Abstract
Objective
To confirm the feasibility and safety of granulocyte colony-stimulating factor (G-CSF) for treating spinal neuropathic pain associated with compression myelopathy, we have initiated an open-label single-center prospective clinical trial.
Methods
Between January 2009 and February 2011, 17 patients were accrued and were divided into two groups. One group included 7 patients who complained of pain associated with worsening symptoms of myelopathy (progressing myelopathy-related pain group). The other group included 10 patients who complained of pain that persisted after surgery for compression myelopathy (post-operative persistent pain group). All patients underwent intravenous administration of G-CSF (10 μg/kg/day) for 5 consecutive days. Pain severity was evaluated using a visual analog scale (VAS) before and after G-CSF administration.
Results
In 14 of the 17 patients, pain was relieved within several days after G-CSF administration. Pain disappeared completely in 3 patients. In the progressing myelopathy-related pain group, the mean VAS score was 71.4/100 before G-CSF administration, and decreased to 35.9/100 at 1 week after G-CSF administration (p < 0.05). In the post-operative persistent pain group, the mean VAS score was 72.0/100 before G-CSF administration, and decreased to 51.7/100 at 1 week after G-CSF administration (p < 0.05). No severe adverse events occurred during or after G-CSF administration.
Conclusions
The present results provide us with the possibility that G-CSF has a pain-relieving effect for neuropathic pain in patients with compression myelopathy.
Keywords: Neuroprotective therapy, Granulocyte colony-stimulating factor, Myelopathy, Neuropathic pain, Clinical trial
Introduction
Granulocyte colony-stimulating factor (G-CSF) is a cytokine that promotes survival, proliferation, and differentiation of cells in the neutrophil lineage [11, 16]. Furthermore, G-CSF can mobilize both immature and mature bone marrow cells into the peripheral blood. As a result, it is used clinically for patients with leukocytopenia and for donors of peripheral blood-derived hematopoietic stem cells for transplantation. Recent studies have indicated that G-CSF also has non-hematopoietic activity and can potentially be used for the treatment of neuronal injury, including stroke and neurodegenerative diseases [3, 5, 7, 18, 19]. We previously demonstrated that G-CSF promoted the restoration of damaged spinal cord tissue and the recovery of neural function in experimental spinal cord injury in both mice and rats [4, 6, 12]. In addition, we showed that G-CSF promoted the migration of bone marrow-derived cells into the damaged spinal cord, suppressed apoptosis of neuronal cells and oligodendrocytes, protected myelin, decreased inflammation, and promoted angiogenesis [4, 6, 12]. Based on these findings, we initiated a clinical trial that evaluated the safety and efficacy of neuroprotective therapy using G-CSF for patients with worsening symptoms of compression myelopathy [17]. In this clinical trial, we intravenously administered G-CSF (5 or 10 μg/kg/day) to 17 patients for 5 consecutive days. G-CSF administration suppressed progression of myelopathy in all patients, and no serious adverse events occurred during or after treatment [17].
During this trial, several cases unexpectedly experienced a dramatic reduction in neuropathic pain associated with thoracic myelopathy after G-CSF administration [22]. Such a pain-relieving effect of G-CSF was not specified as an endpoint of this trial. However, this effect has important implications for future clinical use of G-CSF for compression myelopathy. Thus, we initiated a new clinical trial to verify the feasibility and safety of using G-CSF for spinal neuropathic pain. In the present study, G-CSF was administered to patients who complained of pain associated with compression myelopathy, and the pain-relieving effect of G-CSF for spinal neuropathic pain was analyzed.
Materials and methods
We performed a phase I and IIa clinical trial evaluating G-CSF administration in patients who complained of neuropathic pain associated with compression myelopathy. The trial was initiated following the approval of the Institutional Review Board of our university. According to the inclusion criteria, patients of 20–85 years of age were recruited. Patients in the following categories were excluded: (1) those with intracranial pathologies (e.g., tumors, infection, or ischemia), (2) those with a history of major bleeding requiring blood transfusion or a history of leukopenia, thrombocytopenia, hepatic or renal dysfunction, severe heart failure, or splenomegaly, and (3) those with evidence of malignant disease within the last 5 years. We also excluded patients who were pregnant or nursing. Eligible patients gave informed consent for participation in the trial.
