Abstract
Somatic, also termed adult, stem cells are highly attractive biomedical cell candidates because of their extensive replication potential and functional multilineage differentiation capacity. They can be used for drug and toxicity screenings in preclinical studies, as in vitro model to study differentiation or for regenerative medicine to aid in the repair of tissues or replace tissues that are lost upon disease, injury or ageing. Multipotent adult progenitor cells (MAPCs) and mesenchymal stem cells (MSCs) are two types of adult stem cells derived from bone marrow that are currently being used clinically for tissue regeneration and for their immunomodulatory and trophic effects. This review will give an overview of the phenotypic and functional differences between human MAPCs and MSCs, with a strong emphasis on their immunological characteristics. Finally, we will discuss the clinical studies in which MSCs and MAPCs are already used.
Keywords: multipotent adult progenitor cells, mesenchymal stem cells, phenotype, immunomodulation
Comparison of human MSCs and MAPCs
Mesenchymal stem cells (MSCs) co-exist in the bone marrow (BM) with hematopoietic stem cells (HSCs) and have the potential to differentiate towards lineages of mesenchymal origin, including bone, cartilage, fat, connective tissue, smooth muscle and hematopoietic supportive stroma.1 Although originally isolated from BM, MSCs have since been isolated from many other tissues, including adipose tissue, synovial fluid, periosteum, umbilical cord blood and several fetal tissues.2, 3, 4, 5
Once established in culture, MSCs display a variety of cell surface antigens that may vary depending on the isolation and expansion methods used. They usually express CD73, CD90 and CD105 and lack expression of major histocompatibility complex (MHC) class II surface molecules and endothelial (CD31) and hematopoietic-specific antigens (CD34, CD45, CD14).1, 6 Additional criteria that are used to define MSC are their plastic adherence and their capacity to differentiate in vitro towards osteoblasts, adipocytes and chondrocytes.6
As initial MSC cultures are very heterogeneous, several groups have proposed specific cell surface antigens to prospectively isolate MSCs (for example, STRO1 (stromal precursor antigen 1), VCAM-1 (vascular cell adhesion molecule 1), SH2 (Src homology 2), SH3/SH4, CD271, GD2 (ganglioside 2), SSEA4 (stage-specific embryonic antigen-4)).7, 8, 9, 10, 11 However, it was only recently that the exact nature of human MSCs (hMSCs) in vivo was elucidated. Sachetti et al.12 found that self-renewing osteoprogenitors in human BM, able to generate bone, stroma and organize a hematopoietic microenvironment in vivo, are CD146high (a melanoma-associated cell adhesion molecule). These CD146+ cells are located in the subendothelial layer of BM sinusoids and represent adventitial reticular cells, a subpopulation of pericytes. As pericytes are found in nearly every other organ, it has been hypothesized that all MSCs found in different tissues are also derived from the pericyte fraction in vessels. Indeed, Crisan et al.13 isolated hMSCs based on a combination of pericyte-expressed antigens (CD146, NG2 (nerve/glial antigen 2) and PDGFRβ (platelet-derived growth factor receptor-β)) from multiple organs (including skeletal muscle, BM, umbilical cord blood, placenta, pancreas and white adipose tissue). Interestingly, they demonstrated that these cells, irrespective of the tissue of origin, were skeletal myogenic in vivo and ex vivo and exhibited, at the clonal level, adipo-, osteo- and chondrogenic potential in vitro and formed calcified tissue in vivo.
In 2002, the group of Catherine Verfaillie described the derivation of rare cells from rat and mouse BM with characteristics different from most adult stem cells. The cells, termed multipotent adult progenitor cells (MAPCs), could proliferate without senescence and could, at the single cell level, differentiate in vitro into cells of the three germ layers.14 When injected into the blastocyst, a single MAPC of one of the murine lines could contribute to most somatic tissues, albeit in general, the contribution was very low. Upon transplantation in a non-irradiated recipient, mouse MAPCs engrafted at low levels into the hematopoietic lineage and the epithelium from the lung, gut and liver. Rodent MAPCs were cultured at low density and in the presence of leukemia inhibitory factor, epidermal growth factor (EGF) and platelet-derived growth factor. They were significantly smaller in size than their MSC counterpart and did not express MHC class I and CD44 antigens. Like MSCs, they did not express CD45 or other mature hematopoietic markers.
