Abstract
Introduction
Harvesting bone graft from the iliac crest in spinal fusion surgery is a widely used technique. However, complications can occur and there are also reports of patients with persistent graft site pain after surgery. The aim of this study was to evaluate pain from the donor site (DS) over time, and register associated complications and if it affected health-related quality of life (HRQoL).
Material and methods
One hundred and seven patients participating in an RCT between two different methods of reconstruction after cervical decompression were included in this study. One group underwent surgery with bone graft (BG) from the iliac crest and the other with no bone graft (NBG). All patients were evaluated concerning pain at DS and HRQoL preoperatively, at 4 weeks, 3 months and 1 year. Pain was evaluated with visual analog scale (VAS) and HRQoL with EQ-5D.
Results
A statistically significant difference was found at all times of follow-up in the BG group compared to preoperative levels and the NBG group. The VAS levels at follow-ups at 3 months and 1 year were however of questionable clinical importance. Two patients in the BG group had superficial wound infections postoperatively and five patients still had sensory disturbance in the area of graft site at 12 months. No major complications were registered. No difference could be seen in EQ-5D at any time of follow-up between the groups.
Conclusion
Harvesting of iliac crest bone graft is associated with significant pain. However, at 3 months postoperatively, the negative effect of clinical importance seemed to have disappeared compared to when no bone graft was harvested. The pain from bone graft harvesting does not seem to affect the quality of life at 4 weeks postoperatively and onward.
Keywords: Bone graft, Pain, Complications, Cervical spine
Introduction
Autologous bone is frequently utilized in orthopedic and other reconstructive procedures. A common harvesting site is the anterior iliac crest. However, it is well known that bone graft harvesting may be associated with complications, e.g. local pain in the acute phase, sometimes progressing into a chronic pain problem [1–4], local sensory disturbance, wound infection, postoperative hematoma, iliac fracture, and even bowel perforation [1, 5–8]. These unwanted side effects have, by some authors, been highlighted as a reason to avoid autologous bone graft altogether and instead other, usually expensive, techniques such as allografts, various spacers, and bone substitutes have been advocated [9–15]. However, the true magnitude of the problem with anterior iliac bone harvesting is still unclear as only few controlled studies have been published. Especially the change of the pain level over time has not been fully evaluated. The conduction of a randomized controlled trial (RCT) of surgical treatment of cervical radiculopathy, gave us the opportunity to evaluate pain after bone harvesting in a randomized fashion. In one treatment group the reconstruction was performed with bone graft (BG) from the anterior iliac crest whereas no bone graft (NBG) was used in the other group in which reconstruction was performed with total disc replacement (TDR). The aims of the present study were to evaluate the time scheme and levels of pain from the donor site (DS), to register complications linked to the harvesting of bone, and to evaluate if this affected the health-related quality of life (HRQoL) of the patients.
Patients and methods
One hundred and seven consecutive patients from the ongoing RCT on surgical treatment for cervical spine radiculopathy were followed for 1 year. The demographics are given in Table 1. Inclusion criteria were at least 3 months of radiculopathy in patients aged 18–60 years with correlating MRI findings at 1–2 cervical levels. Previous surgery, severe myelopathy, other cervical spine pathology, known hypersensitivity to implants or drugs used in the study, drug abuse or dementia rendered exclusion. Inclusion was done after informed consent. The patients were given both written and oral information about the two different reconstruction techniques. This also included information about where bone graft was supposed to be taken from in case of randomization to the BG procedure. All the patients underwent discectomy and decompression with an anterior approach. In order to avoid bias on behalf of the surgeon the randomization was made in the operating room after the decompression was finished, using closed envelope technique. In the BG, a tricortical graft was harvested from the right anterior iliac crest and then trimmed to fit in the decompressed disk space. The reconstruction was stabilized with a plate. The patients received a combination of bupivacaine (2.5 mg/ml) and adrenaline (5 mg/ml) via a small catheter at the bone harvesting site for the first 1–2 postoperative days. In the NBG, spinal reconstruction was achieved with TDR (Discover™, DePuy Spine). The postoperative regime was identical in both groups. The patients were allowed free mobilization without neck collar from the first postoperative day, and discharged as soon as this was feasible. No specific physiotherapy was administered.