Granulocyte colony-stimulating factor (10 μg/kg/day) was intravenously administered for 5 consecutive days. This was an open-label study; thus, there was no control group. Spinal neuropathic pain of patients analyzed in the present clinical trial was classified into two categories: at-level pain and below-level pain [1]. At-level pain is characterized as pain located within two or three spinal segments below the neurological level of a spinal cord lesion. In contrast, below-level pain presents diffusely caudal to the level of a spinal cord lesion. The severity of pain was evaluated before and after G-CSF administration using a visual analogue scale (VAS) ranging from 0 to 100. We also evaluated severity of myelopathy using the Japanese Orthopaedic Association (JOA) score (cervical myelopathy scores range from 0 to 17, thoracic myelopathy scores range from 0 to 11) [9]. In the present study, two orthopedic spine surgeons specializing in cervical and thoracic spine surgery evaluated neurological status independently every month until 6 months after G-CSF administration, and calculated the mean data. Hematological data from treated patients were analyzed. Adverse events using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, version 3.0 were also evaluated.
Statistical analysis was performed using a Mann–Whitney U test. A p value less than 0.05 was considered statistically significant. Results are presented as mean ± SD.
Results
Patient data
Between January, 2009 and February, 2011, a total of 18 patients were enrolled in this trial. In one patient, however, fever developed 3 days after the initiation of G-CSF administration, and the administration was discontinued. This patient was excluded from the study. Thus, 17 patients received G-CSF administration and were followed-up for ≥6 months (Tables 1, 2). These 17 patients were divided into two groups. One group included 7 patients (Cases 1–7) who complained of pain associated with worsening symptoms of myelopathy (progressing myelopathy-related pain group) (Table 1). The other group included 10 patients (Cases 8–17) who complained of pain that persisted after surgery for compression myelopathy (post-operative persistent pain group) (Table 2).
Table 1.
Patient data (progressing myelopathy-related pain group)
| Case no. | Age (years)/gender | Diagnosis | Most stenotic level | Surgical procedurea | Time of surgery after G-CSF administration (weeks) |
|---|---|---|---|---|---|
| 1 | 46/M | OPLL | T6–7 | PDF (T2–11) | 8 |
| 2 | 75/M | OPLL | C4–5 | PDF (C2–7) | 7 |
| 3 | 64/M | OPLL | C4–5 | PDF (C2–7) | 2 |
| 4 | 32/M | OPLL | T9–10 | PDF (T7–12) | 4 |
| 5 | 67/M | OLF | T11–12 | PD (T10–12) | 5 |
| 6 | 36/M | OPLL | T5–6 | PDF (T2–10) | 7 |
| 7 | 72/F | OPLL | T11–12 | PDF (T6–L3) | 23 |
OPLL ossification of posterior longitudinal ligament, OLF ossification of ligamentum flavum, PDF posterior decompression with instrumented fusion, PD posterior decompression
aSurgery after G-CSF administration
Table 2.