Since 2003, culture conditions under which rodent MAPCs are isolated have changed, including isolation and maintenance at 5% oxygen, use of a different serum and maintenance at higher cell densities for the first 4 weeks in culture, compared with the previously described MAPCs. These studies have shown that rodent MAPCs can only be detected after 2–3 months of in vitro culture. This is associated with an abrupt change in cell morphology accompanied by the expression of the embryonic stem cell (ESC)-associated transcription factor Oct4 (but not Nanog and Sox2) and the primitive endoderm-specific genes Gata6, Gata4, Sox7 and Sox17.15, 16, 17 As such, rodent MAPCs resemble extraembryonic endoderm cells (Xen cells)18 and extraembryonic endoderm precursor cells (Xen-P cells).19 The levels of Oct4 mRNA in these newly isolated mouse MAPCs range from 1% to 10% compared with mouse ESCs and are nearly equivalent to mouse ESCs for rat MAPCs.15 Compared with the initially described MAPCs, mouse MAPCs now express high levels of c-kit and rat MAPCs express high levels of CD31. These high Oct4 MAPCs also show robust differentiation towards endothelium and hepatocyte-like cells.15 Ross et al.20 showed that MAPCs can form smooth muscle cells, which are phenotypically and functionally similar to primary mature smooth muscle cells. A four-step protocol mimicking the embryonic development of the liver was developed to allow differentiation of rodent high Oct4 MAPCs, in a similar fashion as mouse and human ESCs, towards functional hepatocytes-like cells.21 Rodent MAPCs, like MSCs, also differentiate towards adipo-, chondro- and osteocytes.15, 22 Although the originally isolated rodent MAPCs differentiated robustly towards the neuroectodermal lineage, thereby generating neuron-like cells with electrophysiological properties similar to CNS neurons,23 it is unclear whether the newer MAPC lines can be differentiated beyond the Sox2/Pax6 neural progenitor stage.15
Similarly to rodent MAPCs, human MAPCs (hMAPCs) can also be expanded long term, and several groups have shown that they can differentiate not only towards mesenchymal cell types (adipocytes, osteoblasts, chondrocytes and smooth muscle cells) but also towards endothelium (which can be specified to both venous and arterial), skeletal muscle and hepatocyte-like cells.20, 24, 25, 26, 27, 28 In contrast to rodent MAPCs, hMAPCs do not require LIF for their self-renewal and do not express significant levels of Oct4. When grafted in vivo in a model of severe limb ischemia, hMAPCs significantly increased angiogenesis and endogenous stem cell proliferation, leading to less ischemia and therefore improved skeletal muscle function.29
A recent comparative analysis between hMAPCs and hMSCs has shown that they can be considered as two distinct cell populations (Table 1).30 hMAPCs could be expanded in vitro for >70 population doublings, which was significantly longer than hMSCs (20–25 population doublings). Four surface proteins were found to be differentially expressed: alkaline phosphatase, CD140a and CD140b were not expressed on hMAPCs, whereas hMSCs expressed alkaline phosphatase and CD140a at low levels and CD140b at high levels, and finally MHC class I was highly expressed on hMSCs but at lower levels on hMAPCs.
Table 1. Comparative analysis of hMSCs and hMAPCs.
| Stem cell | hMSCs | hMAPCs |
|---|---|---|
| Isolation and culture | ||
| Source | Bone marrow | Bone marrow/bone |
| % Serum in expansion medium | 10% | 2% |
| Extra growth factors in expansion medium | / | PDGF/EGF |
| Plating density | 5000 cm−2 | 400 cm−2 |
| % Oxygen | 21% | 5% |
| Cell surface phenotype | ||
| CD34 | negative | negative |
| CD45 | negative | negative |
| c-kit | negative | negative |
| KDR | negative | negative |
| CD56 | negative | negative |
| CD271 | negative | negative |
| CD146 | low | low |
| CD44 | high | high |
| CD13 | high | high |
| CD73 | high | high |
| CD90 | high | high |
| CD105 | high | high |
| MHC class I | high | low |
| CD140a | low | negative |
| CD140b | high | negative |
| ALP | low | negative |
| Proliferation capacities | ||
| Population doublings | 20–30 | 70 |
| Differentiation potential | ||
| Adipocytes/osteoblasts/chondrocytes | yes | yes |
| Smooth muscle cells | yes | yes |
| Endothelial cells | no | yes |
Abbreviations: ALP, alkaline phosphatase; EGF, epidermal growth factor; hMAPC, human multipotent adult progenitor cell; hMSC, human mesenchymal stem cell; KDR, kinase insert domain receptor; MHC, major histocompatibility complex; PDGF, platelet-derived growth factor.
Furthermore, both cell types could differentiate into typical mesenchymal cell types, including adipocytes, osteoblasts, chrondocytes and smooth muscle cells, but only hMAPCs showed robust endothelial differentiation. Finally, a transcriptome analysis revealed hMAPCs and hMSCs as two distinct cell populations, with each expressing a set of characteristic genes that correlated with the functional ability of the cells. Whether hMSCs and hMAPCs represent truly different cell types in vivo is not known yet, but the study from Roobrouck et al.30 showed that the in vitro characteristics of the cells are, at least, partially dependent on the culture conditions.
Immunogenicity and immunomodulatory capacities of hMSCs in vitro
Adaptive immune system
MSCs interact with a wide range of immune cells and their interaction with allogeneic T cells has been studied by many groups. First, MSCs have been demonstrated to be poor stimulators of an in vitro allogeneic T-cell response and fail to induce activation of allogeneic T cells. To adequately activate T cells, two signals are required.31 The first signal involves the recognition of MHC molecules together with an antigen on the surface of an antigen-presenting cell (APC) by the T-cell receptor. Subsequently, T-cell activation requires a costimulatory signal involving interaction of CD28 on the T cell with CD80 or CD86 (B7 superfamily) on the APC. MSCs express low levels of MHC class I molecules on their surface but lack the expression of MHC class II and the costimulatory molecules CD80, CD86 or CD40. The expression of both MHC class I and class II can be upregulated upon stimulation with interferon (IFN)-γ. However, this upregulation was not sufficient to enhance the immunogenicity of MSCs.32, 33 In addition, Klyushnenkova et al.32 showed that the lack of T-cell response was not due to a deficiency in co-stimulatory signals, as retroviral transduction of MSCs with B7-1 or B7-2 did not result in T-cell proliferation. Accordingly, Tse et al.34 showed that IFN-γ pretreated MSCs, even in combination with direct costimulation via an anti-CD28 antibody could not induce a T-cell proliferative response. Aside from that, they showed that the lack of proliferation was not due to MSC-induced T-cell apoptosis.
