Abstract
Purpose
Hypothesis that loss of integrity of the membranes in the craniocervical junction might be the cause of neck pain in patients with whiplash-associated disorders (WADs) has been proposed. In recent years, with development of more detailed magnetic resonance imaging (MRI) techniques, morphologic changes of the ligaments and membranes in the craniocervical junction, especially alar and transverse ligaments have been discussed. A meta-analysis was performed to evaluate the relationship of MRI signal changes of alar and transverse ligaments and WADs.
Methods
A systematic search of EMBASE, PUBMED, and Cochrane Library and references from eligible articles were conducted. Comparative studies reporting on evaluating the relationship between MRI high-signal changes of alar and transverse ligaments and WADs were regarded eligible. A pooled estimate of effect size was produced.
Results
Alar ligaments: Six studies (total n = 622) were included. MRI signal changes of alar ligaments did not appear to be related with WADs (P = 0.20, OR = 1.54, 95 % CI = 0.80–2.94). Heterogeneity was present (I2 = 46 %, P = 0.10), which was eliminated upon sensitivity analysis bringing the OR to 1.27 (95 % CI = 0.87–1.86, I2 = 0 %). Transverse ligaments: Four studies (total n = 489) were included. MRI signal changes of transverse ligament did not appear to be related with WADs (P = 0.51, OR = 1.44, 95 % CI = 0.49–4.21). Heterogeneity was present (I2 = 77 %, P = 0.005), which was eliminated upon sensitivity analysis bringing the OR to 0.79 (95 % CI = 0.49–1.28, I2 = 0 %).
Conclusion
MRI signal changes of alar and transverse ligaments are not supposed to be caused by whiplash injury, and MRI examination of alar and transverse ligaments should not be used as the routine workup of patients with WADs.
Keywords: Magnetic resonance imaging, Alar ligaments, Transverse ligament, Craniocervical junction, Whiplash-associated disorders
Introduction
Whiplash-associated disorders (WADs), which describe a variety of clinical manifestations resulting from whiplash injury, have been extensively reported in patients exposed to high-speed neck trauma, especially vehicle collision [1–4]. But the pathogenesis of whiplash complaints is still poorly understood. Injury to longitudinal ligaments [5–7], facet joints [7, 8], discs [7], spinal cord [9], or muscles [10, 11] has been studied as possible sources of chronic pain. However, no detectable findings are significantly different from asymptomatic subjects, and there is no known association between structure damage and symptoms.
At the craniocervical junction, the alar and transverse ligaments provide much stability of the healthy spine. In 2008, a patient with persistent WADs was reported to have alar and transverse ligaments injuries which were supported by patient’s history, examination findings, and proton density-weighted MRI [12]. Biomechanical and postmortem studies have also shown that the alar and transverse ligaments can be injured during neck trauma [13–16]. So the hypothesis that loss of integrity of the membranes in the craniocervical junction might be the cause of neck pain in patients with WADs has been proposed. These structures could be well visualized by magnetic resonance imaging (MRI) [17–19]. In recent years, with the development of more detailed MRI techniques morphologic changes of the ligaments and membranes in the craniocervical junction, especially alar and transverse ligaments have been discussed [18, 20–27]. However, most of these studies include relatively small samples of patients and thus their results were inconsistent and lacked statistical strength. The aim of this meta-analysis was therefore to assess whether MRI signal alterations of alar and transverse ligaments could be responsible for complaints of patients with WADs.
Materials and methods
Search strategy and selection of studies
We carried out a literature search using PUBMED, EMBASE, and Cochrane Library to identify all comparative studies and conference abstracts that evaluated the relationship between MRI high-signal changes of alar and transverse ligaments and WADs. No restrictions were placed on the origin or language of the publications. Both acute and chronic WADs were included in our study. Search filters used were: (1) alar and transverse ligaments (alar ligaments, transverse ligaments, craniovertebral/craniocervical/atlanto-occipital junction, cervical spine/vertebrae, neck); (2) WADs (whiplash injury, whiplash-associated disorder); (3) magnetic resonance imaging. These three concepts were separated by the boolean “AND” (details of search terms are provided in Appendix 1). The reference lists of key studies were manually examined to find additional relevant studies. We also performed a literature search for each author of the included studies, to identify further studies on the same topic.