Patient data (post-operative persistent pain group)
| Case no. | Age (years)/gender | Diagnosis | Most stenotic level | Procedure of previous surgerya | Time of previous surgery before G-CSF administration (years) |
|---|---|---|---|---|---|
| 8 | 58/M | OPLL | C5–6 | PD (C3–6) | 2 |
| 9 | 72/M | DH | T12–L1 | PDF (T9–L3) | 0.5 |
| 10 | 71/M | OPLL | C5–6 | PD (C3–7) | 3 |
| 11 | 78/M | OLF | T10–11 | PDF (T10–12) | 189 |
| 12 | 70/M | OPLL | C4–5 | PD (C3–5) | 30 |
| 13 | 70/F | OPLL | C5–6 | PDF (C2–7) | 1 |
| 14 | 81/F | OLF | T10–11 | PD (T9–11) | 5 |
| 15 | 69/M | CSM | C4–5 | PDF (C4–5) | 4 |
| 16 | 62/M | CSM | C5–6 | PD (C3–7) | 10 |
| 17 | 63/M | OPLL | C5–6 | PDF (C3–7) | 8 |
DH disc herniation, CSM cervical spondylotic myelopathy
aSurgery before G-CSF administration
In the progressing myelopathy-related pain group, worsening of myelopathy occurred due to compression of the spinal cord by ossification of the posterior longitudinal ligament (OPLL) or ossification of the ligamentum flavum (OLF) (Table 1). The mean JOA score for cervical or thoracic myelopathy decreased ≥2 points or more during a recent 1-month period. Of the 7 patients in this group, 6 patients (Cases 1, 3, 4, 5, 6, and 7) complained of at-level pain and 1 patient (Case 2) complained of below-level pain (Table 3). The duration of pain was 0.2–5 years (mean, 1.9 years). In all 7 patients, surgery for myelopathy was performed 2–23 weeks after initial G-CSF administration (Table 1).
Table 3.
Neuropathic pain data
| Case no. | Type of pain | VAS before G-CSF administration | Duration of pain |
|---|---|---|---|
| 1 | At-level | 60 | 0.8 |
| 2 | Below-level | 50 | 3 |
| 3 | At-level | 50 | 0.3 |
| 4 | At-level | 80 | 4 |
| 5 | At-level | 90 | 0.2 |
| 6 | At-level | 100 | 0.2 |
| 7 | At-level | 70 | 5 |
| 8 | At-level | 50 | 1 |
| 9 | Below-level | 90 | 3 |
| 10 | At-level | 80 | 3 |
| 11 | Below-level | 60 | 19 |
| 12 | At-level | 60 | 27 |
| 13 | At-level | 60 | 1 |
| 14 | Below-level | 80 | 5 |
| 15 | Below-level | 80 | 4 |
| 16 | Below-level | 70 | 11 |
| 17 | At-level | 90 | 8 |
VAS visual analogue scale (0–100)
In the post-operative persistent pain group, pain caused by compression to the spinal cord persisted even after myelopathy surgery (Tables 2, 3). Of these 10 patients, 5 patients (Cases 8, 10, 12, 13, and 17) complained of at-level pain and 5 patients (Cases 9, 11, 14, 15, and 16) complained of below-level pain (Table 3). The duration of pain in all 10 patients in this group was 1–27 years (mean, 8.2 years), which was significantly longer than that of the progressing myelopathy-related pain group (p < 0.01).
VAS
In the progressing myelopathy-related pain group, a decrease in VAS score of >10 was obtained in all 7 patients within 1 week after initial G-CSF administration. In 1 patient (Case 4), pain completely disappeared. The mean VAS score immediately before G-CSF administration was 71.4, and it significantly decreased to 35.9 at 1 week after initial G-CSF administration (p < 0.05) (Fig. 1a). The pain-relieving effect of G-CSF was attenuated at 3 months after administration in 3 patients (Cases 2, 3, and 5), and the VAS score returned to the pre-administration level in 1 patient (Case 2). However, 3 and 6 months after G-CSF administration, mean VAS scores were still lower than those before G-CSF administration (p < 0.05) (Fig. 1a).
Fig. 1.
Visual analogue scale before and after G-CSF administration in progressing myelopathy-related pain group (a) and post-operative persistent pain group (b). VAS visual analogue scale, before immediately before G-CSF administration, 1w 1 week after initial G-CSF administration, 1 m 1 month after initial G-CSF administration, 3 m 3 months after initial G-CSF administration, 6 m 6 months after initial G-CSF administration. *p < 0.05 compared with that before G-CSF administration. †p < 0.05 compared with that 1 week after G-CSF administration. ‡p < 0.05 compared with that 1 month after G-CSF administration
Figure 2 shows the change of VAS before and after surgery in seven cases of the progressing myelopathy-related pain group. After surgery, VAS was not altered in four cases (Cases 1, 3, 4 and 6), increased in two cases (Cases 2 and 5), and decreased in one case (Case 7) (Fig. 2).