Second, MSCs have been shown to suppress both naive and memory T lymphocyte activation and proliferation induced by alloantigens,33, 35, 36 mitogens35, 36, 37 and CD3 and CD28 antibodies.33, 38 This suppression is without any MHC restriction as it can be mediated by both autologous and allogeneic MSCs.35, 36, 39 Most studies agree that a soluble factor is involved, as MSCs are suppressive when MSCs and T lymphocytes are separated by a semi-permeable membrane.
Several candidate mediators have been postulated, but the available data are often contradictory. A role for transforming growth factor-β (TGF-β) and hepatocyte growth factor (HGF) has been suggested by Di Nicola et al.36, who found that antibodies against TGF-β and HGF partially restored proliferation of purified T cells stimulated with allogeneic peripheral blood lymphocytes. TGF-β and HGF were not involved when T cells were stimulated with mitogens37 or when peripheral blood mononuclear cells (PBMCs) were used as responder cells.34, 40 Aggarwal et al.35 demonstrated that inhibition of the prostaglandin E2 (PGE2) synthesis abrogated the suppressive effect of MSCs on T-cell proliferation. PGE2 was found to be constitutively produced by MSCs, and the production was even enhanced upon coculture of MSCs with PBMC.34 The attribution of PGE2 in the suppression by MSC was confirmed by Rasmusson et al.41 who showed that indomethacin (an inhibitor of PGE2 synthesis) could restore MSC-mediated suppression of T-cell proliferation, but only when T cells were stimulated with phytohaemagglutinin, and not in a mixed lymphocyte reaction (MLR). Another candidate mediator has been suggested by Meisel et al.39 who described a role for indoleamine 2,3-dioxygenase (IDO) in the suppression by MSCs. The expression of IDO in professional APCs catalyzes conversion of tryptophan to kynurenine and has been identified as a major immunosuppressive effector pathway that inhibits T-cell responses to autoantigens and fetal alloantigens in vivo.42 In MSCs, the expression of IDO is not constitutive but can be induced by IFN-γ. Addition of tryptophan restored T-cell proliferation suppressed by MSCs.39 However, tryptophan depletion was not responsible for the immunosuppressive effect of MSCs when unfractionated PBMCs were used as responder cells.34 Many factors contribute to all these conflicting data. The kind of responder population (unseparated mononuclear cells versus purified T cells) as well as the stimulator used might alter the mechanism of suppression by MSCs. More recently, other mediators in MSC-mediated immunosuppression have been suggested. The human leukocyte antigen (HLA)-G protein, which is a nonclassical HLA class I molecule, was found to mediate the suppressive effect of MSCs through the induction of proliferation of regulatory T cells.43 MSCs have also been reported to induce the production of interleukin (IL)-10 by plasmacytoid dendritic cells (DC), which in turn trigger the generation of regulatory T cells.44 In addition, galectins are now emerging as a main regulator of MSC immunosuppressive function.45
Di Nicola et al.36 demonstrated that the suppressive effect of hMSCs on mitogen-induced allogeneic T-cell proliferation is only transient. This is in contrast to the findings on murine MSCs, which induce a condition of anergy due to divisional arrest of T cells in the G0/G1 phase of the cell cycle.46
Studies on the effect of MSCs on B-cell function have been performed, although less frequent and leading to conflicting results. In vitro experiments showed that B-cell proliferation was inhibited by hMSCs through an arrest in the G0/G1 phase of the cell cycle. In addition, hMSCs inhibited B-cell differentiation because immunoglobulin M (IgM), IgG and IgA production was significantly impaired, as well as chemotaxis.47 By contrast, however, others have reported stimulatory effects on in vitro activated B cells or plasma cells from healthy donors or patients with systemic lupus erythematosus.48
Innate immune system
Natural killer (NK) cells are important effector cells of innate immunity. These cells display spontaneous cytolytic activity against cells that lack the expression of MHC class I molecules. Moreover, NK cells are also important graft-versus-leukemia mediators.49 The function of NK cells is regulated by the balance of the interaction between activating and inhibiting signals with their cell surface receptors. MSCs are known to express low levels of MHC class I molecules, which makes them vulnerable for NK cell-mediated killing.50 Nevertheless, MSCs are not lysed by freshly isolated resting NK cells, even despite killer-cell Ig-like receptor mismatch between MSC and NK cell donors. On the other hand, both autologous and allogeneic MSCs can be successfully killed by activated NK cells.51, 52 This indicates that interactions between MHC class I-specific inhibitory receptors on NK cells and MSCs are not sufficient to protect MSCs from lysis. It is also known that MSCs express ligands for activating NK cell receptors on their surface, like MIC-A (MHC class I-related chain A) and ULBPs (UL16-binding protein; both ligands of NKG2D) as well as PVR (poliovirus receptor) and Nectin-2 (both ligands of DNAM-1 (DNAX accessory molecule-1)). In line with the previously mentioned upregulation of MHC class I on MSCs upon culture with IFN-γ, IFN-γ-treated MSCs were less susceptible to NK cell-mediated lysis.52
