Abstract
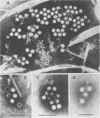
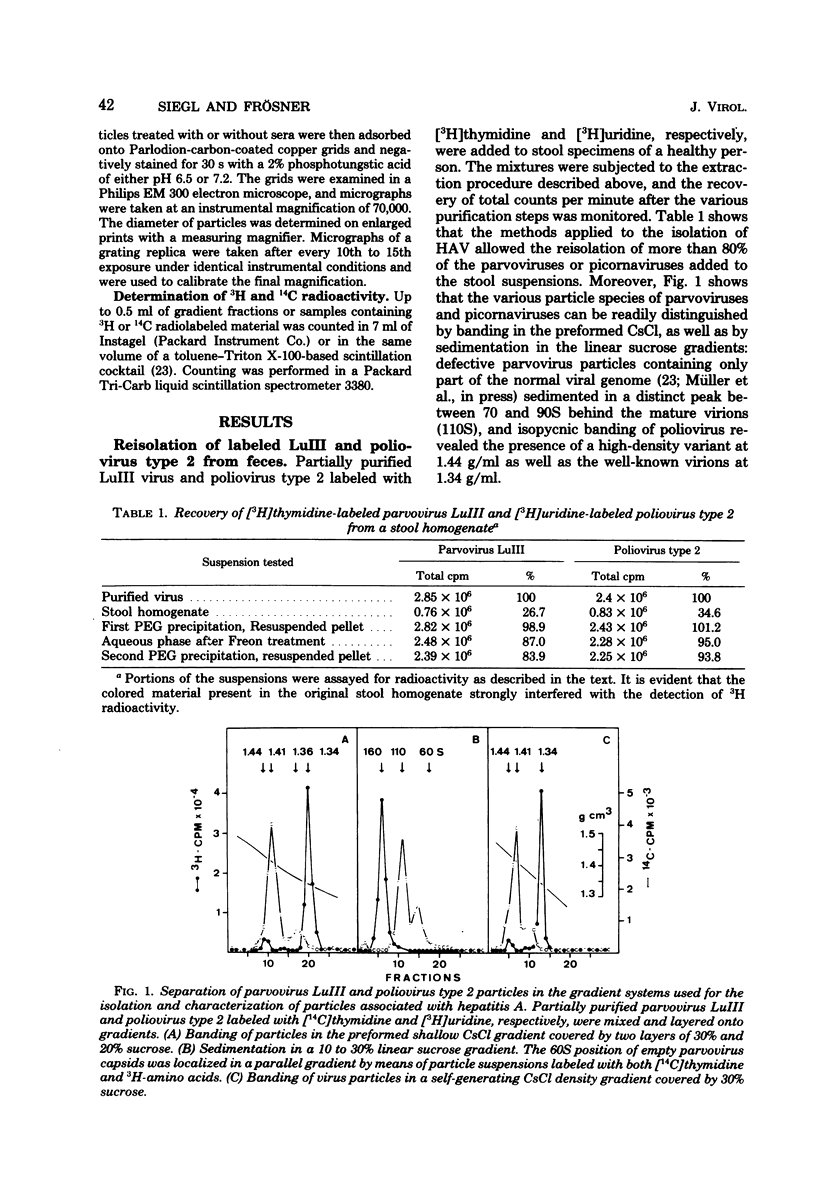
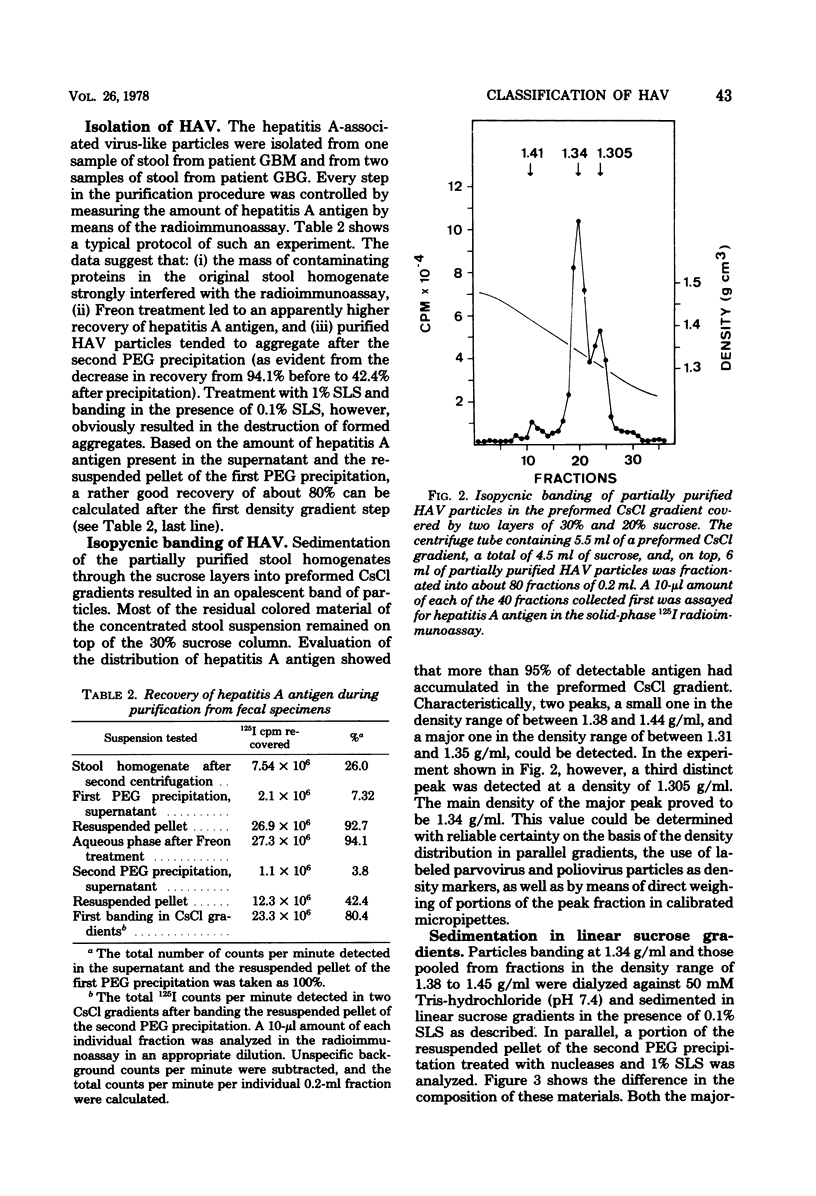
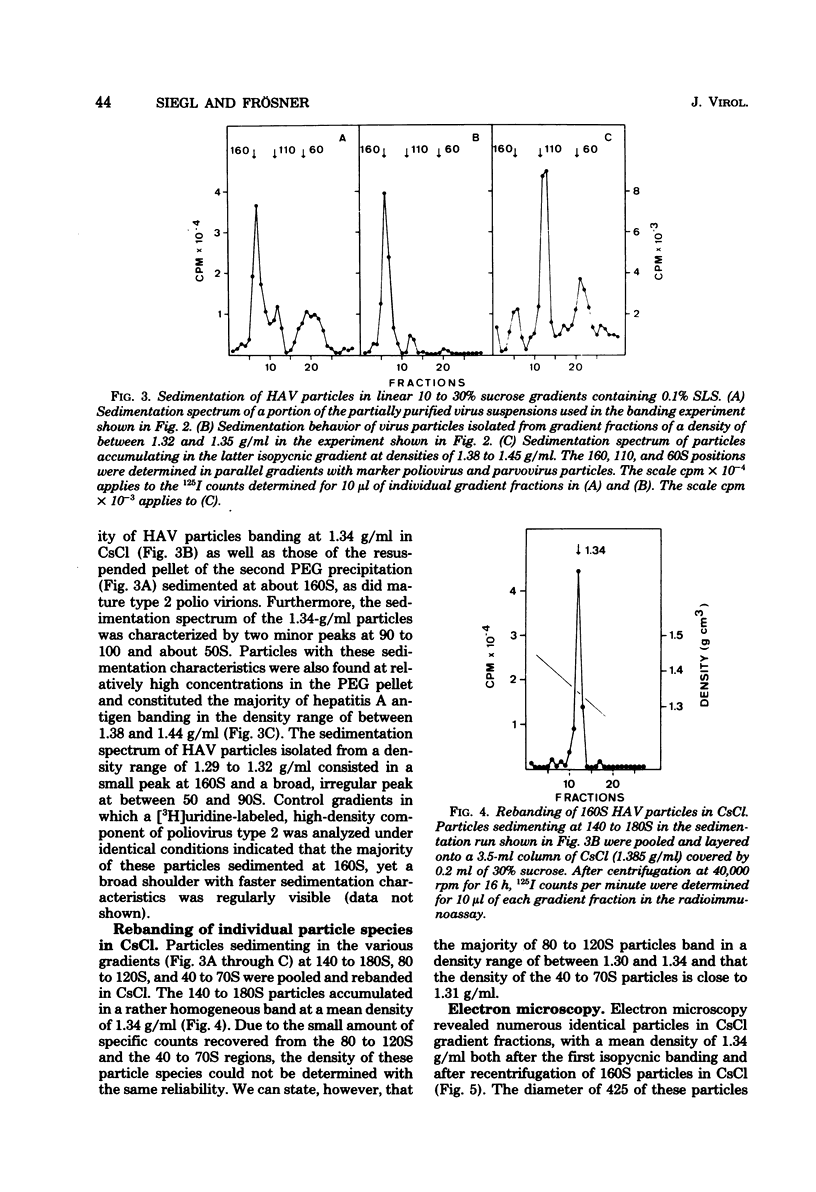
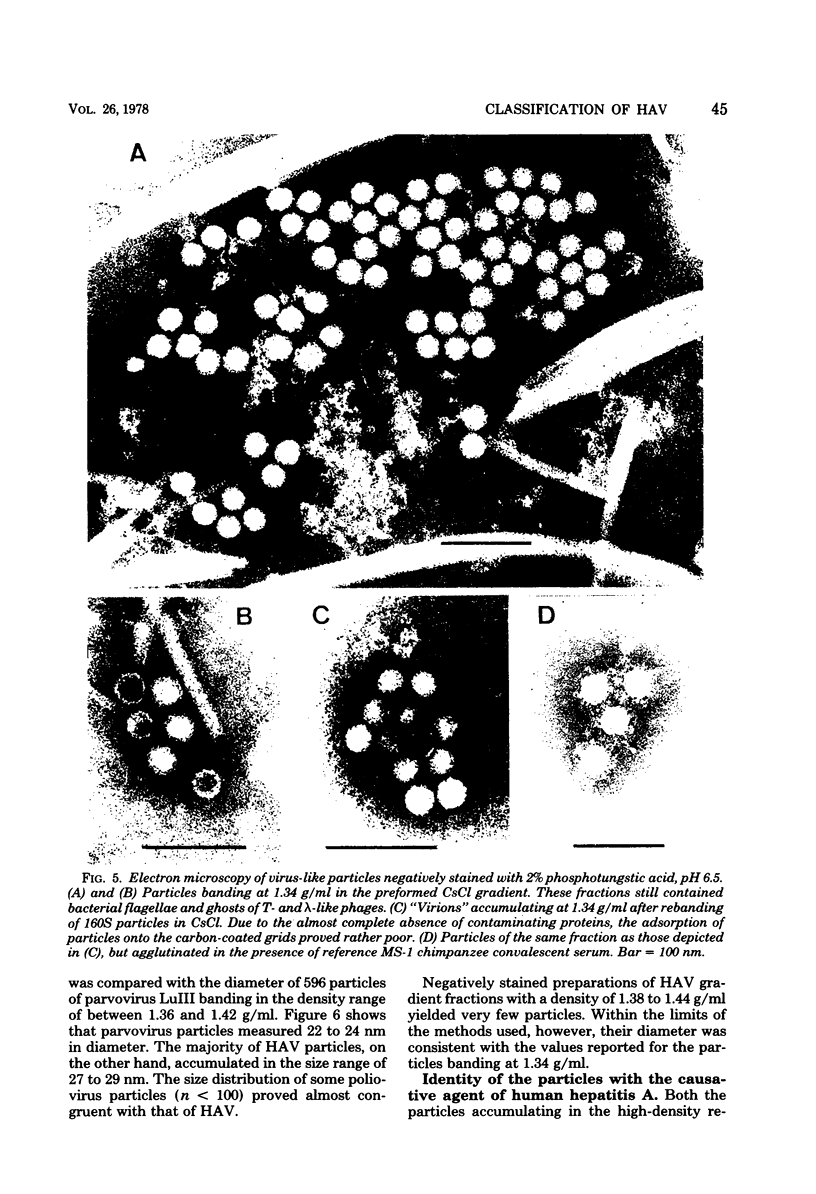
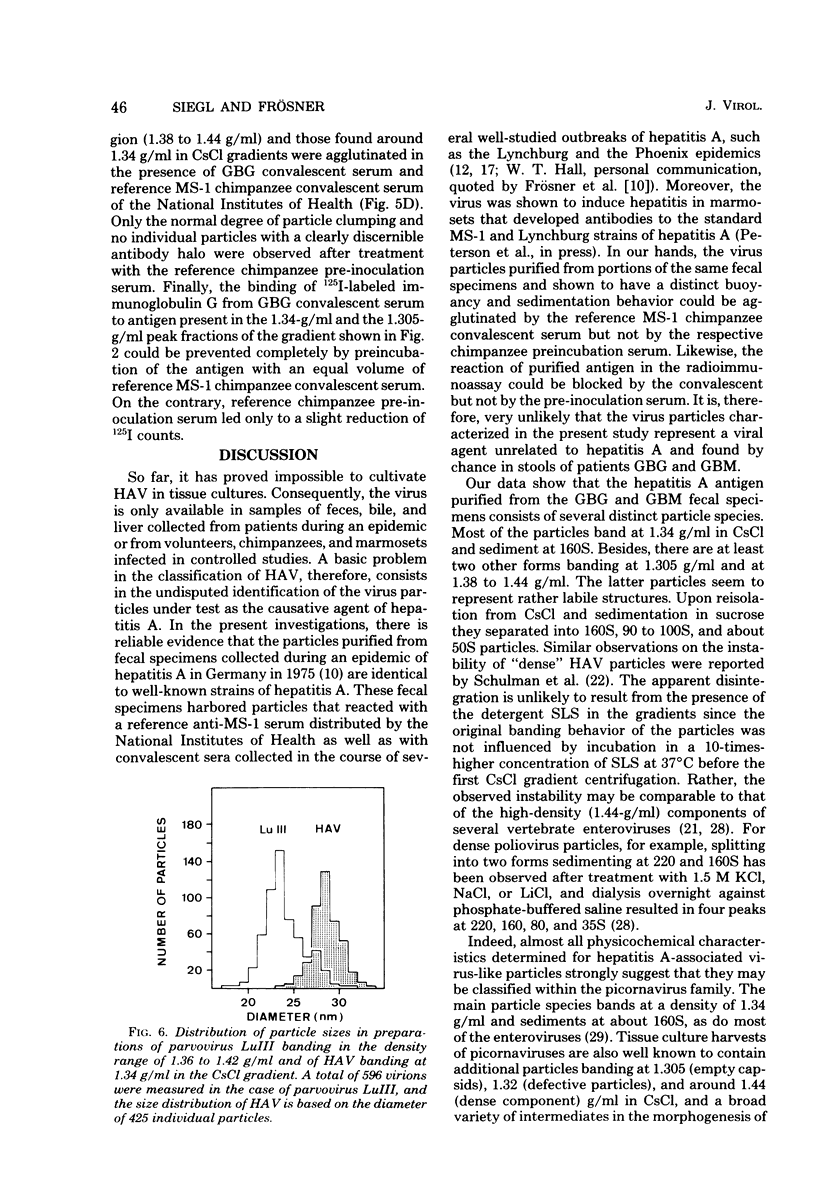
Virus-like particles were purified from stools of patients in an epidemic of hepatitis A in Germany. When reference MS-1 chimpanzee pre-inoculation and convalescent sera were used, the close serological relationship of the purified particles to well-known isolates of hepatitis A could be established. On the other hand, the physicochemical characteristics of the particles were determined in parallel to the characteristics of a marker parvovirus (LuIII) and a marker picornavirus (poliovirus type 2). It could be shown that the majority of the hepatitis A-associated particles band at 1.34 g/ml in CsCl and, like poliovirus, sediment at about 160S. In addition, a distinct hepatitis A antigen was observed, which banded at 1.305 g/ml and sedimented between 50 and 90S. A further component accumulated in the density range of between 1.38 and 1.44 g/ml. However, it seemed to be rather labile. Upon reisolation from CsCl and sedimentation in sucrose, it resolved into