Fig. 2.
Change of visual analogue scale after G-CSF administration in each case of progressing myelopathy-related pain group. VAS visual analogue scale, before immediately before G-CSF administration, 1 w 1 week after initial G-CSF administration, 1 m 1 month after initial G-CSF administration, 3 m 3 months after initial G-CSF administration, 6 m 6 months after initial G-CSF administration. Arrowheads indicate the time of surgery
In the post-operative persistent pain group, a decrease in VAS score of ≥10 was obtained in seven out of ten patients within 10 week after initial G-CSF administration. In three patients (Cases 9, 14, and 16), G-CSF did not show any pain-relieving effect. The mean VAS score immediately before G-CSF administration was 72.0, and it significantly decreased to 51.7 at 1 week after initial G-CSF administration (p < 0.05) (Fig. 1b). The pain-relieving effect of G-CSF was attenuated at 3 months in 4 patients (Cases 11, 13, 15, and 17) and at 6 months in 1 patient (Case 12), and VAS scores returned to pre-administration levels in 4 patients (Cases 11, 13, 15, and 17). The mean VAS score increased to 64.0 at 3 months after G-CSF administration (Fig. 1b).
JOA score
In all 7 patients in the progressing myelopathy-related pain group, the JOA score increased after G-CSF administration. The mean JOA recovery rate at 1 and 6 months after G-CSF administration was 32.3 and 54.2 %, respectively (Table 4).
Table 4.
Recovery rate of JOA score after G-CSF administration
| Group | Time after G-CSF administration | |
|---|---|---|
| 1 month | 6 months | |
| Progressing myelopathy-related pain group | 32.3 ± 27.7* (0–70.6) | 54.2 ± 21.2** (28.6–81.8) |
| Post-operative persistent pain group | 7.3 ± 12.2 (0–28.6) | 7.3 ± 12.2 (0–28.6) |
Data are expressed as the mean ± standard deviation, with the range in parentheses. Recovery rate = (post-operative JOA score − preoperative JOA score/full score − preoperative JOA score) × 100 (%)
JOA score Japanese Orthopaedic Association score (cervical myelopathy 1–17 points, thoracic myelopathy 0–11 points)
* p < 0.05 compared with that of the post-operative persistent pain group
** p < 0.01 compared with that of the post-operative persistent pain group
In the post-operative persistent pain group, an increase in JOA score was observed in only 3 patients (Cases 8, 10, and 11). Three patients (Cases 9, 14, and 16), in whom no pain-relieving effect was observed after G-CSF administration, also did not show any increase in JOA score. The mean JOA recovery rate at 1 month and 6 months after G-CSF administration was 7.3 and 7.3 %, respectively (Table 4). Thus, the neurological improvement after administration of G-CSF in the post-operative persistent pain group was inferior to that in the progressing myelopathy-related pain group (Table 4).
Blood data
White blood cell count dramatically increased the day after G-CSF administration; during G-CSF administration, it increased to 31.2 ± 8.3 (×103/mm3) (Table 5). G-CSF mobilized cells of the neutrophil lineage, but lymphocytes were not affected. G-CSF also caused an increase of monocytes. C-reactive protein levels slightly increased, but this did not appear to be related to any clinical events.
Table 5.