In addition, MSCs exert an inhibitory effect on NK cells, affecting different aspects of NK cell function such as proliferation, cytotoxic activity and cytokine production. MSCs inhibit the cytokine (IL-2 and IL-15)-driven proliferation of purified NK cells in a dose-dependent way.52, 53 Even though MSCs did not inhibit the NK cell-mediated lysis of K562 cells, cytokine-stimulated NK cells cocultured with MSCs exhibit a reduced cytolytic function against K562 cells.51 Sotiropoulou et al.54 could only demonstrate an impaired cytolytic function against MHC class I positive tumor targets. Together with the cytolytic activity, the IFN-γ production by NK cells is impaired after coculture with MSCs.53, 54 As for the effect on T cells, the mechanism of inhibition of MSCs on NK cells is not yet completely unraveled. Different mediators like PGE2, IDO, TGF-β and HLA-G have been proposed.53, 54, 55
DC have a critical role in adaptive immunity acting as the primary APC to initiate a T-cell response. This process is essential to initiate adaptive immunity against foreign antigens, but in case of allogeneic transplantation with a non-HLA-identical donor, DC can promote T-cell alloreactivity, leading to graft rejection. Therefore, the interaction between DC and MSCs has been the subject of intensive research. MSCs have been demonstrated to interfere with DC differentiation, maturation and function. First, MSCs inhibit the differentiation of monocytes to immature DC. This effect is reversible and mediated by PGE2.56, 57 Second, consistent data have shown that MSCs interfere with DC maturation. It has been shown that DC cocultured with MSCs exposed to maturation factors, such as lipopolysaccharide or tumor-necrosis factor-α, did not express CD83 and failed to show upregulation of maturation markers, such as MHC class II, CD40 or CD86.56, 58 In line with these findings, immature DC generated in the presence of MSCs were strongly hampered in their ability to induce activation of T cells. MSC cocultures induced an altered cytokine expression, with reduced IL-12 and increased IL-10 production.35, 58 Additionally, DC cultured with MSCs have been shown to induce indirect expansion of regulatory T cells. Taken together, these results suggest that MSCs suppress the differentiation of DC, resulting in the formation of immature DC that exhibit a suppressor or inhibitory phenotype.
Immunogenicity and immunosuppressive capacities of hMAPCs
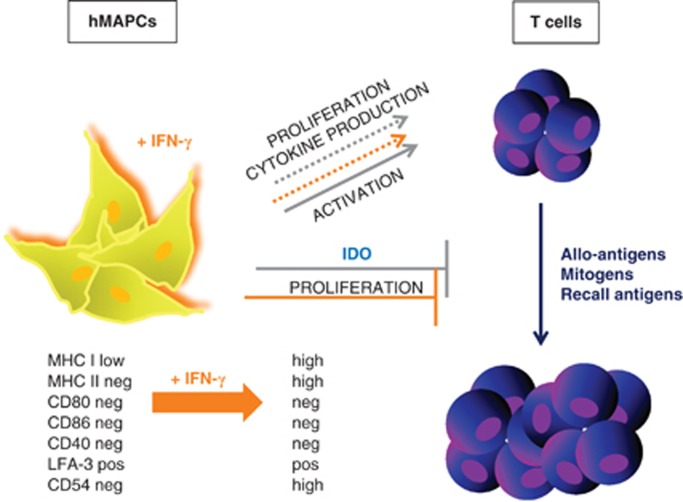
hMAPCs express low levels of MHC class I and LFA-3 (CD58) and are negative for MHC class II, the costimulatory molecules CD80, CD86 and CD40 and the adhesion molecule ICAM-1 (intercellular adhesion molecule 1; CD54).59 Their immune phenotype depends, at least in vitro, on the local inflammatory status of the environment. hMAPCs do not induce proliferation of allogeneic T cells, and this is consistent with their immune phenotypic characteristics, such as the lack of expression of MHC class II and co-stimulatory molecules belonging to the B7-family. Pretreatment with IFN-γ, as a model of an inflammatory environment in which cells might be delivered in vivo, led to the upregulation of MHC class I, MHC class II and ICAM-I. However, despite this IFN-γ pretreatment, hMAPCs still failed to induce alloreactive T-cell proliferation. hMAPCs do not induce production of T helper type 1 or 2 cytokines upon coculture with allogeneic T cells, although low levels of activation markers on responders T cells were induced.
So far, it has been shown that hMAPCs exert strong immunosuppressive effects on T-cell proliferation.59 Furthermore, the suppressive effect was not influenced by MHC compatibility as both autologous and third party stem cells showed similar suppression. These findings are of great clinical relevance because hMAPCs are aimed at being used as an off-the-shelf stem cell product for adoptive cellular therapy. In addition, hMAPCs suppressed T-cell proliferation of memory T cells upon stimulation with recall antigens and of effector T cells during a secondary MLR. Even delayed addition of hMAPCs to a MLR showed similar hMAPC-induced inhibitory effects on alloreactive T cells. These data suggest that hMAPCs can also suppress an ongoing immune response. Importantly, this finding indicates that hMAPCs, when used in clinical settings, can be applied not only for the prevention but also for the treatment of immune-mediated diseases.
The suppressive effect of hMAPCs was not abrogated by pretreatment with IFN-γ. Hence, hMAPCs might remain immunosuppressive when injected into an inflammatory environment in vivo. Third, it was shown that although hMAPCs strongly suppress T-cell allogeneic responses during a primary MLR, they do not prevent priming of T cells, as suppressed T cells show similar secondary responses. hMAPCs did not induce T-cell anergy. Di Nicola et al.36 however, reported that the suppressive effect of hMSCs was reversible, but in their study, T cells were stimulated with a mitogen during the primary MLR instead of allogeneic PBMCs as in our experiments. In addition, exogenous rIL-2 could not restore T-cell proliferation suppressed by hMAPCs.
