA RR | 6.0-24.0 | Student t test | Type 0 and type 1 ISI had a better prognosis than type 2 ISI |
| Park et al. [25] | II | R | 80 | 62.1 | L2 | C | NCSS | 3.0 | Multivariate regression | Multisegmental ISI was independently associated with poorer NCSS recovery rate |
| Shen et al. [27] | II | R | 64 | 58.5 (median) | O2 | C | JOA RR | 34.0 (median) | Student’s t test and ANOVA | ISI in gray and white matter (Group B) was associated with worse outcomes |
| Shin et al. [34] | I | Pr | 70 | 51.1 | Q3 | A | JOA RR | 32.7 | Multivariate regression | Increased ISI grade was related to poorer neurological outcome |
| Singh et al. [30] | II | Pr | 69 | 57.0 (male), 62.0 (female) | L2 | C | Nurick grade, MDI, Ranawat Scale | 3.0 | Correlation | Presence and number of ISI were associated with clinical severity. However, confounders and lack of strong correlation affected analysis of the impact of ISI on surgical outcome |
| Vedantam et al. [13] | II | R | 197 | 48.8 | Q3 | A | Nurick grade change, cure | 35.2 | Multivariate regression | Type 2 ISI was associated with lower probability of complete recovery (Nurick grade 0 or 1) |
| Wada et al. [31] | II | R | 31 | 60.1 | L2 | C | JOA RR | 1.5 | Mann–Whitney U test | Presence of ISI did not correlate with severity of myelopathy or surgical outcome |
| Wada et al. [3] | II | R | 50 | 61.0 | L2 | P | JOA RR | 35.1 | Multivariate regression | Multisegmental ISI correlated with poorer outcomes, but was not a prognostic factor for surgical outcome |
| Yagi et al. [32] | II | R | 71 | 62.9 | L3 | P | JOA RR | 60.6 | Mann–Whitney U test | Long-term surgical outcome was worse in patients with ISI and postoperative expansion of ISI |
| Yukawa et al. [10] | II | Pr | 104 | 61.0 | Q3 | P | JOA RR | 40.0 | Mann–Whitney U test | T2W ISI correlated with postoperative JOA and JOA RR. Intense ISI had a worse prognosis compared to light ISI |
| Yukawa et al. [35] | II | Pr | 104 | 61.0 | Q3 | P | JOA RR | 39.7 | Mann–Whitney U test | Postoperative expansion of ISI was not associated with outcome |
| Zhang et al. [38] | II | R | 73 | 53.2 | O3 | C | JOA RR | 12.0 | Multivariate regression | Increased signal intensity ratio (SIR) with pyramidal signs is associated with poorer prognosis. SIR correlates with JOA RR and postoperative JOA |
NS not specified
aLevels of evidence as described in Table 2
bR retrospective, Pr prospective
cTypes of T2W ISI classifications: Q1–3- qualitative, based on intensity and margins, L1–3 based on longitudinal extent, O1–2 other classifications (refer Table 1)
dType of surgery: P posterior decompression only, A anterior decompression only, C anterior, posterior or combined approaches
eOutcome variables: JOA Japanese Orthopedic Association score, JOA RR JOA recovery rate, SF36 short form-36, NDI neck disability index, NCSS Neurosurgical Cervical Spine score, MDI myelopathy disability index
fANCOVA analysis of co-variance, ANOVA analysis of variance
gIn the study by Chen et al. [5] although the figures in the report suggested that posterior decompression was performed, the exact surgical procedure was not specified
Types of studies
Of the 22 articles reviewed, 11 were prospective studies (Table 3). Of the retrospective reports, one report also described 17 patients who were assessed prospectively [25]. The mean (±SE) follow-up duration in 18 studies was 27.8 ± 4.6 months. One study only provided a range of follow-up duration of 6 months to 2 years [26], another reported the median follow-up as 34 months [27], while two other studies [28, 29] did not provide the follow-up interval. There was no difference in the mean follow-up intervals between prospective (n = 10) and retrospective studies (n = 8) (28.4 vs. 27.2 months, P = 0.90).
Patient population
Demographic features of patients studied in the selected articles are shown in Table 3. A total of 1,508 patients were studied in the 22 reports reviewed. In all studies, the majority of the patients were males (n = 1,100, 72.9 %). The mean age based on 20 studies where the mean age was provided was 57.4 ± 1.0 years.
Type of T2W ISI classification
Two major classifications were used to evaluate the effect of T2W ISI on outcome in patients with CSM (Table 4).
Table 4.