a 160S, a 90 to 100S, and a 50S form. The size of the 160S particles (27 to 29 nm) could be readily distinguished from that of the parvovirus (22 to 24 nm). It is concluded, therefore, that hepatitis A-associated virus particles are more likely to be classified with the picornaviruses than with the parvoviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. W., Hollinger F. B., Hornbeck C. L., Maynard J. E. Isolation and characterization of hepatitis A virus. Am J Clin Pathol. 1976 May;65(5 Suppl):876–889. [PubMed] [Google Scholar]
- Bradley D. W., Hornbeck C. L., Cood E. H., Maynard J. E., Gravelle C. R. CsCl banding of hepatitis A-associated virus-like particles. J Infect Dis. 1975 Mar;131(3):304–306. doi: 10.1093/infdis/131.3.304. [DOI] [PubMed] [Google Scholar]
- Bradley D. W., Hornbeck C. L., Cook E. H., Maynard J. E. Purification of hepatitis A virus from chimpanzee stools. J Virol. 1977 Apr;22(1):228–231. doi: 10.1128/jvi.22.1.228-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag J. L., Feinstone S. M., Purcell R. H., Hoofnagle J. H., Barker L. F., London W. T., Popper H., Peterson J. M., Kapikian A. Z. Experimental infection of chimpanzees with hepatitis A virus. J Infect Dis. 1975 Nov;132(5):532–545. doi: 10.1093/infdis/132.5.532. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Schulman A. N., Gerety R. J., Hoofnagle J. H., Lorenz D. E., Purcell R. H., Barker L. F. Hepatitis A antigen isolated from liver and stool: immunologic comparison of antisera prepared in guinea pigs. J Immunol. 1976 Sep;117(3):876–881. [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Gerin J. L., Purcell R. H. Buoyant density of the hepatitis A virus-like particle in cesium chloride. J Virol. 1974 Jun;13(6):1412–1414. doi: 10.1128/jvi.13.6.1412-1414.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelle C. R., Hornbeck C. L., Maynard J. E., Schable C. A., Cook E. H., Bradley D. W. Hepatitis A: report of a common-source outbreak with recovery of a possible etiologic agent. II. Laboratory studies. J Infect Dis. 1975 Feb;131(2):167–171. doi: 10.1093/infdis/131.2.167. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hoey E. M., Martin S. J. A possible precursor containing RNA of a bovine enterovirus: the provirion 11. J Gen Virol. 1974 Sep;24(3):515–524. doi: 10.1099/0022-1317-24-3-515. [DOI] [PubMed] [Google Scholar]
- Hollinger F. B., Bradley D. W., Dreesman G. R., Melnick J. L. Detection of viral hepatitis type A. Am J Clin Pathol. 1976 May;65(5 Suppl):854–865. [PubMed] [Google Scholar]
- Hollinger F. B., Bradley D. W., Maynard J. E., Dreesman G. R., Melnick J. L. Detection of hepatitis A viral antigen by radioimmunoassay. J Immunol. 1975 Nov;115(5):1464–1466. [PubMed] [Google Scholar]
- Matthew E. B., Dietzman D. E., Madden D. L., Newman S. J., Sever J. L., Nagler B., Bouton S. M., Rostafinski M. A major epidmeic of infectious hepatitis in an institution for the mentally retarded. Am J Epidemiol. 1973 Sep;98(3):199–215. doi: 10.1093/oxfordjournals.aje.a121549. [DOI] [PubMed] [Google Scholar]
- Maynard J. E., Bradley D. W., Gravelle C. R., Ebert J. W., Krushak D. H. Preliminary studies of hepatitis A in chimpanzees. J Infect Dis. 1975 Feb;131(2):194–197. doi: 10.1093/infdis/131.2.194. [DOI] [PubMed] [Google Scholar]
- Moritsugu Y., Dienstag J. L., Valdesuso J., Wong D. C., Wagner J., Routenberg J. A., Purcell R. H. Purification of hepatitis A antigen from feces and detection of antigen and antibody by immune adherence hemagglutination. Infect Immun. 1976 Mar;13(3):898–908. doi: 10.1128/iai.13.3.898-908.