Table 1.
The demographics of the groups
| n | BG group | NBG group | p |
|---|---|---|---|
| 45 | 62 | ||
| Age, (years), mean (SD) | 47.1 (6.9) | 46.5 (6.6) | 0.98 |
| Women, n (%) | 22 (48.9) | 31 (50.0) | 0.53 |
| 1-level/2-level surgery, n (%) | 29/16 (64) | 40/22 (64) | 0.57 |
| BMI, mean | 24.7 | 25.5 | 0.39 |
| Smokers, n (%) | 13 (29) | 20 (32) | 0.80 |
| Preoperative EQ-5D mean | 0.44 | 0.38 | 0.36 |
The patients were evaluated with regard to pain at DS and HRQoL preoperatively, at 4 weeks, 3 months, and 1 year after surgery. Questionnaires were sent to the patients by mail. Pain was evaluated with a 100 mm visual analog scale (VAS) specifying pain at DS where 0 represented “no pain” and 100 “worst imaginable pain”. The patients marked with a pen on a line and the actual measurement was done by a research nurse using a millimeter graded ruler. All patients also made a pain drawing [16, 17] as a supplement to the VAS to get a graphic representation of the pain and its physical distribution. Consumption of analgesics was also registered but not the type of medication. HRQoL was evaluated with the EQ-5D questionnaire [18]. The patients also attended a follow-up visit at 3 months and 1 year, where problems or complications related to the graft harvesting were registered.
The study was approved by the Regional Ethics Committee in Stockholm, no: 2006/1266-31/3.
Statistics
Statistical analysis was done using the Statistica software package version 10. Statsoft Inc. 2300 East 14th Street Tulsa, OK 74104, USA. VAS and EQ-5D levels were compared and computed with repeated measurement ANOVA, but as it can be argued that the VAS scale does not fulfill the criteria for ANOVA, statistical analysis was repeated using non-parametric tests, i.e. Mann–Whitney U test for unpaired samples and ordinal data and Wilcoxon’s sign test for paired samples. Student’s t test was used for comparison of numeric data and Fisher’s exact test was used for comparison of proportions. Level of significance was set at p < 0.05. Ninety-five percent confidence interval for the probability of complications were calculated with the Wilson Quadratic method [19].
Minimal clinically important difference for VAS when used in a similar setting is 10–18 mm and standard deviation according to various studies is 23 [20–22]. With a significance level of 0.05 and a power of 0.90, sample size calculation required 36 patients in each group to detect a change in VAS of 18. We wanted to increase this number slightly, to be on the safer side so we stopped the sampling when we had reached 45 patients in the smallest group. As the randomization was skew at this point we ended up with a total of 107 patients in the study.