Comparison of studies that used the two major types of ISI classifications
| Study characteristics | Number of studies using a particular type of ISI classification (%) | |
|---|---|---|
| L2 classification | Q3 classification | |
| Total number of studies | 10 | 6 |
| Prospective | 6 (60.0) | 4 (66.6) |
| Retrospective | 4 (40.0) | 2 (33.3) |
| Blinding of assessors | 4 (40.0)a | 2 (33.3) |
| Uniform surgical procedure performed | 4 (40.0) | 5 (83.3) |
| Multivariate analysis used | 2 (20.0) | 3 (50.0)b |
| Type of ISI correlated with outcome | 4 (40.0) | 4 (66.6) |
| Type of ISI was an independent predictor of outcome | 1 (10.0) | 3 (50.0) |
| Classes of evidence (Class I/II) | 0/10 | 1/5 |
Q3 classification—type 0, no ISI; type 1, faint, fuzzy border; type 2, intense, well-defined border
L2 classification—type 0, no ISI; type 1, focal/single segment ISI; type 2, multisegmental ISI
aOnly one assessor was blinded in the study by Papadopoulos et al. [26]
bIn the study by Chen et al. [5], ANCOVA was used to identify the independent predictive value of ISI. All other studies used the multiple regression analyses
Longitudinal extent of ISI
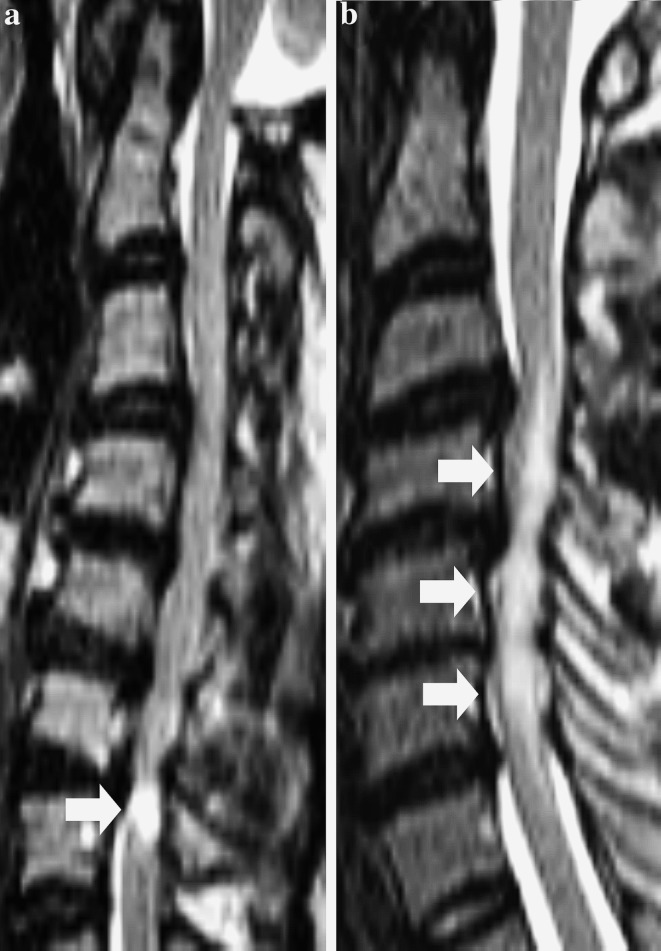
The first type of classification assessed the longitudinal extent of the T2W ISI. The most frequently used system classified MR changes as absent, focal (1 segment) and multisegmental (≥2 segments) (Fig. 1). Some authors defined a ‘segment’ as a vertebral level at which the spinal cord was compressed [3, 25, 26, 30, 31], while others defined it as a single disc space [2, 32]. In 12 reports that classified the size of ISI, the prevalence of focal ISI ranged from 14.0 to 61.7 %, while multisegmental ISI was seen in 8.7–45.7 % of all patients. In all but two reports [32, 33], the prevalence of multisegmental ISI was lower than that of focal ISI. However, the classification (L3, refer Table 1) used by Yagi et al. [32] did not specify what type of T2W ISI was seen in patients with T1W hypointensity and so the actual prevalence of focal or multisegmental ISI was unclear.
Fig. 1.
T2-weighted (T2W) sagittal MR images showing the L2 classification of T2W increased signal intensity (ISI). a Type 1, focal T2W ISI (arrow). b Type 2, multisegmental T2W ISI (arrows)
Qualitative classification of ISI
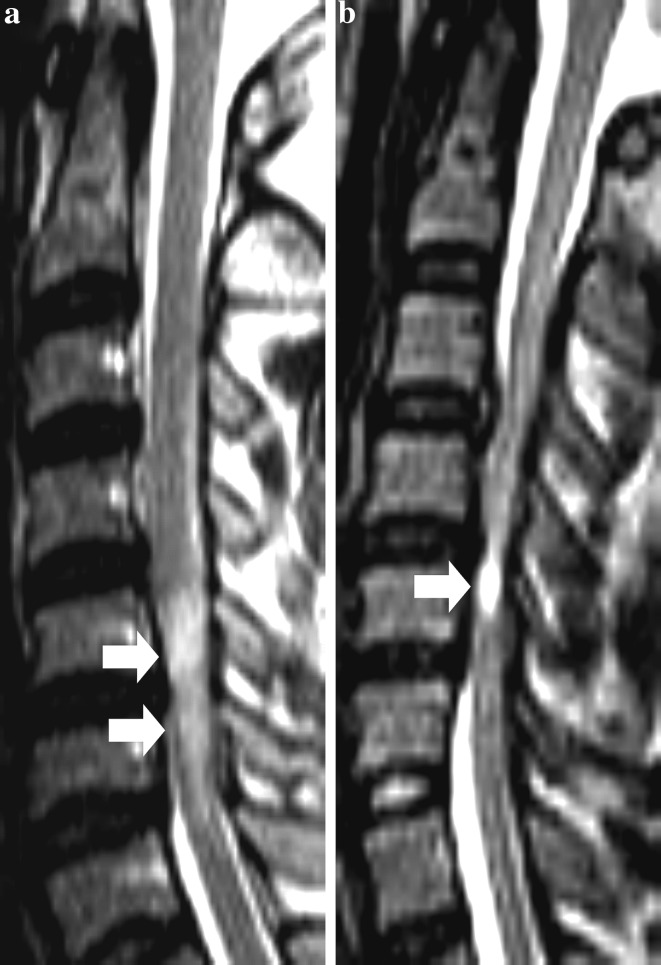
The second major type of classification was based on a qualitative description of the T2W ISI on sagittal MR images of the cervical spine (Table 1). The qualitative classification of T2W ISI involved an assessment of the intensity of ISI, marginal pattern or both. The most popular classification, which was first described by Chen et al. [5], was used in six reports (Fig. 2) [2, 5, 10, 13, 34, 35]. In these studies, the proportion of patients with type I (faint, fuzzy) ISI ranged from 35.7 to 52.8 %, while the prevalence of type 2 (intense, sharp) ISI ranged from 32.0 to 60.0 %.