While it is clearly preferable to include only randomized controlled trials in meta-analyses, the paucity of such studies precluded the use of this standard. We screened all studies and selected articles that satisfied the following inclusion criteria: (1) comparative study (trials, cohorts, case–controls); (2) the population study consisted of adults with whiplash injury; (3) the study reported MRI signal changes in normal and whiplash-injured patients. The following were excluded: (1) letters, editorial, case reports, guidelines and reviews; (2) animal or biomechanical studies; (3) studies containing previously published data; (4) studies containing participants who had undergone a previous cervical spine surgery or severe head injury. Two reviewers independently assessed each of the studies for eligibility for inclusion. If the title or the abstract was judged by either reviewer to be potentially eligible, the full article would be examined. All disagreements were resolved by consensus. This meta-analysis was performed in accordance with Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [28].
Data extraction and study quality
From each eligible article, the two reviewers extracted all pertinent information regarding participants, examination, and outcome. Participants’ data included age, gender (the rate of males in all participants), WADs grade according to Québec Task Force [29], mean duration between injury and MRI examination, and image evaluation criteria of ligaments. Examination data were MRI scan protocol. Outcome date comprised the number of ligaments with high-signal changes in both WADs and control groups.
Newcastle–Ottawa scale was used for the assessment of the methodological quality of each study. This assessment was made by the two reviewers who were blinded regarding the source institution, the journal, and the authors for each included publication. Any disagreement was resolved by discussion between the two reviewers.
Data analysis
All analyses were performed on STATA version 11.0 (Stata/MP, College Station, TX). Differences observed between the two groups were expressed as the odds ratio (OR) with its 95 % confidence interval (CI). Forest plots were used to graphically present the results of individual studies and the respective pooled estimate of effect size.
The impact of heterogeneity on the pooled estimates of the individual outcomes of the meta-analysis was assessed with the Cochran Q statistic and I2 test. As the Cochran Q test has a low sensitivity for detecting heterogeneity, a P value of <0.1 was considered significant for the presence of statistical heterogeneity [30]. An I2 value more than 50 % was considered as evidence of high heterogeneity, between 50 and 25 % as moderate heterogeneity, and less than 25 % as low heterogeneity. In the low heterogeneity, only the OR by the fixed-effect model is given in the results. In case of lower P or higher I2 value indicating significant heterogeneity, a DerSimonian and Laird random-effect model was used for the analysis.
