Abstract
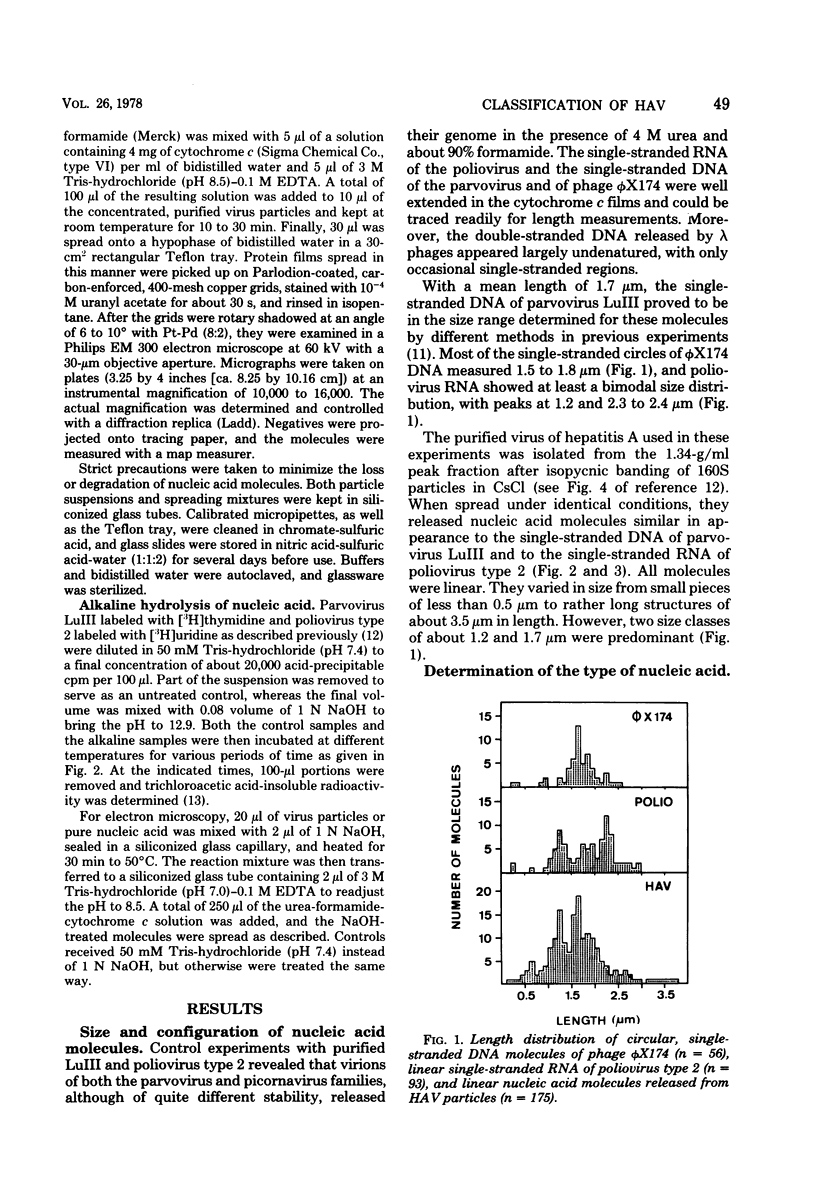
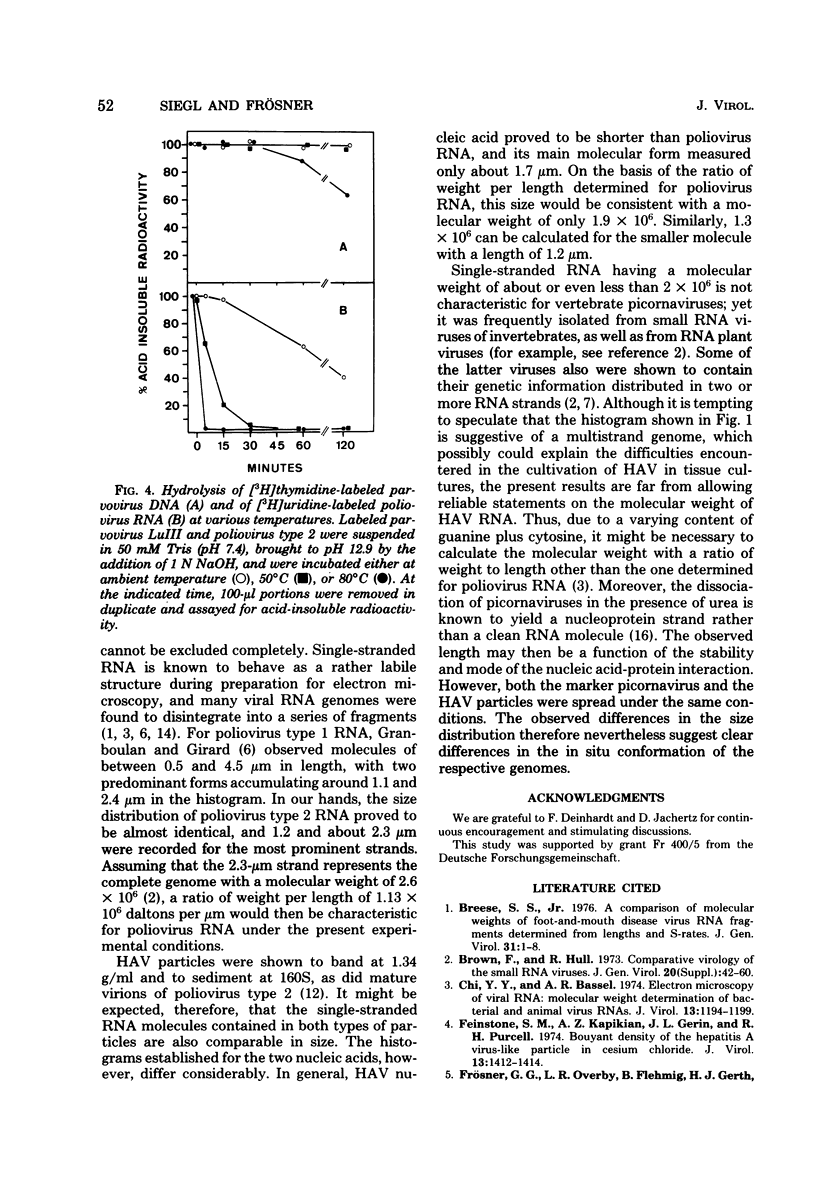
Virus particles banding at 1.34 g/ml in CsCl and sedimenting at 160S in sucrose gradients were isolated from fecal specimens of patients suffering from hepatitis. In the presence of 4 M urea and about 90% formamide, these particles released linear nucleic acid molecules of the kinked appearance characteristic of single-stranded RNA or single-stranded DNA. They could be distinguished from the nucleic acid of phage lambda added to the preparation as a marker for double-stranded configuration. Experiments in which the virus particles under investigation were incubated at pH 12.9 at 50 degrees C for 30 min revealed that their nucleic acid molecules were hydrolyzed as readily as the RNA genome of poliovirus type 2 analyzed in parallel. Both the single-stranded DNA of phage phiX174 and that of parvovirus LuIII, however, proved unaffected by this treatment, and the double-stranded DNA of phage lambda was denatured to single-stranded molecules. It was concluded, therefore, that the virus of human hepatitis A contains a linear genome of single-stranded RNA and has to be classified with the picornaviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chi Y. Y., Bassel A. R. Electron microscopy of viral RNA: molecular weight determination of bacterial and animal virus RNAs. J Virol. 1974 Jun;13(6):1194–1199. doi: 10.1128/jvi.13.6.1194-1199.