Fig. 2.
Preoperative T2W sagittal MR images showing the Q3 classification of T2W increased signal intensity (ISI). a Type 1, faint ISI with fuzzy border (arrows). b Type 2, intense ISI with well-defined border (arrow)
Combination of extent and quality of ISI
Three studies evaluated MR changes using classifications of both the intensity and longitudinal extent of T2W ISI in the same group of patients [2, 9, 33].
Blinded/non-blinded assessment
T2W ISI changes were evaluated by two assessors in 11 studies [2, 3, 5, 9, 10, 13, 26, 27, 30, 35, 36]. In six of these reports, the assessors were blinded to the clinical outcome [3, 5, 9, 13, 30, 36]. In one report, only one assessor was blinded, and in four other reports [26], assessor blinding was not specified [2, 10, 27, 35]. The interobserver agreement (κ value) for assessment of the longitudinal extent of ISI ranged from 0.77 to 0.98 [2, 30], while the κ value for qualitative assessment of T2W ISI was 0.8–0.82 [2, 5, 10, 35].
Type of surgery
The majority of studies evaluated surgical outcome following both anterior and posterior decompressive procedures (Table 3). In seven reports, only posterior decompression was performed, such as laminoplasty [3, 32, 33], laminectomy [2] and expansive laminoplasty [10, 35, 37]. In five studies, only patients who had undergone anterior cervical surgery such as anterior cervical discectomy and fusion (ACDF) [9, 34], corpectomy [13] or a combination [4, 29] of the two were studied. In nine reports, patients underwent anterior or posterior decompression or combined approaches [8, 25–28, 30, 31, 36, 38]; in one report, although it appears that all patients underwent posterior decompression, the exact surgical procedure performed was not specified [5].
Outcome variables
The modified Japanese Orthopedic Association score (JOA) [39] was the commonest scale used to record the neurological status, while the JOA recovery rate as calculated by Hirabayashi et al. [40]. was the commonest outcome measure used. Other outcome variables used are shown in Table 3.
Type of analysis
Regression analysis was performed in nine studies to identify the impact of ISI on surgical outcome. Six of these reports used multiple regression analysis to control for other factors like age, duration of symptoms and preoperative neurological status (Table 3) [3, 13, 25, 34, 36, 38]. One study used ANCOVA (analysis of covariance) to study the effect of ISI on surgical outcome, and controlled only for age and preoperative JOA score [5]. In three reports, linear regression analysis was performed without controlling for other factors [2, 4, 8]. Other studies used parametric and non-parametric tests to compare the functional outcomes based on the type of T2W ISI.
Association of type of ISI and surgical outcome
Five reports concluded that multisegmental T2W ISI was associated with worse functional outcomes. In six studies that used the qualitative classification (Q1–3) of T2W ISI, the sharp, intense, well-circumscribed ISI was associated with poorer functional status at follow-up. Other individual reports showed that snake-eye appearance on axial T2W MR images [29], ISI in gray and white matter [27] as well as increased SIR [38] correlated with inferior surgical outcomes. In three studies [9, 28, 32], only patients without T2W ISI or with postoperative regression of ISI had better results. In five other studies, functional outcomes were not associated with any type of T2W ISI [2, 3, 30, 31, 35].
Postoperative imaging
In ten reports, the authors looked at the evolution of T2W ISI after surgery by studying both pre- and postoperative MR images. Postoperative MR imaging was done at mean intervals ranging from 3 to 60.6 months in ten studies [2, 5, 9, 25, 26, 29, 32, 35–37]. In six reports, the majority of patients (74.4–84 %) had no alteration in the grade/type of T2W ISI postoperatively [2, 5, 9, 35–37]. Postoperatively, regression of T2W ISI was seen in 11.5–51.4 % of cases [2, 5, 9, 25, 26, 28, 35, 36], while worsening of ISI was seen in 5.7–34 % of patients [2, 28, 32, 35, 36].
Levels of evidence
Twenty studies were graded Class II, one study provided Class I evidence and another was graded Class IV. The majority of studies did not evaluate patients at a uniform time in their disease (17 studies) and did not account for confounding variables in their statistical analyses (15 studies).