A priori potential sources of heterogeneity were identified as mean duration between injury and MR examination (<2 weeks vs. >6 months), grade of WADs patients, image evaluation criteria of ligaments, and MRI scan protocol. If heterogeneity was found, we assessed differences by random-effects meta-regression using the above-mentioned variables. Subgroup analyses were performed if the source of heterogeneity was determined. If the source of heterogeneity was not identified by meta-regression, we performed sensitivity analysis by eliminating one study at a time checking for resolution of heterogeneity.
We checked for the presence of publication bias with the Begg’s test and funnel plot [31]. A P value of <0.05 was considered as evidence of significant publication bias.
Results
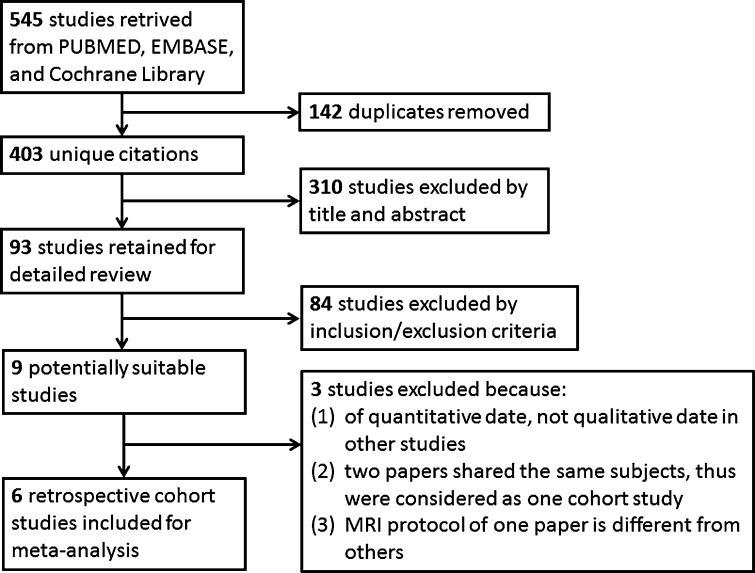
The search revealed 545 citations, but only 9 were selected for further text review to determine eligibility for the meta-analysis. One study was further excluded after detailed screening of full text articles because the comparison was performed in quantitative date of signal alterations of the transverse ligament [32], not qualitative date in other studies. Another two papers by the same authors, evaluating alar and transverse ligaments, respectively, shared the same subjects, thus were combined and considered as one study [20, 23]. Because MRI scan protocol and evaluation criteria for ligament changes used in the paper by Lindgren et al. [24] (1.5 T dynamic kine MRI and two-point grading scale and movement) was completely different from other six papers (1.5 T fast spin-echo proton density-weighted sequences and four-point grading scale), this paper was excluded in order to minimize clinical bias. In total, data from six retrospective case–control studies involving 622 patients were included in our analyses [20, 22, 23, 25–27, 33] (Fig. 1). The characteristics of the included studies were shown in Table 1 and the scores of the study quality were shown in Table 2. Patients were classified according to Québec Task Force WAD Grade [29]. Ligaments were considered to be injured with grade 2 or 3, according to a four-point grading scale based on maximal cross-section involvement in proton density-weighted images (0, low signal throughout the entire cross-section area of the alar ligaments; 1, high-signal in less than one-third of the cross-section; 2, high-signal in one-third–two-third of the cross-section; 3, high-signal in more than two-third of the cross-section) [20, 22, 23, 25, 26, 33], with grade 3 or 4 according to another four-point grading scale based on unilateral thinning or interruption of the ligament (1, clearly normal; 2, probably normal; 3, probably abnormal; 4, clearly abnormal) [27].