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Gerin J. L., Purcell R. H. Buoyant density of the hepatitis A virus-like particle in cesium chloride. J Virol. 1974 Jun;13(6):1412–1414. doi: 10.1128/jvi.13.6.1412-1414.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frösner G. G., Overby L. R., Flehmig B., Gerth H. J., Haas H., Decker R. H., Ling C. M., Zuckerman A. J., Frösner H. R. Seroepidemiological investigation of patients and family contacts in an epidemic of hepatitis A. J Med Virol. 1977;1(3):163–173. doi: 10.1002/jmv.1890010303. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Wolanski B. S., Miller W. J., Ittensohn O. L., McAleer W. J., Hilleman M. R. Physical, chemical and morphologic dimensions of human hepatitis A virus strain CR326 (38578). Proc Soc Exp Biol Med. 1975 Feb;148(2):532–539. doi: 10.3181/00379727-148-38578. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Shirley M. W., Sangar D. V., Brown F. A high density component in several vertebrate enteroviruses. J Gen Virol. 1975 Nov;29(2):223–234. doi: 10.1099/0022-1317-29-2-223. [DOI] [PubMed] [Google Scholar]
- Weber G. H., Dahlberg J. E., Cottler-Fox M., Heine U. Electron microscopy of single-stranded RNA from vesicular stomatitis virus. Virology. 1974 Nov;62(1):284–287. doi: 10.1016/0042-6822(74)90324-9. [DOI] [PubMed] [Google Scholar]
- Wiegers K. J., Yamaguchi-Koll U., Drzeniek R. A complex between poliovirus RNA and the structural polypeptide VP1. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1308–1312. doi: 10.1016/0006-291x(76)90797-x. [DOI] [PubMed] [Google Scholar]