Discussion
Longitudinal extent of ISI
Wada et al. [31] provided one of the earliest classifications of the longitudinal extent of ISI: focal (restricted to the compressed level) and linear (extending beyond the compressed level, multisegmental). In a subsequent study [3], the authors found that although patients with multisegmental ISI had significantly poorer outcomes compared to those with focal ISI, ISI was not a predictor of surgical outcome when analyzed using a multiple regression analysis. Five of 12 reports that studied the significance of T2W ISI size found multisegmental ISI to be associated with significantly poorer surgical outcomes [4, 8, 25, 26, 33]. Additionally, Ahn et al. [33] demonstrated that the number of segments showing ISI correlated inversely with the recovery rate. All reports studying this type of ISI were scored Class II.
It has been shown that patients with multisegmental ISI have longer duration of symptoms [8], more severely compressed cords [8, 26] as well as poorer preoperative functional status [26, 30]. These findings seem to indicate that multisegmental ISI represents an advanced pathological process that should translate into poorer surgical outcomes. However, it is possible that in some cases the longitudinal extension of ISI represents a reversible pathology such as edema. As a result, the association between surgical outcome and ISI size has not been conclusively proven and more robust studies are required to confirm this relationship.
Qualitative type of ISI
Mehalic et al. [28]. described one of the earliest qualitative classifications of T2W ISI using five grades (Grade 0–4) based on signal intensity (Q4, refer Table 1). Although the authors asserted that repeated evaluations of MR images resulted in a similar grading, the subjective nature of the classification meant that it was used in only one other study [9].
In 2001, Chen et al. [5] provided a simpler classification of T2W ISI based on intensity and border pattern (Q3, refer Table 1), which had high interobserver agreement (k = 0.81). This classification is the most popular qualitative ISI grading system and has been used in six studies to date. The classifications used by Ahn et al. [33]. and Arvin et al. [36]. (Q1, Q2 refer Table 1) were similar to that described by Chen et al. [5]. Of the six studies that used the classification described by Chen et al. [5], four found that the sharp, intense ISI correlated with worse outcomes [5, 10, 13, 34]. In three of these studies [5, 13, 34], the authors used multivariate analysis to identify the independent predictive value of sharp ISI on surgical outcome. In the study by Yukawa et al. [10], patients with intense ISI were older and had longer duration of illness. Moreover, the authors did not use multilevel regression analysis to derive their results, thereby raising the question of whether the type of ISI affected the outcome independent of age and duration of illness. Among the studies with negative results, Avadhani et al. [2]. found that no type of T2W ISI (sharp or multisegmental) was predictive of outcome, while Yukawa et al. [35] studying only postoperative T2W ISI concluded that the intensity of ISI after surgery did not impact the functional status at the final follow-up. Among these six reports, one study was scored Class I [34], while the rest were graded Class II [2, 5, 10, 13, 35].
T2W ISI reflects a wide range of pathological changes in the cord. These changes can range from edema and demyelination to gliosis and microcavitation [29, 41, 42]. Ohshio et al. [42] showed that while high T2W signals reflected severe neural damage in the spinal cord, less intense T2W ISI was associated with milder nerve injury. Additionally, Shin et al. [34] demonstrated that CSM patients with intense T2W ISI had significantly poorer preoperative mJOA scores as compared to those with faint or no T2W ISI. The results of most clinical studies seem to indicate that the sharp, more intense T2W ISI is associated with worse clinical outcomes, thereby suggesting that this type of ISI represents severe neural damage.
Other classifications
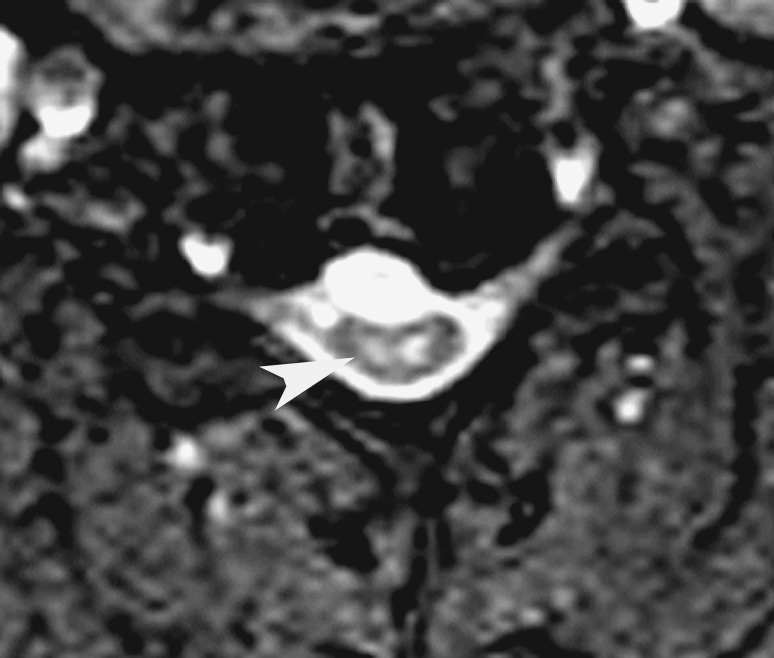
Shen et al. [27] used sagittal images of the cervical spine to classify T2W ISI based on its position in the cord (O1, refer Table 1) and patients with ISI in both white and gray matter (entire width of the cord) had the worst outcomes at 2 years after surgery. Zhang et al. [38] calculated the signal intensity ratio by dividing the signal intensity at the level of ISI or severely compressed cord (in cases with no ISI) by the signal intensity at the C7-T1 disc level. Intense ISI was associated with higher SIR, and patients with the highest SIR (group 3) were older and had worse preoperative JOA scores. Using multiple linear regression, the authors concluded that a high SIR correlated with poor surgical outcome. In another study, Mizuno et al. [29] found that patients with bilateral foci of ISI (snake-eye appearance, SEA) (Fig. 3) on preoperative axial T2W MRI had poorer surgical outcomes. Although several authors have described SEA within the cord in patients with CSM/OPLL [41, 43–48], this type of ISI is uncommon and has only been evaluated in this study.