Fig. 1.
Outline of the literature search and selection
Table 1.
Characteristics of the included studies
| Study | Patient no. | Mean age (years) | Male (%) | WADs grade* | Mean duration between injury and MRI examination | Evaluation criteria for ligament changes | MRI scan protocol |
|---|---|---|---|---|---|---|---|
| Dullerud | 55 | 37 | 45.5 | NA | >6 months | Four-point grading scale | 1.5 T fast spin-echo proton density-weighted sequences |
| Knackstedt | 40 | 41.9 | 22.0 | NA | >6 months | Four-point grading scale | Fast spin-echo T2 and proton density-weighted sequences |
| Krakenes | 122 | 41.5 | 36.1 | II | >6 months | Four-point grading scale | 1.5 T fast spin-echo proton density-weighted sequences |
| Myran | 116 | 37.9 | 45.7 | I–II | >6 months | Four-point grading scale | 1.5 T fast spin-echo proton density-weighted sequences |
| Vetti | 271 | 38.9 | 48.3 | I–II | <2 weeks | Four-point grading scale | 1.5 T fast spin-echo proton density-weighted sequences |
| Wilmink | 18 | 38.3 | 50 | NA | >6 months | Another four-point grading scale | 0.5 T fast spin-echo proton density/T2 weighted sequences |
WADs whiplash-associated disorders, MRI magnetic resonance imaging, NA not applicable
* Classified according to Québec Task Force WAD grade
Table 2.
Methodologic quality of the included studies (Newcastle–Ottawa Scale)
| Study | Selection | Comparability | Exposure |
|---|---|---|---|
| Dullerud | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Knackstedt | ☆☆☆ | ☆ | ☆☆☆ |
| Krakenes | ☆☆☆☆ | ☆☆ | ☆☆ |
| Myran | ☆☆☆☆ | ☆☆ | ☆☆ |
| Vetti | ☆☆☆☆ | ☆☆ | ☆☆☆ |
| Wilmink | ☆☆☆ | ☆☆ | ☆☆ |
Alar ligaments
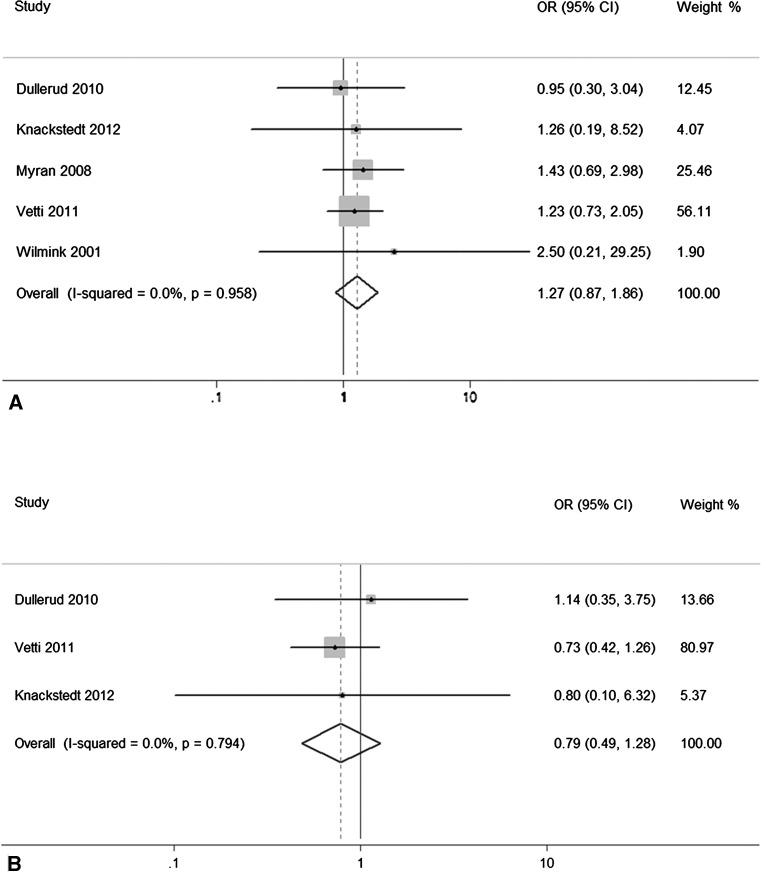
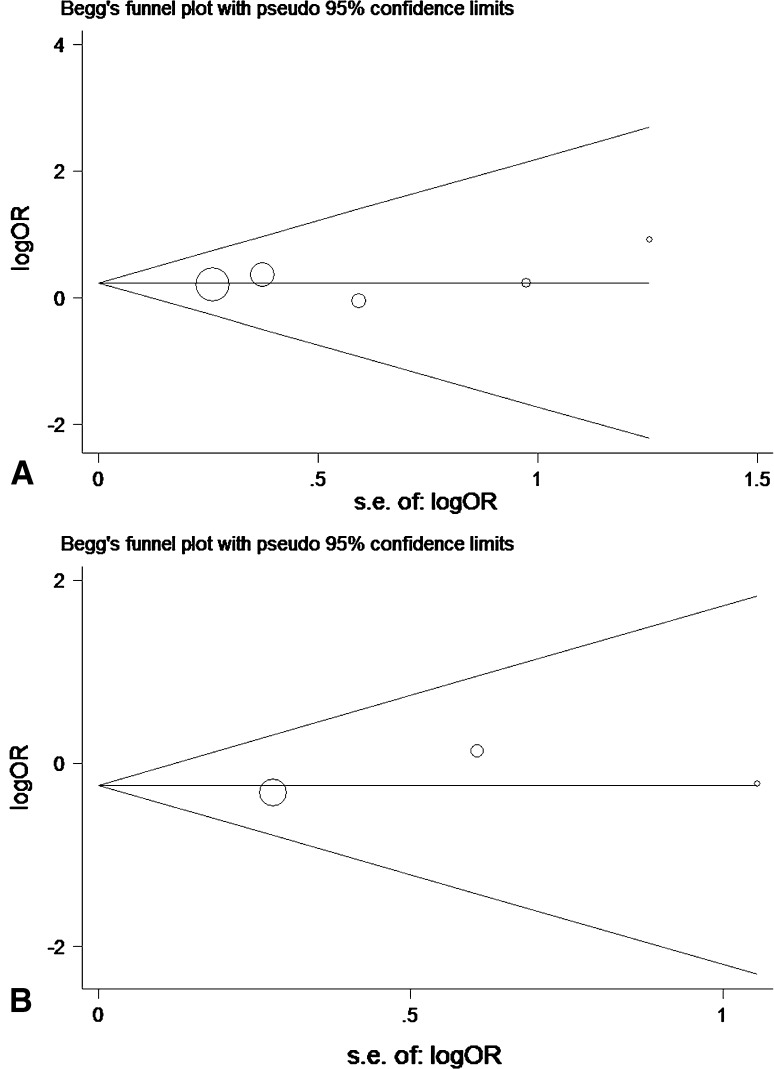
Six studies (total n = 622) were included in the analysis related to alar ligaments evaluation. The pooled estimate of effect size demonstrated that MRI signal changes of alar ligaments were not associated with WADs (P = 0.20, OR = 1.54, 95 % CI = 0.80–2.94). Moderate statistical heterogeneity was detected (I2 = 46 %, P = 0.10). Meta-regression with the a priori variables demonstrated none of them to be significantly associated with the outcome. Upon sensitivity analysis, removal of the study by Krakenes and co-workers eliminated heterogeneity (I2 = 0 %, P = 0.96), and no statistically significant difference was documented between WADs and control groups with regard to alar ligaments injury (P = 0.22, OR = 1.27, 95 % CI = 0.87–1.86) (Fig. 2a). We found no evidence of publication bias by means of Begg’s test (P = 0.64) and visual inspection of the Begg plot (Fig. 3a).