Blood data before and after G-CSF administration
| Normal range | Before | Peak value after G-CSF administrationa | p | |
|---|---|---|---|---|
| WBC (×103/mm3) | 4.0–9.0 | 6.2 ± 2.0 (3.3–12.5) | 31.2 ± 8.3 (19.2–47.3) | <0.01 |
| Neutrophil (×103/mm3) | 1.8–5.0 | 3.6 ± 1.2 (2.0–6.6) | 25.8 ± 5.4 (16.6–34.1) | <0.01 |
| Lymphocyte (×103/mm3) | 1.0–4.1 | 2.0 ± 1.0 (0.9–5.4) | 2.1 ± 1.1 (0.7–5.9) | 0.25 |
| Monocyte (×103/mm3) | 0.1–0.6 | 0.4 ± 0.1 (0.2–0.6) | 1.2 ± 0.9 (0.3–3.4) | <0.01 |
| CRP (×103/mm3) | <0.5 | 0.1 ± 0.1 (0.0–0.4) | 0.3 ± 0.4 (0.0–1.3) | <0.01 |
WBC white blood cell, CRP C-reactive protein
aPeak value within 7 days after initiating G-CSF administration
Adverse events
In this series, no patient experienced bone pain or hepatic dysfunction after G-CSF administration. No other severe adverse events occurred during or after G-CSF administration.
Case presentation
Case 6 (progressing myelopathy-related pain group)
A 36-year-old man was admitted to our hospital complaining of progressive motor weakness of his lower extremities and gait disturbance. On admission, his JOA score for thoracic myelopathy was 3/11 points. He also showed spontaneous severe back pain (at-level pain). Magnetic resonance (MR) and computed tomography (CT) images showed beak-type OPLL and OLF that compressed his spinal cord anteriorly and posteriorly at T5–6 (Fig. 3a, b). Beginning on the day of admission, he received G-CSF. Six days after initial G-CSF administration, he felt relief of his back pain. His pain VAS score was 100 before G-CSF administration, and it decreased to 70 1 week after initial treatment. At 1 month after initial administration, his VAS score further decreased to 50. He also felt improved muscle strength of his legs, and his JOA score increased to 3.5 points. At 7 weeks after G-CSF administration, he underwent surgery for spinal cord decompression using a posterior approach and T2–T10 posterior instrumented fusion. At 6 months after G-CSF administration, he showed recovery from myelopathy (JOA score = 8 points) and his VAS score was 50.
Fig. 3.
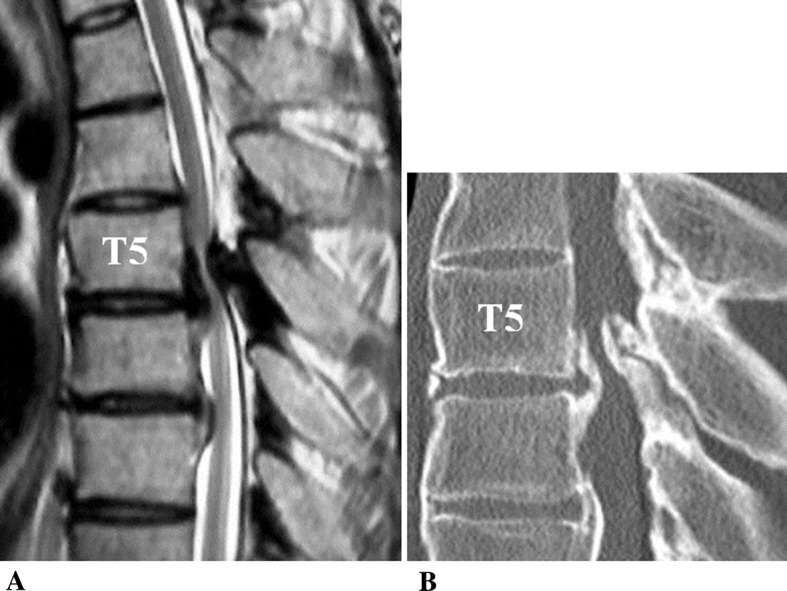
Case 6. T2-weighted midsagittal MR image (a) and CT midsagittal reconstruction plane (b) showing anterior and posterior compression of the spinal cord by beak-type OPLL and OLF at T5–T6
Case 11 (post-operative persistent pain group)
A 78-year-old man was admitted to our hospital complaining of motor weakness of his lower extremities and gait disturbance. Nineteen years prior, he had undergone T10–12 laminectomy for thoracic myelopathy due to OLF. After surgery, pain persisted in his lower extremities. On admission, his JOA score was 4/11 points. In addition to myelopathy symptoms, he complained of spontaneous severe bilateral pain at the level of his thigh and leg (below-level pain). MR images showed that his spinal cord was decompressed, but was atrophic at T10–11 (Fig. 4a, b). Beginning on the day of admission, he received G-CSF. One day after initial G-CSF administration, he felt pain relief in his bilateral thigh and leg. His VAS score for pain was 60 before G-CSF administration. At 1 week after initial administration, the VAS score was reduced to zero and his pain was diminished. His myelopathy also improved, and his JOA score increased to 6/11 points 1 month after G-CSF administration. At 3 months after administration, however, he felt recurrence of his pain and his VAS score returned to 60.