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost P. J., Wolanski B. S., Miller W. J., Ittensohn O. L., McAleer W. J., Hilleman M. R. Physical, chemical and morphologic dimensions of human hepatitis A virus strain CR326 (38578). Proc Soc Exp Biol Med. 1975 Feb;148(2):532–539. doi: 10.3181/00379727-148-38578. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Shirley M. W., Sangar D. V., Brown F. A high density component in several vertebrate enteroviruses. J Gen Virol. 1975 Nov;29(2):223–234. doi: 10.1099/0022-1317-29-2-223. [DOI] [PubMed] [Google Scholar]
- Schulman A. N., Dienstag J. L., Jackson D. R., Hoofnagle J. H., Gerety R. J., Purcell R. H., Barker L. F. Hepatitis A antigen particles in liver, bile, and stool of chimpanzees. J Infect Dis. 1976 Jul;134(1):80–84. doi: 10.1093/infdis/134.1.80. [DOI] [PubMed] [Google Scholar]
- Su R. T., Taylor M. W. Morphogenesis of picornaviruses: characterization and assembly of bovine enterovirus subviral particles. J Gen Virol. 1976 Mar;30(3):317–328. doi: 10.1099/0022-1317-30-3-317. [DOI] [PubMed] [Google Scholar]
- Wiegers K. J., Yamaguchi-Koll U., Drzeniek R. Differences in the physical properties of dense and standard poliovirus particles. J Gen Virol. 1977 Mar;34(3):465–473. doi: 10.1099/0022-1317-34-3-465. [DOI] [PubMed] [Google Scholar]