Fig. 2.
Forest plot with risk ratios and 95 % CI of the meta-analysis evaluating: a alar ligaments, excluding the study by Krakenes and coworkers; b transverse ligaments, excluding the study by Krakenes and coworkers
Fig. 3.
Begg’s funnel plot with 95 % CI for publication bias of: a alar ligaments, and b transverse ligaments
Transverse ligament
Four studies (total n = 489) were included in the analysis related to transverse ligament evaluation. The pooled estimate of effect size demonstrated that MRI signal changes of transverse ligament were not associated with WADs (P = 0.51, OR = 1.44, 95 % CI = 0.49–4.21). High heterogeneity was detected (I2 = 77 %, P = 0.005). Meta-regression with the a priori variables demonstrated none of them to be significantly associated with the outcome. Upon sensitivity analysis, removal of the study by Krakenes and coworkers eliminated heterogeneity (I2 = 0 %, P = 0.79) and no statistically significant difference was documented between WADs and control groups with regard to transverse ligament injury (P = 0.34, OR = 0.79, 95 % CI = 0.49–1.28) (Fig. 2b). We found no evidence of publication bias by means of Begg’s test (P = 0.58) and visual inspection of the Begg plot (Fig. 3b).
Discussion
Whether MRI signal changes of the alar or transverse ligaments could be responsible for complaints of patients having whiplash injury in the upper cervical spine was still controversial. Assumption that MRI signal changes of alar and transverse ligaments were due to a whiplash injury was not supported by our results.
MRI signal changes of alar and transverse ligaments were usually expected to reflect structure alternation. However, not only trauma but also degeneration, edema, bleeding, and inflammation could cause structure alternation of ligaments. It is well documented that degeneration of tendons and anterior cruciate ligaments could cause high-signal changes on MRI [34–36]. Although Vetti et al. [37] reported that high-signal changes were not related to age and spinal degeneration in their study, histological degeneration of ligaments that were not supposed to increase by age [38, 39] still could be a potential reason of MRI signal changes. Except for trauma, any factors that cause edema, bleeding, or inflammation of surround structure of alar or transverse ligaments could also lead to signal changes and create a disturbance on ligament evaluation between WADs patients and control group. MRI artifacts and the magnetic field strength may also influence the image quality of small structural changes in the ligaments. Using high-resolution sequences in three orthogonal planes could reduce MRI artifacts and provide detailed structural information [40]. Since above influencing factors exist, high-signal intensity changes among asymptomatic individuals were not rare [17, 41], and MRI signal changes of alar and transverse ligaments were not significantly associated with clinical test [42] and prognosis of acute whiplash injury [43].
The main limitation of this meta-analysis is the presence of statistical and clinical heterogeneity, although we did try to compensate for the statistical heterogeneity using a random-effects model and perform meta-regression and sensitivity analysis. Ideally a meta-analysis should be considered only when the individual studies are sufficiently homogeneous in terms of participants, interventions, and outcomes, so that one can reasonably expect the same magnitude of effect across the range of patients, interventions, and outcomes of the various studies. However, one can also argue that since clinical diversity always occurs in any two studies included in a meta-analysis, statistical heterogeneity is inevitable. Since the six included studies differed with respect to definition of WAD, evaluation criteria for ligament changes and MRI scan protocol, significant heterogeneity was observed in this meta-analysis. We have to admit that the retrospective case–control studies could be responsible for biased and flawed results. However, a meta-analysis of such studies might still be useful in discovering the relationship between WADs and MRI signal changes of alar and transverse ligaments.
Conclusion
Based on the results of this meta-analysis, MRI signal changes of alar and transverse ligaments are not supposed to be caused by whiplash injury, and MRI examination of alar and transverse ligaments should not be used as the routine workup of patients with WADs. The clinical relevance of these findings is unknown and causation should be evaluated in prospective designed studies.