Fig. 4.

Case 11. T2-weighted MR midsagittal image (a) and axial image at T10–T11 (b) showing that the spinal cord was decompressed but was atrophic at T10–T11
Discussion
Neuropathic pain has been defined as a type of pain arising as a direct consequence of a lesion affecting parts of the somatosensory system, such as the brain, spinal cord, and peripheral nerves [1, 10, 21]. Among numerous diseases of the spinal cord, neuropathic pain following spinal cord injury (SCI) has been studied most extensively [10, 21]. Baastrup and Finnerup [1] reviewed pharmacological management of neuropathic pain following SCI. Based on the data from several randomized controlled trials, these investigators suggested that pregabalin, gabapentin, and tricyclic antidepressants (TCAs) are optimal first-line treatments for neuropathic pain associated with SCI. Furthermore, they considered that serotonin–norepinephrine reuptake inhibitors (SNRIs) are second-line choices, and that tramadol, opioids, and lamotrigine are third-line options. However, these researchers concluded that such oral pharmacological intervention is often inadequate, commonly resulting in a reduction of only 20–30 % in pain intensity. Thus, no established cure for spinal neuropathic pain currently exists.
The present study is the first to report the results of a clinical trial that evaluated the therapeutic effect of G-CSF on neuropathic pain associated with compression myelopathy. G-CSF was administered to two distinct groups of spinal neuropathic pain patients: the progressing myelopathy-related pain group and the post-operative persistent pain group. In the 7 patients in the progressing myelopathy-related pain group, G-CSF administration reduced neuropathic pain within several days in all patients. The mean VAS score was 71.4/100 before G-CSF administration, and it significantly decreased to 35.9/100 at 1 week after administration, indicating that the severity of pain decreased to 50 % of the pre-administration level. In all 7 patients, surgery for compression myelopathy was performed ≥2 weeks after initial G-CSF administration. Thus, the pain-relieving effect of G-CSF occurred prior to surgery. We suggest that the pain reduction observed within 1 week of administration in these seven cases was caused by the pharmacological effect of G-CSF and not by surgery.
To the best of our knowledge, no report has fully determined the effect of surgery itself on spinal neuropathic pain associated with compression myelopathy. In the present study, all seven patients in the progressing myelopathy-related pain group underwent surgery ≥2 weeks after the initial G-CSF administration. At the time of surgery, pain reduction had already been achieved in all the patients receiving G-CSF. After surgery, further decreases of pain were not obtained. These findings suggest the possibility that surgery itself does not have pain-relieving effects exceeding G-CSF. However, there are several limitations to this hypothesis. In this study, surgeries were performed a rather long time after myelopathy worsening. In addition, the number of patients analyzed in the present study was too small for definitive conclusions. Further studies with a larger number of patients will be required. We will determine the effect of much earlier times of surgery on the reduction of neuropathic pain due to compression myelopathy.