Fig. 3.
Postoperative axial MR image of a patient with distal type of cervical spondylotic amyotrophy, 11 months after C6 central corpectomy, showing “snake eye” ISI (arrowhead)
The above studies do not provide data on blinding or interobserver agreement. It is not possible to confirm the prognostic value of these types of ISI using the results from a single study. However, they provide a novel dimension to the interpretation of T2W ISI and with refinement may provide useful results in future studies.
Postoperative imaging
It has been suggested that tracking changes in T2W ISI after decompressive surgery could predict long-term functional outcome. Mehalic et al. [28] were one of the first to provide evidence for this relationship. Five studies concluded that complete or partial regression of T2W ISI postoperatively was associated with better outcomes, when compared with patients who had no change in ISI after surgery [9, 25, 28, 36, 37]. However, it is clinically more important to assess if the relative change in ISI on postoperative imaging can predict the functional outcome in the future. Only three of ten studies have looked at the functional outcome at a time point after postoperative imaging [9, 32, 36]. Mastronardi et al. [9] performed MR imaging on all patients immediately after surgery and found ISI regression in only four patients. Since these four patients did not show improvement more than the other patients at the final follow-up (mean 40.2 months), the authors concluded that the timing of ISI regression was not a factor in predicting outcome. Yagi et al. [32] demonstrated that the postoperative expansion of ISI at 1 year predicted poorer recovery at the final follow-up (mean 60.6 months) in 71 patients after laminoplasty. The authors also stated that the risk factor for postoperative expansion of ISI was cervical instability. Arvin et al. [36] showed that an improvement in the grade of T2W ISI at 6 months postoperatively predicted better functional status at 1 year after surgery. Overall, there is Class II evidence to suggest that the regression of ISI after surgery is associated with a higher probability of functional recovery.
Additionally, some authors have tried to identify which types of ISI were more likely to regress after surgery. Chen et al. [5]. and Yukawa et al. [35] showed that the faint, fuzzy type of ISI (type 1 ISI, Q3, refer to Table 1) was more likely to regress after surgery. In contrast, Avadhani et al. [2] found that diminished MR changes postoperatively were seen predominantly in patients with type 2 ISI (sharp, intense MR change) and multisegmental ISI. In the series by Papadopoulos et al. [26], all patients who showed regression of ISI after surgery had focal ISI preoperatively. Mastronardi et al. [9] demonstrated that only those patients without associated T1W hypointensity had diminished ISI after surgery. There seems to be no definite conclusion as to which types of ISI are more likely to regress after surgery and this needs to be addressed in future studies.
Statistical analysis and outcomes
Evaluation of the independent predictive value of T2W ISI in patients with CSM/OPLL depends largely on the type of statistical analysis used. A number of factors affect the surgical outcome in these patients including age, duration of symptoms and preoperative neurological status [11, 12, 49–51]. In six reports, the impact of T2W ISI was assessed using a multivariate analysis with the above variables, thereby reducing the risk of spurious correlations. Three reports focused on the qualitative ISI classification [13, 34, 36], two reports on the longitudinal extent of ISI [3, 25] and one study used SIR to classify ISI [38]. Among the reports that looked at ISI shape and intensity, all three studies found that focal, sharp ISI predicted poorer postoperative functional status. The results of two studies, which used multivariate analysis, found conflicting results regarding the independent predictive value of the longitudinal extent of T2W ISI [3, 25]. Overall, a multivariate analysis using preoperative clinical variables is essential to independently evaluate the prognostic value of T2W ISI, and future studies should incorporate this statistical method.
Presently, it is difficult to identify which type of ISI classification is best able to predict surgical outcomes in patients with CSM. Previous studies have considerable variability in terms of preoperative variables, surgical procedures, outcome measures, follow-up intervals and statistical analyses. Studies that have used the two major classifications of T2W ISI have provided Class II evidence (Table 4). In 2009, Mummaneni et al. [1] published a systematic review on the predictive value of preoperative imaging in patients undergoing cervical surgery (articles from 1966 to 2007 were included) and graded the evidence according to a classification used for studies on therapeutic effectiveness [52]. The authors concluded that multisegmental T2W ISI predicted a poor surgical outcome, while there was conflicting evidence regarding discrete T2W ISI. It was unclear if ‘discrete’ T2W ISI referred to the qualitative description of ISI or a focal/single segment ISI. The review, however, did not sufficiently analyze the predictive value of the qualitative type of ISI. Subsequently, more studies have looked at ISI qualitatively, and the present review retrieved 14 additional studies, 8 of which used a qualitative classification of ISI.