Conflict of interest
None.
Appendix: Search terms
PUBMED
((((“Ligaments” [Mesh] OR “Ligaments, Articular” [Mesh]) OR ligament*) AND (alar OR transverse)) OR (craniovertebral OR craniocervical OR (Atlanto*Occipital) OR cervical* OR neck)) AND (MRI OR (Magnetic Resonance) OR (“Magnetic Resonance Imaging” [Mesh])) AND ((whiplash) OR (“Whiplash Injurie” [Mesh]))
EMBASE
craniovertebral OR craniocervical OR cervical* OR ‘neck’/exp OR neck OR atlanto*occipital OR ‘cervical spine’/exp OR (‘alar’/exp OR alar OR transverse AND (‘ligament’/exp OR ‘ligament’ OR ligament*)) AND (‘mri’/exp OR mri OR (magnetic AND resonance AND (‘imaging’/exp OR imaging))) AND (‘whiplash injury’/exp OR whiplash)
Cochrane library
(ligament* OR craniovertebral OR craniocervical OR (cervical spine) OR neck OR (Cervical Vertebrae) OR (Atlanto*Occipital)) AND (MRI OR (Magnetic Resonance Imaging)) AND whiplash
Contributor Information
Quan Li, Phone: +86-13-764764980, Email: quan.li@mail.com.
Hongxing Shen, Phone: +86-13-601814912, Email: shenhxgk@126.com.
Ming Li, Phone: +86-13-801681322, Email: limingch@21cn.com, Email: limingch@mail.com.
References
- 1.Crouch R, Whitewick R, Clancy M, Wright P, Thomas P. Whiplash associated disorder: incidence and natural history over the first month for patients presenting to a UK emergency department. Emerg Med J. 2006;23:114–118. doi: 10.1136/emj.2004.022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari R, Russell AS, Carroll LJ, Cassidy JD. A re-examination of the whiplash associated disorders (WAD) as a systemic illness. Ann Rheum Dis. 2005;64:1337–1342. doi: 10.1136/ard.2004.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miettinen T, Leino E, Airaksinen O, Lindgren KA. Whiplash injuries in Finland: the situation 3 years later. Eur Spine J. 2004;13:415–418. doi: 10.1007/s00586-004-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt MA, van Meeteren NL, de Wijer A, Helders PJ, Graaf Y. Functional health status in subjects after a motor vehicle accident, with emphasis on whiplash associated disorders: design of a descriptive, prospective inception cohort study. BMC Musculoskelet Disord. 2008;9:168. doi: 10.1186/1471-2474-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivancic PC, Pearson AM, Panjabi MM, Ito S. Injury of the anterior longitudinal ligament during whiplash simulation. Eur Spine J. 2004;13:61–68. doi: 10.1007/s00586-003-0590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichihara D, Okada E, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Hashimoto T, Ogawa J, Watanabe M, Takahata T, Matsumoto M. Longitudinal magnetic resonance imaging study on whiplash injury patients: minimum 10-year follow-up. J Orthop Sci: official journal of the Japanese Orthopaedic Association. 2009;14:602–610. doi: 10.1007/s00776-009-1378-z. [DOI] [PubMed] [Google Scholar]
- 7.Yoganandan N, Cusick JF, Pintar FA, Rao RD. Whiplash injury determination with conventional spine imaging and cryomicrotomy. Spine (Phila Pa 1976) 2001;26:2443–2448. doi: 10.1097/00007632-200111150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ivancic PC, Ito S, Tominaga Y, Rubin W, Coe MP, Ndu AB, Carlson EJ, Panjabi MM. Whiplash causes increased laxity of cervical capsular ligament. Clin Biomech (Bristol, Avon) 2008;23:159–165. doi: 10.1016/j.clinbiomech.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, Panjabi MM, Ivancic PC, Pearson AM. Spinal canal narrowing during simulated whiplash. Spine (Phila Pa 1976) 2004;29:1330–1339. doi: 10.1097/01.BRS.0000127186.81814.4A. [DOI] [PubMed] [Google Scholar]
- 10.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976) 2006;31:847–855. doi: 10.1097/01.brs.0000240841.07050.34. [DOI] [PubMed] [Google Scholar]