In the present study, we employed only one pain measure, VAS, to evaluate the severity of pain before and after the G-CSF administration. A number of pain measures have been reported for evaluating the intensity and quality of pain in patients with spinal neuropathic pain [2, 8, 14, 20]. Previous clinical trials analyzing the effect of amitriptyline [2, 14], gabapentin [8, 14], and pregabalin [20] on neuropathic pain associated with SCI employed multiple pain measures in addition to VAS, such as the McGill Pain Questionnaire (MPQ) and the Center for Epidemiologic Studies Depression Scale (CESD). They combined several pain measures based on the characteristics of each tool, and adequately evaluated the efficacy of the drugs for neuropathic pain. Since our present study was a phase I and IIa clinical trial, we utilized only VAS. In the subsequent phase IIb clinical trial of G-CSF neuroprotective therapy, we are planning to employ multiple pain measures in addition to VAS to evaluate the details of the effect of G-CSF on spinal neuropathic pain.
In the 10 patients in the post-operative persistent pain group, G-CSF administration did not have a pain-relieving effect in 3 patients. Since no improvement of myelopathy was observed in these 3 patients, we speculate that the pain-relieving and neuroprotective effects with respect to improvement of motor and sensory deficits of G-CSF are correlated. However, a pain-relieving effect was observed in the other 7 patients within 1 week after initial G-CSF administration. The mean VAS score of all 10 patients in the post-operative persistent pain group was 72.0/100 prior to G-CSF administration, and it significantly decreased to 51.7/100 at 1 week after administration. This indicates that the severity of pain decreased to 72 % of the pre-administration level. Based on this finding, we suggest that G-CSF may have a certain pain-relieving effect in patients who complain of post-operative persistent pain, although this effect is not as pronounced as that for patients with worsening symptoms of compression myelopathy.
Of the 17 patients analyzed in the present study, a pain-relieving effect associated with G-CSF was detected in 14 patients. However, recurrence of pain occurred in 8 out of these 14 patients during the follow-up period. Notably, pain returned to pre-administration levels in 5 patients. The recurrence of pain was detected at 3 months after G-CSF administration in 7 patients and at 6 months after G-CSF administration in 1 patient. This finding suggests that the pain-relieving effect by G-CSF only lasts for at most 3–4 months. Therefore, when the clinical utility of G-CSF for spinal neuropathic pain is evaluated in the future, administration every 3–4 months should be considered.
Previous studies reported the presence of placebo effects in patients suffering from neuropathic pain, although the duration of the placebo effect was not fully established [13]. In the present study, the pain-relieving effect of G-CSF continued for 3–4 months. Because the study design was open label, we cannot deny the contribution of the placebo effect of injection for reducing the spinal neuropathic pain. To verify the pharmaceutical pain-relieving effect of G-CSF on spinal neuropathic pain, a subsequent clinical trial with double-blind placebo-controlled study design will be necessary.
To the best of our knowledge, no reports of experimental studies of G-CSF administration in an animal model of spinal neuropathic pain have been published. In our studies using animal models of compression-induced and contusive SCI, intravenously administered G-CSF resulted in functional recovery by (1) promoting the migration of bone marrow-derived cells into the damaged spinal cord, (2) directly suppressing the neural apoptosis that occurs via G-CSF receptors at the injured spinal cord, and (3) decreasing the expression of inflammatory cytokines such as IL-1β and TNF-α [4, 6, 12]. Ro et al. [15] administered G-CSF to animal models of peripheral neuropathic pain, and demonstrated that G-CSF increased the number of opioid-contained polymorphonuclear cells and relieved neuropathic pain. We suggest that such mechanisms may participate in the pain-relieving effect of G-CSF on spinal neuropathic pain, although further studies are required to fully clarify all of the underlying mechanisms.