Subsequently, transwell experiments demonstrated that hMAPCs still exert a suppressive effect, albeit lower, when they are separated from the effector T cells via a membrane. This suggests that the suppression is mediated, at least in part, via a soluble factor, which blocks T-cell proliferation. This hypothesis is supported by the immunosuppressive activity of added supernatants from hMAPC-cultures. However, similar as was shown for hMSCs,34, 36 the degree of suppression was stronger when cell–cell contact was present. This points to a parallel cell-contact-dependent suppressive activity or to adhesion of immune cells to MAPCs, which strengthen their suppressive function through soluble factors. Several candidate molecules have been proposed as the soluble immunosuppressive factor produced by hMSCs, although data are contradictory. hMAPCs displayed increased IDO activity upon IFN-γ pretreatment and partially lost their suppressive effect on T-cell proliferation by blocking IDO activity. This confirmed that the hMAPC-mediated suppressive effect is dependent, at least in part, on IDO activity. Neither a role for PGE2 nor for TGF-β and IL-10 was found. Overall, hMAPCs have immunosuppressive properties on T-cell alloreactivity similar as hMSCs, at least in vitro (Figure 1).
Figure 1.
Summarizing figure of the interactions of hMAPCs with T cells. Full lines indicate that there was an effect observed, dotted lines indicate that there was no effect or interaction. The influence of IFN-γ is indicated in orange. LFA, lymphocyte function-associated antigen.
Clinical experience on immunomodulation by MSCs
Over the last two decades, MSCs have been brought into the clinic for several purposes. Initially, MSCs were used in a number of studies to create cartilage or bone tissue for cell replacement therapy because of their potential to differentiate towards chondrocytes and osteocytes in vivo.60 MSCs also contribute to the formation of vessels due to their capacity to differentiate into vascular smooth muscle cells and their secretion of ‘trophic' factors that promote angiogenesis.61, 62 Therefore, MSCs are currently being tested for their ability to aid in the revascularization of ischemic tissues, such as the myocardium, the brain and the peripheral limb.63, 64, 65
Subsequently, the use of MSCs gained interest in the field of hematopoietic stem cell transplantation (HSCT) as it was shown that in immunodeficient mice hematopoietic engraftment could be enhanced by co-transplanting MSCs.66, 67 Furthermore, MSCs produce several growth factors and cytokines that create a supportive microenvironment of hematopoietic stem/progenitor cells.68
In the mid-nineties, the first studies were performed to test the safety of intravenous infusion of autologous hMSCs.69, 70 In the beginning, only patients with hematological malignancies and breast cancer were studied because of the fear of adverse effects and tumor formation. Researchers observed that autologous MSC infusion at the time of HSCT was feasible and safe. Many studies followed and so far there are no reports of any infusion-related toxicity or ectopic tissue formation.
Meanwhile, others have studied the influence of MSCs on the engraftment of HSCs in a BM graft. In 2002, Lee et al.71 were the first to report the use of allogeneic MSCs in a patient with a hematological malignancy. They reported a patient with high-risk acute myeloid leukemia who was transplanted with mobilized peripheral blood hematopoietic stem cells in combination with BM-derived MSCs from a HLA-haploidentical donor. The patient engrafted rapidly with no acute or chronic graft-versus-host disease (GVHD) and was without any leukemia relapse at 31 months after transplantation. The group of Le Blanc transplanted seven patients in a pilot study together with HLA-identical MSCs in three cases and haploidentical MSCs in four cases to enhance engraftment.72 Despite notable differences in the patient population, sources of HSCs and MSCs and HLA compatibility, all patients had full donor chimerism within 100 days. Ball et al.73 co-transplanted 14 pediatric patients with hematological malignancy and immune deficiency or nonmalignant disorder with haploidentical HSCs together with donor-derived MSCs. Although graft failure in 47 historical controls was 15%, all patients given MSCs showed sustained hematopoietic engraftment without any adverse reaction. Haploidentical MSCs have also been used to enhance hematopoietic reconstitution in a patient suffering from severe aplastic anemia and a second patient with graft failure secondary to incomplete engraftment after autologous HSCT for acute myeloid leukemia.74, 75 Despite back-up BM infusion, the graft failure persisted in the latter patient, and this patient was infused with MSCs 3 years after the initial HSCT. The authors observed hematopoietic recovery of polymorphonuclear cells and platelets in the absence of additional HSC support. The first patient only showed histological improvement in the BM after MSC administration. Meuleman et al.76 transplanted six patients with graft failure after allogeneic HSCT with MSCs without HSC co-infusion. Two of the patients, both transplanted in first complete remission, showed rapid hematopoietic recovery, whereas other patients transplanted at later stages of their disease were unresponsive.