The results of our review indicate that both multisegmental T2W ISI and ‘sharp, intense’ T2W ISI are associated with poorer functional outcome after decompressive surgery for CSM (Class II). Although this review focused on T2W ISI, it is possible that other MR findings may add to the predictive value of T2W hyperintensities. There is increasing evidence that T1W intramedullary hypointensities predict the worst clinical outcome in patients with CSM [2, 5, 8, 12, 13]. However, it has been suggested that since T2W ISI is much commoner than T1W changes, T2W ISI is a better candidate for prognostication [8]. Other authors have shown that gadolinium enhancement of the compressed cord is associated with less favorable outcomes [53, 54]. Clinical signs such as clonus and leg spasticity have also been correlated with poor functional status postoperatively [55]. Overall, only few studies have evaluated the prognostic value of a combination of MR findings and clinical signs in CSM patients [55, 56], and more studies are required to determine if this approach is more reliable than MR changes alone. Additionally, the underlying pathology such as disc protrusion, bony compression or OPLL may impact surgical outcome independently. To better evaluate the prognostic value of T2W ISI, we recommend that future studies use a uniform ISI grading system, a standardized outcome measure and multivariate analyses controlling for other preoperative clinical variables.
Conclusions
Identifying the type of T2W ISI on preoperative MR imaging is gaining importance in terms of predicting surgical outcome in patients with CSM. Methodological variations in previous studies with regard to ISI classifications, surgical procedures, outcome measures, follow-up intervals and statistical analyses meant that it was difficult to compare studies and results. Preoperative MR images that show multisegmental T2W ISI or ‘sharp’ T2W ISI indicate a poorer prognosis in patients with CSM (Class II evidence). The regression of T2W ISI postoperatively correlates with better functional outcomes (Class II).
Conflict of interest
The authors report no conflict of interest. No funding or grants were received for this study.
References
- 1.Mummaneni PV, Kaiser MG, Matz PG, Anderson PA, Groff M, Heary R, Holly L, Ryken T, Choudhri T, Vresilovic E, Resnick D. Preoperative patient selection with magnetic resonance imaging, computed tomography, and electroencephalography: does the test predict outcome after cervical surgery? J Neurosurg Spine. 2009;11(2):119–129. doi: 10.3171/2009.3.SPINE08717. [DOI] [PubMed] [Google Scholar]
- 2.Avadhani A, Rajasekaran S, Shetty AP. Comparison of prognostic value of different MRI classifications of signal intensity change in cervical spondylotic myelopathy. Spine J. 2010;10(6):475–485. doi: 10.1016/j.spinee.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Wada E, Yonenobu K, Suzuki S, Kanazawa A, Ochi T. Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 1999;24(5):455–456. doi: 10.1097/00007632-199903010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Chatley A, Kumar R, Jain VK, Behari S, Sahu RN. Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(5):562–567. doi: 10.3171/2009.6.SPINE091. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. 2001;221(3):789–794. doi: 10.1148/radiol.2213010365. [DOI] [PubMed] [Google Scholar]
- 6.Yone K, Sakou T, Yanase M, Ijiri K. Preoperative and postoperative magnetic resonance image evaluations of the spinal cord in cervical myelopathy. Spine (Phila Pa 1976) 1992;17(10 Suppl):S388–S392. doi: 10.1097/00007632-199210001-00008. [DOI] [PubMed] [Google Scholar]
- 7.Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52(5):1081–1087. doi: 10.1227/01.NEU.0000057746.74779.55. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez de Rota JJ, Meschian S, Fernandez de Rota A, Urbano V, Baron M. Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine. 2007;6(1):17–22. doi: 10.3171/spi.2007.6.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Mastronardi L, Elsawaf A, Roperto R, Bozzao A, Caroli M, Ferrante M, Ferrante L. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7(6):615–622. doi: 10.3171/SPI-07/12/615. [DOI] [PubMed] [Google Scholar]
- 10.Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K. MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine (Phila Pa 1976) 2007;32(15):1675–1678. doi: 10.1097/BRS.0b013e318074d62e. [DOI] [PubMed] [Google Scholar]
- 11.Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33–45. doi: 10.1016/S1529-9430(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 12.Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine (Phila Pa 1976) 2001;26(11):1238–1245. doi: 10.1097/00007632-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 13.Vedantam A, Jonathan A, Rajshekhar V. Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. J Neurosurg Spine. 2011;15(6):660–666. doi: 10.3171/2011.8.SPINE11452. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, Matsuo M. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg. 1991;74(6):887. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 15.Morio Y, Yamamoto K, Kuranobu K, Murata M, Tuda K. Does increased signal intensity of the spinal cord on MR images due to cervical myelopathy predict prognosis? Arch Orthop Trauma Surg. 1994;113(5):254–259. doi: 10.1007/BF00443813. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Yamashita Y, Sakamoto Y, Kojima R. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology. 1989;173(1):219–224. doi: 10.1148/radiology.173.1.2781011. [DOI] [PubMed] [Google Scholar]