To date, no effective therapies for spinal neuropathic pain have been established. To the best of our knowledge, this is the first report showing the possibility of a therapeutic effect of G-CSF on neuropathic pain associated with compression myelopathy. The biggest limitation of the present study was that this was an open-label study, so no comparison with a control group was performed. We cannot deny the possibility that a placebo effect of injection and surgical intervention contributed to pain relief. Based on the experience of the present findings, however, we intend to advance to a further clinical trial to verify the feasibility of using G-CSF for relief of spinal neuropathic pain. This will be a multi-center, double-blind, controlled clinical trial; the control group will receive placebo injection. If the efficacy and safety of G-CSF treatment for spinal neuropathic pain is confirmed and clinical use of G-CSF therapy is approved, a novel and effective approach for the treatment of this disorder will be available.
Acknowledgments
This work was supported by a Health Labour Science Research Grant of Japan.
Conflict of interest
No funds were received in support of this study.
References
- 1.Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas DD, Warms CA, Turner JA, et al. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–373. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 3.Gibson CL, Jones NC, Prior MJ, et al. G-CSF suppresses edema formation and reduces interleukin-1β expression after cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- 4.Kawabe J, Koda M, Hashimoto M, et al. Granulocyte colony-stimulating factor (G-CSF) exerts neuroprotective effects via promoting angiogenesis after spinal cord injury in rats. J Neurosurg Spine. 2011;15:414–421. doi: 10.3171/2011.5.SPINE10421. [DOI] [PubMed] [Google Scholar]
- 5.Kawada H, Takizawa S, Takanashi T, et al. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 6.Koda M, Nishio Y, Kamada T, et al. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223–231. doi: 10.1016/j.brainres.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 7.Komine-Kobayashi M, Zhang N, et al. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26:402–413. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 8.Levendoglu F, Ogün CO, Ozerbil O, et al. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743–751. doi: 10.1097/01.BRS.0000112068.16108.3A. [DOI] [PubMed] [Google Scholar]
- 9.Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7–13. doi: 10.1097/01.bsd.0000211260.28497.35. [DOI] [PubMed] [Google Scholar]
- 10.New PW, Lim TC, Hill ST, et al. A survey of pain during rehabilitation after acute spinal cord injury. Spinal Cord. 2007;35:658–663. doi: 10.1038/sj.sc.3100472. [DOI] [PubMed] [Google Scholar]
- 11.Nicola NA, Metcalf D, Matsumoto M, et al. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983;258:9017–9023. [PubMed] [Google Scholar]
- 12.Nishio Y, Koda M, Kamada T, et al. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2007;66:724–731. doi: 10.1097/nen.0b013e3181257176. [DOI] [PubMed] [Google Scholar]
- 13.Petersen GL, Finnerup NB, Nørskov KN, et al. Placebo manipulations reduce hyperalgesia in neuropathic pain. Pain. 2012;153:1292–1300. doi: 10.1016/j.pain.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Rintala DH, Holmes SA, Courtade D, et al. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1547–1560. doi: 10.1016/j.apmr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Ro LS, Chen SR, Chao PK, et al. The potential application of granulocyte colony stimulating factor therapy on neuropathic pain. Chang Gung Med J. 2009;32:235–246. [PubMed] [Google Scholar]
- 16.Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma T, Yamazaki M, Okawa A, et al. Neuroprotective therapy using granulocyte-colony stimulating factor for patients with worsening symptoms of compression myelopathy, part 1: a phase I and IIa clinical trial. Eur Spine J. 2011;21:482–489. doi: 10.1007/s00586-011-2020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schäbitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 19.Schneider A, Kuhn HG, Schäbitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 20.Siddall PJ, Cousins MJ, Otte A, et al. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–1800. doi: 10.1212/01.wnl.0000244422.45278.ff. [DOI] [PubMed] [Google Scholar]
- 21.Störmer S, Gerner HJ, Grüninger W, et al. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 2007;35:446–455. doi: 10.1038/sj.sc.3100411. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki M, Sakuma T, Kato K et al (2012) Granulocyte colony-stimulating factor reduced neuropathic pain associated with thoracic compression myelopathy: report of 2 cases. J Spinal Cord Med (in press) [DOI] [PMC free article] [PubMed]