Results
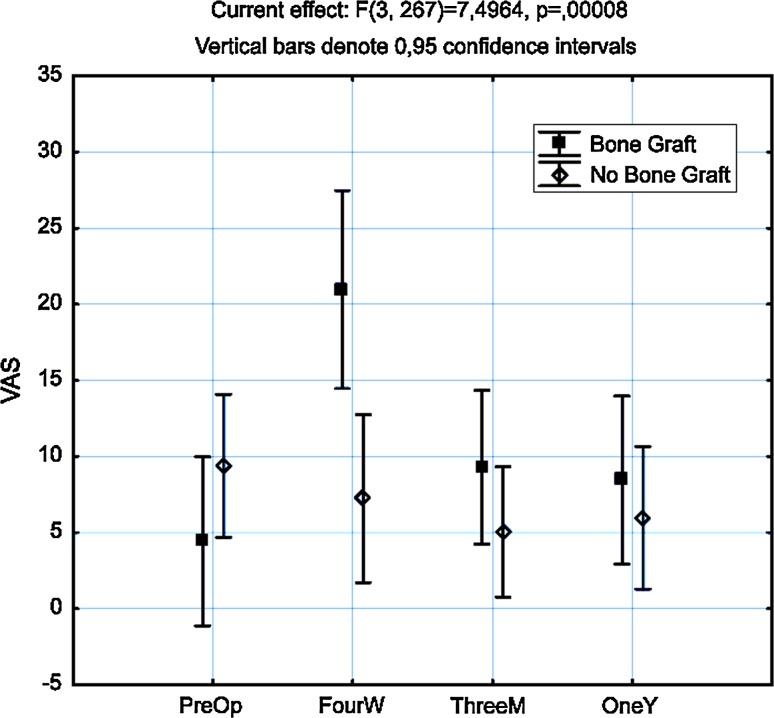
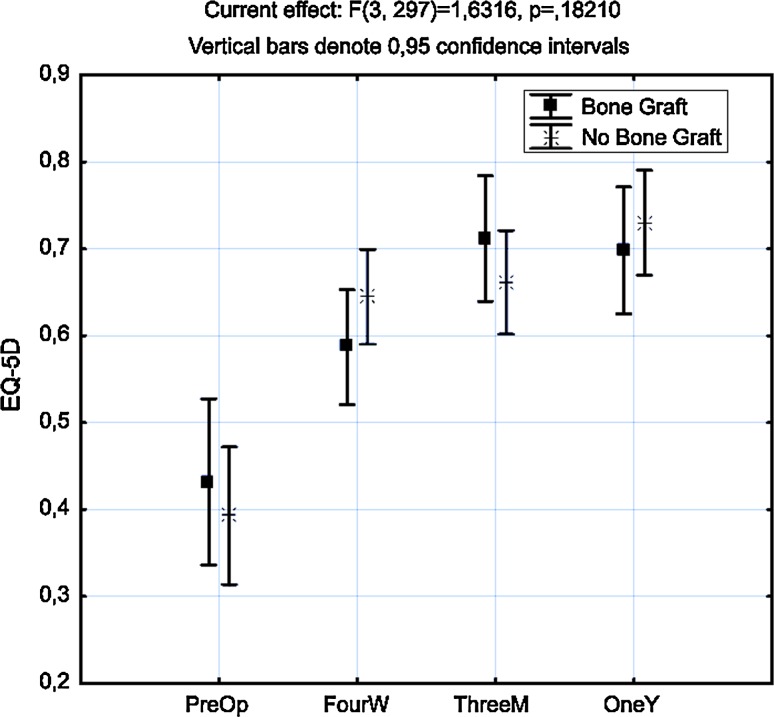
Preoperative VAS levels at donor site were generally low with an average of 9 mm on VAS with no significant differences between the BG and the NBG group, p = 0.27. Seventeen patients scored VAS more than 40 mm preoperative, 6 (13 %) in the BG group and 11 (18 %) in the NBG group. Statistical significant differences in VAS levels could be detected in the BG group at all occasions of follow-up compared to preoperative levels, p < 0.05. There were also statistical significant differences between both the groups at all occasions after surgery, p < 0.05 (Fig. 1). The VAS levels in the NBG group did not differ significantly from preoperative levels at any time of follow-up (Table 3). At 4 weeks, 15 patients in the BG group scored over 40 mm compared to 6 in the NBG group which was statistically significant, p = 0.003. At 3 months and 1 year, there were equally small numbers of patients scoring over 40 mm in both groups (Table 2). Thirty-three (73 %) patients in the BG group still took analgesics after 4 weeks compared to 37 (60 %) in the NBG group. Since the data concerning intake of analgesics was insufficient regarding the type of medication and dosage, and the obvious problem of appreciating if the analgesic was taken for pain at the donor site or from pain related to the index operation, we did not analyze this further as the interpretation would be too uncertain. There were no major complications registered with connection to bone harvesting. However, two patients (4 %) had superficial wound infections at the graft site. Both were treated with oral antibiotics and healed without any further complications. Five patients (11 %) had areas of sensory disturbance close to the donor site which persisted at the final follow-up at 12 months. Thus 7/45 patients in the BG group developed a complication making the 95 % confidence interval for risk of complication between 8 and 29 %. The mean preoperative EQ-5D was 0.41. It increased to 0.61 at 4 weeks, 0.68 at 3 months, and 0.71 at 1 year. There was no statistical difference in EQ-5D between the two treatment groups at any time point (Fig. 2).