As it became clear that MSCs have also immunomodulatory properties, they are currently being evaluated in the clinic for the prevention and treatment of GVHD, as well as for a number of autoimmune disorders (for example, Crohn's disease, multiple sclerosis, and so on).77, 78
In 2005, Lazarus et al.70 reported on a large multicenter clinical trial with 46 patients with hematological malignancies receiving HLA-identical sibling HSCs together with donor-derived MSCs. MSC infusions were well tolerated, without any infusion-related adverse event, but stromal cell chimerism could only be demonstrated in 2 of 19 examined patients at 6 and 18 months after transplantation. Moderate-to-severe acute GVHD was seen in 28% of patients, and chronic GVHD was observed in 61%. Later, in an open-label randomized clinical trial, HLA-identical sibling-matched HSCs were transplanted or cotransplanted with MSCs.79 They showed that cotransplantation of MSCs with HSCs prevented GVHD (11% in MSC group versus 53% in non-MSC group grades II-IV GVHD). On the other hand, the relapse rate was obviously higher in the MSC group than in the control group (60% versus 20%). More recently, Liu et al.80 conducted a randomized controlled phase II study. A total of 55 patients diagnosed with leukemia received MSCs at the same time as haploidentical HSCT. Within 100 days in the treatment group, platelet recovery was faster, incidence of acute GVHD higher (51.8% versus 38.9%, respectively), whereas there was less chronic GVHD (51.4% versus 74.1%, respectively). The overall survival rate did not significantly differ. The authors claim that the heavily pretreated status of their patients together with the low dose of MSCs (105 cells kg−1) are responsible for the absence of a beneficial effect of MSCs on the prevention of GVHD.
Based on the demonstration of the ability of MSCs to suppress the proliferation of activated alloreactive T cells, MSCs were used for the treatment of acute GVHD. In a landmark case report, Le Blanc et al.81 described the successful therapy of a 9-year-old boy with steroid-resistant grade IV acute GVHD using haploidentical third-party MSCs. Reversal of severe gut and liver GVHD was documented and sustained complete response occurred after the second infusion of MSCs. Subsequently, the group of Le Blanc treated eight additional patients with treatment-resistant acute GVHD of which six patients showed complete response after MSC infusion.82 Some less impressive reports were published by Muller et al.83 who noted improvement of GVHD in only two out of seven pediatric patients receiving haploidentical parental MSCs. Von Bonin et al.84 reported beneficial effects of third-party MSCs expanded in platelet lysate-containing medium in 2 of 13 adult patients with acute GVHD. Fang et al.85 reported on five of six steroid-refractory acute GVHD patients given adipose-tissue-derived third-party MSCs. Those reports indicate that patient population, timing of MSC infusion and culture and expansion methods can largely influence outcomes. In 2008, the results of a large multicenter non-randomized phase II trial of the European Blood and Marrow Transplant MSC consortium, using the same expansion protocol for the cells and common reagents, were published and confirmed a beneficial effect.77 Fifty-five patients with steroid-resistant severe acute GVHD were included. Patients received one (n=27) or more (n=28) MSC infusions with a median dose of 106 cells kg−1. MSCs were obtained from HLA-identical, haploidentical or unrelated HLA-mismatched donors. A complete response was seen in 30 patients (55%). Most interestingly, there was no difference in response rates with respect to the source of MSCs. This finding paves the way for the establishment of large banks of MSCs enabling rapid availability without the need for HLA typing. In 2010, the abstract of Martin et al.86 reported on the first randomized placebo-controlled multicenter phase III trial for the treatment of steroid-resistant acute GVHD using large scale expanded MSCs derived from a healthy third-party donor (Prochymal). In total, 244 patients were enrolled, 163 patients received eight infusions of Prochymal (dose 2 × 106 cells kg−1) over a period of 4 weeks versus 81 patients who received placebo. Durable complete remission did not differ between the study groups. However, subgroup analysis paradoxically showed that patients with gut and liver involvement had better response rates with Prochymal compared with patients with skin involvement. Kebriaei et al.87 studied the use of MSCs to treat newly diagnosed acute GVHD. Patients with grade II–IV were randomized to receive 2 infusions of either low-dose (2 × 106 cells kg−1) or high-dose (8 × 106 cells kg−1) third-party MSCs (Prochymal) in combination with corticosteroids. The infusions were well tolerated and the combination of high-dose corticosteroids and Prochymal resulted in complete and partial response in 77% and 16% of cases, respectively. There was no difference with respect to safety or efficacy between the low and high dose of MSCs. Recently, Prochymal was used to treat 12 children with steroid-refractory grade III–IV acute GVHD.88 They received 2 or 8 × 106 cells kg−1 twice a week for 4 weeks. Partial and mixed responders received subsequent therapy for 4 weeks. Clinical response, particularly in the gastro-intestinal system, was seen in the majority of children (58% complete response, 17% partial response).
Taken together, all studies focusing on the treatment of acute GVHD with MSC infusions could see a complete or partial response in the majority of patients. There seemed to be no differences between using low versus high doses of MSCs or between a single or multiple infusions of MSCs. In addition, the HLA compatibility between MSC donor and recipient was not of major importance.
As chronic GVHD still represents substantial morbidity and mortality after allogeneic HSCT, the number of sporadic reports on the use of MSCs to treat chronic GVHD are emerging.82, 89 Weng et al.90 reported on 19 patients with steroid-refractory chronic GVHD treated with MSCs. Partial or complete response was seen in 74% of the patients. The question whether the established mechanism of graft-versus-leukemia (GVL) will remain in patients with chronic GVHD who have been successfully treated needs to be further addressed.
At the moment, GVHD is by far the most studied therapeutic application for MSCs. However, clinical trials using MSCs to treat other auto-immune diseases, for example, Crohn's disease, multiple sclerosis, systemic lupus erythematosus and arthritis, are very promising.63, 91, 92, 93
Clinical experience on immunomodulation by MAPCs
The extensive proliferation potential of hMAPCs allows banking of large amounts of cells for their clinical use. Moreover, the immunomodulatory properties of hMAPCs make it possible to use them as a universal donor. Therefore, a clinical grade, large-scale expanded product has been developed based on the MAPC technology (Multistem). Genomic and epigenetic stability of Multistem during long-term culture expansion has been confirmed, and preclinical studies demonstrated the safety of administration in repeat dosing regimens in BM transplant settings.94, 95
Multistem is currently evaluated in a number of phase I/II clinical trials in patients with stroke, acute myocardial infarction, inflammatory bowel disease and for the prevention of GVHD in patients undergoing BM transplantation.