- 11.Elliott J, Jull G, Noteboom JT, Galloway G. MRI study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD) Manual therapy. 2008;13:258–265. doi: 10.1016/j.math.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Elliott JM, Cherry J. Upper cervical ligamentous disruption in a patient with persistent whiplash associated disorders. J Orthop Sports Phys Ther. 2008;38:377. doi: 10.2519/jospt.2008.0406. [DOI] [PubMed] [Google Scholar]
- 13.Dickman CA, Greene KA, Sonntag VK. Injuries involving the transverse atlantal ligament: classification and treatment guidelines based upon experience with 39 injuries. Neurosurgery. 1996;38:44–50. doi: 10.1097/00006123-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Fielding JW, Cochran GB, Lawsing JF, 3rd, Hohl M. Tears of the transverse ligament of the atlas. A clinical and biomechanical study. J Bone Joint Surg Am. 1974;56:1683–1691. [PubMed] [Google Scholar]
- 15.Saternus KS, Thrun C. Traumatology of the alar ligaments. Aktuelle Traumatologie. 1987;17:214–218. [PubMed] [Google Scholar]
- 16.Obenauer S, Herold T, Fischer U, Fadjasch G, Koebke J, Grabbe E, Saternus KS. The evaluation of experimentally induced injuries to the upper cervical spine with a digital X-ray technic, computed tomography and magnetic resonance tomography. Rofo. 1999;171:473–479. doi: 10.1055/s-1999-271. [DOI] [PubMed] [Google Scholar]
- 17.Pfirrmann CW, Binkert CA, Zanetti M, Boos N, Hodler J. MR morphology of alar ligaments and occipitoatlantoaxial joints: study in 50 asymptomatic subjects. Radiology. 2001;218:133–137. doi: 10.1148/radiology.218.1.r01ja36133. [DOI] [PubMed] [Google Scholar]
- 18.Krakenes J, Kaale BR. Magnetic resonance imaging assessment of craniovertebral ligaments and membranes after whiplash trauma. Spine. 2006;31:2820–2826. doi: 10.1097/01.brs.0000245871.15696.1f. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti PF, Fahr LM, Kuhns LR, Hayman LA. MR imaging findings in spinal ligamentous injury. AJR Am J Roentgenol. 2000;175:661–665. doi: 10.2214/ajr.175.3.1750661. [DOI] [PubMed] [Google Scholar]
- 20.Krakenes J, Kaale BR, Moen G, Nordli H, Gilhus NE, Rorvik J. MRI assessment of the alar ligaments in the late stage of whiplash injury—a study of structural abnormalities and observer agreement. Neuroradiology. 2002;44:617–624. doi: 10.1007/s00234-002-0799-6. [DOI] [PubMed] [Google Scholar]
- 21.Krakenes J, Kaale BR, Moen G, Nordli H, Gilhus NE, Rorvik J. MRI of the tectorial and posterior atlanto-occipital membranes in the late stage of whiplash injury. Neuroradiology. 2003;45:585–591. doi: 10.1007/s00234-003-1036-7. [DOI] [PubMed] [Google Scholar]
- 22.Dullerud R, Gjertsen O, Server A. Magnetic resonance imaging of ligaments and membranes in the craniocervical junction in whiplash-associated injury and in healthy control subjects. Acta Radiol (Stockholm, Sweden: 1987) 2010;51:207–212. doi: 10.3109/02841850903321617. [DOI] [PubMed] [Google Scholar]
- 23.Krakenes J, Kaale BR, Nordli H, Moen G, Rorvik J, Gilhus NE. MR analysis of the transverse ligament in the late stage of whiplash injury. Acta Radiol (Stockholm, Sweden: 1987) 2003;44:637–644. doi: 10.1080/02841850312331287739. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren KA, Kettunen JA, Paatelma M, Mikkonen RH. Dynamic kine magnetic resonance imaging in whiplash patients and in age- and sex-matched controls. Pain Res Manag. 2009;14:427–432. doi: 10.1155/2009/369612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myran R, Kvistad KA, Nygaard OP, Andresen H, Folvik M, Zwart JA. Magnetic resonance imaging assessment of the alar ligaments in whiplash injuries: a case–control study. Spine. 2008;33:2012–2016. doi: 10.1097/BRS.0b013e31817bb0bd. [DOI] [PubMed] [Google Scholar]