Fig. 1.
ANOVA analysis of pain from donor site over time in both groups
Table 3.
VAS levels presented as means (SD) and medians (range) in both groups, respectively
| Bone graft | No bone graft | p | |||
|---|---|---|---|---|---|
| Preoperative | 5 (13) | 0 (0–50) | 11 (23) | 0 (0–83) | 0.27 |
| 4 weeks | 27 (28) | 17 (0–100) | 7 (18) | 0 (0–75) | <0.01 |
| 3 months | 10 (19) | 2 (0–66) | 7 (16) | 0 (0–65) | 0.038 |
| 1 year | 11 (19) | 0 (0–66) | 7 (20) | 0 (0–85) | 0.034 |
Table 2.
Number of patients scoring 40 mm or above on the VAS scale concerning pain from donor site (%)
| BG group | NBG group | p | |
|---|---|---|---|
| 4 weeks | 15 (33.3) | 6 (13.3) | 0.003 |
| 3 months | 5 (11.1) | 5 (8.0) | 0.74 |
| 1 year | 5 (11.1) | 6 (9.7) | 1.00 |
Fig. 2.
ANOVA analysis of EQ-5D over time in both groups
Discussion
Bone harvesting from the iliac crest is a widely used technique, not just in spine surgery. It is frequently used when autologous bone graft is needed, i.e. for reconstruction in maxillary facial surgery or for healing when dealing with pseudarthrosis in fractures. The use of other spinal implants such as cages and disk arthroplasty has to some extent reduced the need for bone graft, although cages are used by many surgeons together with autologous bone graft due to higher rates of solid fusion [12, 23, 24]. Pain from the donor site seems to be a problem and the first postoperative period and alternative surgical and pain reducing methods have been proposed [25–28]. Chronic pain and other complications have been reported but with very varying incidence. Pain from donor site over time after surgery has also been described [4]. Lofgren et al. [24] presented a study in 2010 with a similar design evaluating patients from a RCT between fusion with autograft and fusion with trabecular metal implant. However, they did not evaluate the pain from DS within the first 4 months after surgery and they could not find any statistical difference between the groups after this time period. Another study in a randomized fashion was presented in 2003 by Baskin et al. They compared rhBMP-2 to iliac crest bone in cervical fusions [15]. They found significant higher pain levels in the BG group at discharge from the hospital and after 6 weeks but similar to us they could not find a difference in pain at follow-up after 6 months. In this study, we used the patients who were randomized to TDR as controls with self-evaluation of pain in the area of a theoretical site of surgery. We used VAS to evaluate pain over time which is a widely accepted method but can be difficult to manage because statistical significant difference is not always the same as clinical significant difference. The minimally clinical important difference when using VAS in pain evaluation may vary with the level of pain [29, 30], but several authors suggest that the changes in VAS <18 mm cannot be regarded as a clinical relevant change [20, 31, 32]. It can also be doubtful to use these limits to changes in VAS on a group level since significant individual variations may not be detected [33]. It is possible to present individual changes in graphs, but it may not add much for the understanding of this material since it will be too complex. In the BG, group there was an increase from mean VAS 5 (SD 13) to 27 (SD 28) between evaluations preoperatively and after 4 weeks. The increase of 23 mm indicates that this group experiences more pain than if no bone graft was harvested. It is probably safe to assume that the DS pain was even higher, and thus the difference between the two groups is larger, during the first few weeks after surgery, but this was never documented in the present study. The BG group received locally administrated bupivacaine which may affect pain even in the longer term, and therefore also could have had an impact on the results, although the conclusions from previous studies are somewhat conflicting [34–37]. There is also a possibility that the infusion may have contributed to increase in the risk for postoperative infection. The randomized controls on postoperative local infusion all had placebo groups with catheters and thus we cannot determine if this influenced the results. The 17 patients who scored VAS more than 40 mm preoperative were analyzed further using the pain drawings to see if there were graphical distributions of the pain correlating to the