- 17.Haines SJ. Evidence-based neurosurgery. Neurosurgery. 2003;52(1):36–47. doi: 10.1097/00006123-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Aarabi B, Alden TD, Chestnut RM, Downs JH, III, Ecklund JM, Eisenberg HM, Farace E, Florin RE, Jane JA, Jr, Kreiger MD, Maas AIR, Narayan RK, Potapov AA, Salazar AM, Shaffrey ME, Walters BC.Management and prognosis of penetrating brain injury J Trauma 200151Suppl 2S44–S49.11505200 [Google Scholar]
- 19.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Jt Surg Am. 2003;85-A(1):1–3. [PubMed] [Google Scholar]
- 20.Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976) 2010;35(9 Suppl):S37–S46. doi: 10.1097/BRS.0b013e3181d8338e. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld AJ, Sieg RN, Li G, Bader JO, Belmont PJ, Jr, Bono CM. Outcomes after spine surgery among racial/ethnic minorities: a meta-analysis of the literature. Spine J. 2011;11(5):381–388. doi: 10.1016/j.spinee.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld AJ, Bono CM, McGuire KJ, Warholic N, Harris MB. Computed tomography alone versus computed tomography and magnetic resonance imaging in the identification of occult injuries to the cervical spine: a meta-analysis. J Trauma. 2010;68(1):109–113. doi: 10.1097/TA.0b013e3181c0b67a. [DOI] [PubMed] [Google Scholar]
- 23.Riley LH,, 3rd, Vaccaro AR, Dettori JR, Hashimoto R. Postoperative dysphagia in anterior cervical spine surgery. Spine (Phila Pa 1976) 2010;35(9 Suppl):S76–S85. doi: 10.1097/BRS.0b013e3181d81a96. [DOI] [PubMed] [Google Scholar]
- 24.Wupperman R, Davis R, Obremskey WT. Level of evidence in spine compared to other orthopedic journals. Spine. 2007;32(3):388–393. doi: 10.1097/01.brs.0000254109.12449.6c. [DOI] [PubMed] [Google Scholar]
- 25.Park YS, Nakase H, Kawaguchi S, Sakaki T, Nikaido Y, Morimoto T. Predictors of outcome of surgery for cervical compressive myelopathy: retrospective analysis and prospective study. Neurol Med Chir (Tokyo) 2006;46(5):231–238. doi: 10.2176/nmc.46.231. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulos C, Katonis P, Papagelopoulos P, Karampekios S, Hadjipavlou A. Surgical decompression for cervical spondylotic myelopathy: correlation between operative outcomes and MRI of the spinal cord. Orthopedics. 2004;27(10):1087–1091. doi: 10.3928/0147-7447-20041001-19. [DOI] [PubMed] [Google Scholar]
- 27.Shen HX, Li L, Yang ZG, Hou TS. Position of increased signal intensity in the spinal cord on MR images: does it predict the outcome of cervical spondylotic myelopathy? Chin Med J (Engl) 2009;122(12):1418–1422. [PubMed] [Google Scholar]
- 28.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26(2):217–226. doi: 10.1227/00006123-199002000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno J, Nakagawa H, Inoue T, Hashizume Y. Clinicopathological study of “snake-eye appearance” in compressive myelopathy of the cervical spinal cord. J Neurosurg Spine. 2003;99(2):162–168. doi: 10.3171/spi.2003.99.2.0162. [DOI] [PubMed] [Google Scholar]
- 30.Singh A, Crockard HA, Platts A, Stevens J. Clinical and radiological correlates of severity and surgery-related outcome in cervical spondylosis. J Neurosurg. 2001;94(2 Suppl):189–198. doi: 10.3171/spi.2001.94.2.0189. [DOI] [PubMed] [Google Scholar]
- 31.Wada E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 1995;20(20):2226–2232. doi: 10.1097/00007632-199510001-00009. [DOI] [PubMed] [Google Scholar]
- 32.Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on magnetic resonance imaging. J Neurosurg Spine. 2010;12(1):59–65. doi: 10.3171/2009.5.SPINE08940. [DOI] [PubMed] [Google Scholar]
- 33.Ahn JS, Lee JK, Kim BK. Prognostic factors that affect the surgical outcome of the laminoplasty in cervical spondylotic myelopathy. Clin Orthop Surg. 2010;2(2):98–104. doi: 10.4055/cios.2010.2.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin J, Jin B, Kim K, Cho Y, Cho W. Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir. 2010;152(10):1687–1694. doi: 10.1007/s00701-010-0692-8. [DOI] [PubMed] [Google Scholar]
- 35.Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Machino M, Ito ZY, Matsuyama Y. Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy: comparison between preoperative and postoperative magnetic resonance images. J Neurosurg Spine. 2008;8(6):524–528. doi: 10.3171/SPI/2008/8/6/524. [DOI] [PubMed] [Google Scholar]