- 26.Vetti N, Krakenes J, Damsgaard E, Rorvik J, Gilhus NE, Espeland A. Magnetic resonance imaging of the alar and transverse ligaments in acute whiplash-associated disorders 1 and 2: a cross-sectional controlled study. Spine. 2011;36:E434–E440. doi: 10.1097/BRS.0b013e3181da21a9. [DOI] [PubMed] [Google Scholar]
- 27.Wilmink JT, Patijn J. MR imaging of alar ligament in whiplash-associated disorders: an observer study. Neuroradiology. 2001;43:859–863. doi: 10.1007/s002340100600. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining “whiplash” and its management. Spine (Phila Pa 1976) 1995;20:1S–73S. doi: 10.1097/00007632-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 32.Ulbrich EJ, Eigenheer S, Boesch C, Hodler J, Busato A, Schraner C, Anderson SE, Bonel H, Zimmermann H, Sturzenegger M. Alterations of the transverse ligament: an MRI study comparing patients with acute whiplash and matched control subjects. AJR Am J Roentgenol. 2011;197:961–967. doi: 10.2214/AJR.10.6321. [DOI] [PubMed] [Google Scholar]
- 33.Knackstedt H, Krakenes J, Bansevicius D, Russell MB. Magnetic resonance imaging of craniovertebral structures: clinical significance in cervicogenic headaches. J Headache Pain. 2012;13:39–44. doi: 10.1007/s10194-011-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjellin I, Ho CP, Cervilla V, Haghighi P, Kerr R, Vangness CT, Friedman RJ, Trudell D, Resnick D. Alterations in the supraspinatus tendon at MR imaging: correlation with histopathologic findings in cadavers. Radiology. 1991;181:837–841. doi: 10.1148/radiology.181.3.1947107. [DOI] [PubMed] [Google Scholar]
- 35.Makino A, Pascual-Garrido C, Rolon A, Isola M, Muscolo DL. Mucoid degeneration of the anterior cruciate ligament: MRI, clinical, intraoperative, and histological findings. Knee Surg Sports Traumatol Arthrosc. 2011;19:408–411. doi: 10.1007/s00167-010-1239-5. [DOI] [PubMed] [Google Scholar]
- 36.Schweitzer ME, Mitchell DG, Ehrlich SM. The patellar tendon: thickening, internal signal buckling, and other MR variants. Skeletal Radiol. 1993;22:411–416. doi: 10.1007/BF00538442. [DOI] [PubMed] [Google Scholar]
- 37.Vetti N, Krakenes J, Eide GE, Rorvik J, Gilhus NE, Espeland A. MRI of the alar and transverse ligaments in whiplash-associated disorders (WAD) grades 1–2: high-signal changes by age, gender, event and time since trauma. Neuroradiology. 2009;51:227–235. doi: 10.1007/s00234-008-0482-7. [DOI] [PubMed] [Google Scholar]
- 38.Cushner FD, La Rosa DF, Vigorita VJ, Scuderi GR, Scott WN, Insall JN. A quantitative histologic comparison: ACL degeneration in the osteoarthritic knee. J Arthroplasty. 2003;18:687–692. doi: 10.1016/S0883-5403(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 39.Mullaji AB, Marawar SV, Simha M, Jindal G. Cruciate ligaments in arthritic knees: a histologic study with radiologic correlation. J Arthroplasty. 2008;23:567–572. doi: 10.1016/j.arth.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Krakenes J, Kaale BR, Rorvik J, Gilhus NE. MRI assessment of normal ligamentous structures in the craniovertebral junction. Neuroradiology. 2001;43:1089–1097. doi: 10.1007/s002340100648. [DOI] [PubMed] [Google Scholar]
- 41.Roy S, Hol PK, Laerum LT, Tillung T. Pitfalls of magnetic resonance imaging of alar ligament. Neuroradiology. 2004;46:392–398. doi: 10.1007/s00234-004-1193-3. [DOI] [PubMed] [Google Scholar]
- 42.Kaale BR, Krakenes J, Albrektsen G, Wester K. Clinical assessment techniques for detecting ligament and membrane injuries in the upper cervical spine region—a comparison with MRI results. Man Ther. 2008;13:397–403. doi: 10.1016/j.math.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Vetti N, Krakenes J, Eide GE, Rorvik J, Gilhus NE, Espeland A. Are MRI high-signal changes of alar and transverse ligaments in acute whiplash injury related to outcome? BMC musculoskeletal disorders. 2010;11:260. doi: 10.1186/1471-2474-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]