donor site pain that was set on the VAS. In a majority of the pain drawings with high VAS at DS location, the patients also had marked pain in the area of the lumbar spine. We interpreted the pain at DS in these cases as a pain associated to a lumbar pain problem (Fig. 3). In another group of patients scoring high preoperative, the pain was drawn very widespread which could indicate a generalized pain syndrome with a more uncertain aetiology of the pain. Two patients with very high VAS preoperative did not mark pain at DS at all on the pain drawing and after a telephone interview we could establish that this was due to a misunderstanding of the questionnaire. This influence of other factors contributing to pain at DS might of course also affect the results during the whole study period and is a source of systematic bias which in that case is a weakness. On the other hand, it is a randomized material and the strength is that confounding factors are minimized with this study design. The difference in preoperative pain between the BG group and the NBG group was not statistically significant, p = 0.27, which indicates that the influence of possible confounders was equal in both groups. A statistic significant improvement in EQ-5D was seen in both groups indicating a good effect by surgery for cervical disk disease on HRQoL. However, the difference in pain and discomfort caused by the harvesting of bone graft from the iliac crest cannot be detected in differences in EQ-5D, thus indicating that the effect is of limited or of no importance on HRQoL.
Fig. 3.
Preoperative pain drawing where pain at DS has been marked. Example of a patient where pain could be associated to a lumbar problem
Conclusion
Bone harvesting from anterior iliac crest seems to be associated with significant pain within the first 3 months after surgery. Minor complications are not uncommon and this should be communicated with the patients before surgery, other techniques may be discussed. Pain from donor site does not seem to affect HRQoL.
Acknowledgment
We thank Eva Gulle for invaluable help with keeping track of all the pain drawings and data.
Conflict of interest
Corporate research grants were received from DePuy Spine in support of this work.
References
- 1.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50:510–516. doi: 10.1097/00006123-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Hill NM, Horne JG, Devane PA. Donor site morbidity in the iliac crest bone graft. Aust N Z J Surg. 1999;69:726–728. doi: 10.1046/j.1440-1622.1999.01674.x. [DOI] [PubMed] [Google Scholar]
- 4.Sasso RC, LeHuec JC, Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl):S77–S81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 5.Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin Orthop Relat Res. 1994;309:208–213. [PubMed] [Google Scholar]
- 6.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 7.Porchet F, Jaques B. Unusual complications at iliac crest bone graft donor site: experience with two cases. Neurosurgery. 1996;39:856–859. doi: 10.1097/00006123-199610000-00043. [DOI] [PubMed] [Google Scholar]
- 8.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine (Phila Pa 1976) 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Grauer JN, Beiner JM, Kwon BK, Vaccaro AR. Bone graft alternatives for spinal fusion. BioDrugs. 2003;17:391–394. doi: 10.2165/00063030-200317060-00002. [DOI] [PubMed] [Google Scholar]
- 10.Savolainen S, Usenius JP, Hernesniemi J. Iliac crest versus artificial bone grafts in 250 cervical fusions. Acta Neurochir (Wien) 1994;129:54–57. doi: 10.1007/BF01400873. [DOI] [PubMed] [Google Scholar]
- 11.Heneghan HM, McCabe JP. Use of autologous bone graft in anterior cervical decompression: morbidity & quality of life analysis. BMC Musculoskelet Disord. 2009;10:158. doi: 10.1186/1471-2474-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9:398–403. doi: 10.1007/s005860000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lind BI, Zoega B, Rosen H. Autograft versus interbody fusion cage without plate fixation in the cervical spine: a randomized clinical study using radiostereometry. Eur Spine J. 2007;16:1251–1256. doi: 10.1007/s00586-007-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thalgott JS, Fritts K, Giuffre JM, Timlin M. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine (Phila Pa 1976) 1999;24:1295–1299. doi: 10.1097/00007632-199907010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine (Phila Pa 1976) 2003;28:1219–1224. doi: 10.1097/01.BRS.0000065486.22141.CA. [DOI] [PubMed] [Google Scholar]