- 36.Arvin B, Kalsi-Ryan S, Karpova A, Mercier D, Furlan JC, Massicotte EM, Fehlings MG. Post-operative magnetic resonance imaging can predict neurological recovery following surgery for cervical spondylotic myelopathy: a prospective study with blinded assessments. Neurosurgery. 2011;69(2):362–368. doi: 10.1227/NEU.0b013e31821a418c. [DOI] [PubMed] [Google Scholar]
- 37.Kohno K, Kumon Y, Oka Y, Matsui S, Ohue S, Sakaki S. Evaluation of prognostic factors following expansive laminoplasty for cervical spinal stenotic myelopathy. Surg Neurol. 1997;48(3):237. doi: 10.1016/S0090-3019(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YZ, Shen Y, Wang LF, Ding WY, Xu JX, He J. Magnetic resonance T2 image signal intensity ratio and clinical manifestation predict prognosis after surgical intervention for cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2010;35(10):E396–E399. doi: 10.1097/BRS.0b013e3181c6dbc4. [DOI] [PubMed] [Google Scholar]
- 39.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4(3):286–295. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976) 1981;6(4):354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Al-Mefty O, Harkey LH, Middleton TH, Smith RR, Fox JL. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg. 1988;68(2):217–222. doi: 10.3171/jns.1988.68.2.0217. [DOI] [PubMed] [Google Scholar]
- 42.Ohshio I, Hatayama A, Kaneda K, Takahara M, Nagashima K. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine (Phila Pa 1976) 1993;18(9):1140–1149. doi: 10.1097/00007632-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Faiss J, Schroth G, Grodd W, Koenig E, Will B, Thron A. Central spinal cord lesions in stenosis of the cervical canal. Neuroradiology. 1990;32(2):117–123. doi: 10.1007/BF00588561. [DOI] [PubMed] [Google Scholar]
- 44.Ramanauskas WL, Wilner HI, Metes JJ, Lazo A, Kelly JK. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1989;13(3):399–404. doi: 10.1097/00004728-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Choi S, Lee SH, Lee JY, Choi WG, Choi WC, Choi G, Jung B, Lee SC. Factors affecting prognosis of patients who underwent corpectomy and fusion for treatment of cervical ossification of the posterior longitudinal ligament: analysis of 47 patients. J Spinal Disord Tech. 2005;18(4):309–314. doi: 10.1097/01.bsd.0000161236.94894.fc. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasa Rao NV, Rajshekhar V. Distal-type cervical spondylotic amyotrophy: incidence and outcome after central corpectomy. J Neurosurg Spine. 2009;10(4):374–379. doi: 10.3171/2008.12.SPINE08526. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara Y, Tanaka N, Fujimoto Y, Nakanishi K, Kamei N, Ochi M. Surgical outcome of posterior decompression for cervical spondylosis with unilateral upper extremity amyotrophy. Spine (Phila Pa 1976) 2006;31(20):E728–E732. doi: 10.1097/01.brs.0000240207.00747.82. [DOI] [PubMed] [Google Scholar]
- 48.Kameyama T, Ando T, Yanagi T, Yasui K, Sobue G. Cervical spondylotic amyotrophy. Magnetic resonance imaging demonstration of intrinsic cord pathology. Spine (Phila Pa 1976) 1998;23(4):448–452. doi: 10.1097/00007632-199802150-00008. [DOI] [PubMed] [Google Scholar]
- 49.Rajshekhar V, Kumar GS. Functional outcome after central corpectomy in poor-grade patients with cervical spondylotic myelopathy or ossified posterior longitudinal ligament. Neurosurgery. 2005;56(6):1279–1284. doi: 10.1227/01.NEU.0000159713.20597.0F. [DOI] [PubMed] [Google Scholar]
- 50.Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine (Phila Pa 1976) 1993;18(14):2024–2029. doi: 10.1097/00007632-199310001-00016. [DOI] [PubMed] [Google Scholar]
- 51.Koyanagi T, Hirabayashi K, Satomi K, Toyama Y, Fujimura Y. Predictability of operative results of cervical compression myelopathy based on preoperative computed tomographic myelography. Spine (Phila Pa 1976) 1993;18(14):1958–1963. doi: 10.1097/00007632-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 52.Matz PG, Anderson PA, Kaiser MG, Holly LT, Groff MW, Heary RF, Mummaneni PV, Ryken TC, Choudhri TF, Vresilovic EJ. Introduction and methodology: guidelines for the surgical management of cervical degenerative disease. J Neurosurg Spine. 2009;11(2):101–103. doi: 10.3171/2009.1.SPINE08712. [DOI] [PubMed] [Google Scholar]
- 53.Ozawa H, Sato T, Hyodo H, Ishii Y, Morozumi N, Koizumi Y, Matsumoto F, Kasama F, Aizawa T, Itoi E, Kokubun S. Clinical significance of intramedullary Gd-DTPA enhancement in cervical myelopathy. Spinal Cord. 2010;48(5):415–422. doi: 10.1038/sc.2009.152. [DOI] [PubMed] [Google Scholar]
- 54.Cho YE, Shin JJ, Kim KS, Chin DK, Kuh SU, Lee JH, Cho WH. The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J. 2011;20(12):2267–2274. doi: 10.1007/s00586-011-1878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17(4):315–322. doi: 10.1111/j.1552-6569.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y-z, Wang L-f, Shen Y, Ding WY, Xu JX, He J. The effects of MRI signal intensity changes and clinical manifestations on prognosis after surgical intervention for cervical spondylotic myelopathy. Orthop Surg. 2009;1(2):101–106. doi: 10.1111/j.1757-7861.2009.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]