Last year, clinical results from the phase I trial in acute myocardial infarction demonstrated the safety of Multistem. Moreover, a beneficial effect was seen, in part, because of their ability to induce neovascularization through secretion of trophic factors such as VEGF (vascular endothelial growth factor), IL8 and CXCL5 (C-X-C motif chemokine ligand 5).96, 97 Earlier this year, the results of the phase I trial for the prevention of GVHD were announced. Single dose and repeat dose administration of MultiStem was well-tolerated, and a low incidence of severe acute GVHD was observed.98
Conclusion
To bring stem cell-based adoptive therapy with hMAPCs to a successful clinical application for the control of immunological disorders, some main concerns need to be further addressed. A first major concern about the in vivo use of hMAPCs is that hMAPCs might interfere with the normal protective immune responses against pathogens. We observed that hMAPCs suppress T-cell proliferation induced by recall antigens. This indicates that the adoptive treatment with hMAPCs might render the host more vulnerable to infections. However, different data derived from the clinical use of MSC therapy in GVHD and allogeneic HSCT showed that anti-viral immune reactions may normally occur following systemic administration.73, 77
A second question regarding the adoptive transfer of immunosuppressive cells to prevent GVHD after HSCT involves the influence of these immunosuppressive cells on the desired GVL effect. So far, this has not been studied for hMAPCs. On the contrary and as mentioned before, suppression of the GVL effect by hMSC therapy has been reported. Ning et al.79 reported on a randomized clinical trial in which patients with hematological malignancies received HSCs with or without hMSCs. They observed that hMSC therapy had a beneficial effect on the occurrence of GVHD but was associated with a higher relapse rate. This observation highlights the critical effects of hMSC therapy on the balance between GVHD and GVL and underscores the importance of studying further the influence of hMAPCs on the GVL effect in allogeneic HSCT.
A third issue is the question of whether the immunosuppressive capacities of hMAPCs will be influenced by other immunosuppressive drugs, and this has thus far not been studied for hMAPCs. Standard immunosuppressive treatment following allogeneic HSCT or organ transplantation involves the administration of calcineurin inhibitors, such as cyclosporine A (CSA), tacrolimus or mycophenolate mofetil (MMF). These drugs might interfere with the immunosuppressive effects of hMSCs after adoptive transfer. Le Blanc et al.37 were the first to show an in vitro synergistic effect of CSA on the hMSC-mediated immunosuppression of T-cell reactivity. However, by contrast, Buron et al.99 observed that CSA, tacrolimus and rapamycin antagonized hMSC inhibitory effects, whereas MMF promoted the hMSC inhibitory effects. In addition, Eggerhofer et al.100 demonstrated in a rat model of heart transplantation that MSCs and MMF synergistically prevented infiltration of APC and T cells into the graft. By contrast, calcineurin inhibitors have been shown to abrogate the immunosuppressive effect of rat MSC therapy.101 These observations underline the need to study the appropriate drugs in combination with the adoptive stem cell-mediated immunotherapy.
In conclusion, the use of stem cell-based immunotherapy is very promising. So far, in vitro studies showed comparable suppressive effects for hMSCs and hMAPCs, although the broader expansion capacities of hMAPCs make them more attractive for clinical use.
The authors declare no conflict of interest.
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- In't Anker PS, Scherjon SA, Kleijburg-van der KC, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Ulloa-Montoya F, Kidder BL, Pauwelyn KA, Chase LG, Luttun A, Crabbe A, et al. Comparative transcriptome analysis of embryonic and adult stem cells with extended and limited differentiation capacity. Genome Biol. 2007;8:R163. doi: 10.1186/gb-2007-8-8-r163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K, Geraerts M, Pauwelyn KA, Park Y, Owens DJ, Muijtjens M, et al. Isolation procedure and characterization of multipotent adult progenitor cells from rat bone marrow. Methods Mol Biol. 2010;636:55–78. doi: 10.1007/978-1-60761-691-7_4. [DOI] [PubMed] [Google Scholar]
- Lo Nigro A, Geraerts M, Notelaers T, Roobrouck VD, Muijtjens M, Eggermont K, et al. MAPC culture conditions support the derivation of cells with nascent hypoblast features from bone marrow and blastocysts J Mol Cell Biol(e-pub ahead of print 9 August 2012). [DOI] [PMC free article] [PubMed]
- Kunath T, Arnaud D, Uy GD, Okamoto I, Chureau C, Yamanaka Y, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- Debeb BG, Galat V, Epple-Farmer J, Innaccone S, Woodward WA, et al. Isolation of Oct4-expressing extraembryonic endoderm precursor cell lines. PLoS One. 2009;4:e7216. doi: 10.1371/journal.pone.0007216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelandt P, Pauwelyn KA, Sancho-Bru P, Subramanian K, Bose B, Ordovas L, et al. Human embryonic and rat adult stem cells with primitive endoderm-like phenotype can be fated to definitive endoderm, and finally hepatocyte-like cells. PLoS One. 2010;5:e12101. doi: 10.1371/journal.pone.0012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Coenegrachts L, Stockmans I, Daci E, Luttun A, Petryk A, et al. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest. 2006;116:1230–1242. doi: 10.1172/JCI26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc Natl Acad Sci USA. 2003;100 (Suppl 1:11854–11860. doi: 10.1073/pnas.1834196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranguren XL, Luttun A, Clavel C, Moreno C, Abizanda G, Barajas MA, et al. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood. 2007;109:2634–2642. doi: 10.1182/blood-2006-06-030411. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31:1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Qi H, Aguiar DJ, Williams SM, La PA, Pan W, Verfaillie CM. Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci USA. 2003;100:3305–3310. doi: 10.1073/pnas.0532693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranguren XL, McCue JD, Hendrickx B, Zhu XH, Du F, Chen E, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–514. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobrouck VD, Clavel C, Jacobs SA, Ulloa-Montoya F, Grippa S, Sohni A, et al. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29:871–882. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N, et al. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87:S62–S66. doi: 10.1097/TP.0b013e3181a2a4b3. [DOI] [PubMed] [Google Scholar]
- Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- Sioud M, Mobergslien A, Boudabous A, Floisand Y. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011;38:385–390. doi: 10.3892/ijo.2010.869. [DOI] [PubMed] [Google Scholar]
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Mancusi A, Burchielli E, Capanni M, Carotti A, Aloisi T, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40:84–90. doi: 10.1016/j.bcmd.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell. Nat Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Jacobs SA, Pinxteren J, Roobrouck VD, Luyckx A, Van't Hof W, Deans R, et al. Human multipotent adult progenitor cells are non-immunogenic and exert potent immunomodulatory effects on alloreactive T cell responses Cell Transplant(e-pub ahead of print, 1 october 2012). [DOI] [PubMed]
- Noel D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs. 2002;3:1000–1004. [PubMed] [Google Scholar]
- Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, Yu G, et al. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell Transplant. 2007;16:159–169. [PubMed] [Google Scholar]
- Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503–515. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, et al. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103:3313–3319. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- Lee ST, Jang JH, Cheong JW, Kim JS, Maemg HY, Hahn JS, et al. Treatment of high-risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co-infusion of T cell-depleted haematopoietic stem cells and culture-expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol. 2002;118:1128–1131. doi: 10.1046/j.1365-2141.2002.03767.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- Fouillard L, Chapel A, Bories D, Bouchet S, Costa JM, Rouard H, et al. Infusion of allogeneic-related HLA mismatched mesenchymal stem cells for the treatment of incomplete engraftment following autologous haematopoietic stem cell transplantation. Leukemia. 2007;21:568–570. doi: 10.1038/sj.leu.2404550. [DOI] [PubMed] [Google Scholar]
- Meuleman N, Tondreau T, Ahmad I, Kwan J, Crokaert F, Delforge A, et al. Infusion of mesenchymal stromal cells can aid hematopoietic recovery following allogeneic hematopoietic stem cell myeloablative transplant: a pilot study. Stem Cells Dev. 2009;18:1247–1252. doi: 10.1089/scd.2009.0029. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA, nilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- Liu K, Chen Y, Zeng Y, Xu L, Liu D, Chen H, et al. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev. 2011;20:1679–1685. doi: 10.1089/scd.2010.0447. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- Muller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- von Bonin M, Stolzel F, Goedecke A, Richter K, Wuschek N, Holig K, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- Fang B, Song Y, Lin Q, Zhang Y, Cao Y, Zhao RC, et al. Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatr Transplant. 2007;11:814–817. doi: 10.1111/j.1399-3046.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- Martin PJ, Uberti JP, Soiffer RJ, Klingemann H, Waller EK, Daly AS, et al. Improves response rates in patients with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial in GVHD. Biol Blood Marrow Transplant. 2010;16:S169–S170. [Google Scholar]
- Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, et al. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant. 2010;16:403–412. doi: 10.1016/j.bbmt.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45:1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- Tyndall A, van Laar JM. Stem cells in the treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010;24:565–574. doi: 10.1016/j.berh.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Boozer S, Lehman N, Lakshmipathy U, Love B, Raber A, Maitra A, et al. Global characterization and genomic stability of human multistem, a multipotent adult progenitor cell. J Stem Cells. 2009;4:17–28. [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M, Mauch K, Raber A, Streeter PR, Deans RJ, Maziarz RT, et al. Pre-clinical safety testing supporting clinical use of allogeneic multipotent adult progenitor cells. Cytotherapy. 2008;10:730–742. doi: 10.1080/14653240802320245. [DOI] [PubMed] [Google Scholar]
- Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- Lehman N, Cutrone R, Raber A, Perry R, van't Hof W, Deans R, et al. Development of a surrogate angiogenic potency assay for clinical-grade stem cell production. Cytotherapy. 2012;14:994–1004. doi: 10.3109/14653249.2012.688945. [DOI] [PubMed] [Google Scholar]
- Maziarz. poster abstract 165. American Society for Bone Marrow Transplantation Tandem meeting San Diego; 2012 [Google Scholar]
- Buron F, Perrin H, Malcus C, Hequet O, Thaunat O, Kholopp-Sarda MN, et al. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplant Proc. 2009;41:3347–3352. doi: 10.1016/j.transproceed.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Eggenhofer E, Steinmann JF, Renner P, Slowik P, Piso P, Geissler EK, et al. Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl Immunol. 2011;24:157–163. doi: 10.1016/j.trim.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]