- 16.Bertilson B, Grunnesjo M, Johansson SE, Strender LE. Pain drawing in the assessment of neurogenic pain and dysfunction in the neck/shoulder region: inter-examiner reliability and concordance with clinical examination. Pain Med. 2007;8:134–146. doi: 10.1111/j.1526-4637.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohnmeiss DD, Vanharanta H, Ekholm J. Relationship of pain drawings to invasive tests assessing intervertebral disc pathology. Eur Spine J. 1999;8:126–131. doi: 10.1007/s005860050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 19.Dorey F, Nasser S, Amstutz H. The need for confidence intervals in the presentation of orthopaedic data. J Bone Joint Surg Am. 1993;75:1844–1852. doi: 10.2106/00004623-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–638. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 22.Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 23.An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine (Phila Pa 1976) 1995;20:2211–2216. doi: 10.1097/00007632-199510001-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lofgren H, Engquist M, Hoffmann P, Sigstedt B, Vavruch L. Clinical and radiological evaluation of Trabecular Metal and the Smith-Robinson technique in anterior cervical fusion for degenerative disease: a prospective, randomized, controlled study with 2-year follow-up. Eur Spine J. 2010;19:464–473. doi: 10.1007/s00586-009-1161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanishima T, Yoshimasu N, Ogai M. A technique for prevention of donor site pain associated with harvesting iliac bone grafts. Surg Neurol. 1995;44:131–132. doi: 10.1016/0090-3019(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 26.Dusseldorp JR, Mobbs RJ. Iliac crest reconstruction to reduce donor-site morbidity: technical note. Eur Spine J. 2009;18:1386–1390. doi: 10.1007/s00586-009-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuben SS, Vieira P, Faruqi S, Verghis A, Kilaru PA, Maciolek H. Local administration of morphine for analgesia after iliac bone graft harvest. Anesthesiology. 2001;95:390–394. doi: 10.1097/00000542-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–S15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 30.Stauffer ME, Taylor SD, Watson DJ, Peloso PM, Morrison A. Definition of nonresponse to analgesic treatment of arthritic pain: an analytical literature review of the smallest detectable difference, the minimal detectable change, and the minimal clinically important difference on the pain visual analog scale. Int J Inflam. 2011;2011:231926. doi: 10.4061/2011/231926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med. 1996;3:142–146. doi: 10.1111/j.1553-2712.1996.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 32.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 33.Svensson E. Ordinal invariant measures for individual and group changes in ordered categorical data. Stat Med. 1998;17:2923–2936. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2923::AID-SIM104>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Gundes H, Kilickan L, Gurkan Y, Sarlak A, Toker K. Short- and long-term effects of regional application of morphine and bupivacaine on the iliac crest donor site. Acta Orthop Belg. 2000;66:341–344. [PubMed] [Google Scholar]
- 35.Singh K, Phillips FM, Kuo E, Campbell M. A prospective, randomized, double-blind study of the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after posterior spinal arthrodesis: a minimum of 4-year follow-up. Spine (Phila Pa 1976) 2007;32:2790–2796. doi: 10.1097/BRS.0b013e31815b7650. [DOI] [PubMed] [Google Scholar]
- 36.Coulthard P, Oliver R, Khan Afridi KA, Jackson-Leech D, Adamson L, Worthington H. The efficacy of local anaesthetic for pain after iliac bone harvesting: a randomised controlled trial. Int J Surg. 2008;6:57–63. doi: 10.1016/j.ijsu.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Morgan SJ, Jeray KJ, Saliman LH, Miller HJ, Williams AE, Tanner SL, Smith WR, Broderick JS. Continuous infusion of local anesthetic at iliac crest bone-graft sites for postoperative pain relief. A randomized, double-blind study. J Bone Joint Surg Am. 2006;88:2606–2612. doi: 10.2106/JBJS.E.00984. [DOI] [PubMed] [Google Scholar]