Abstract
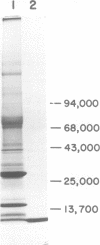
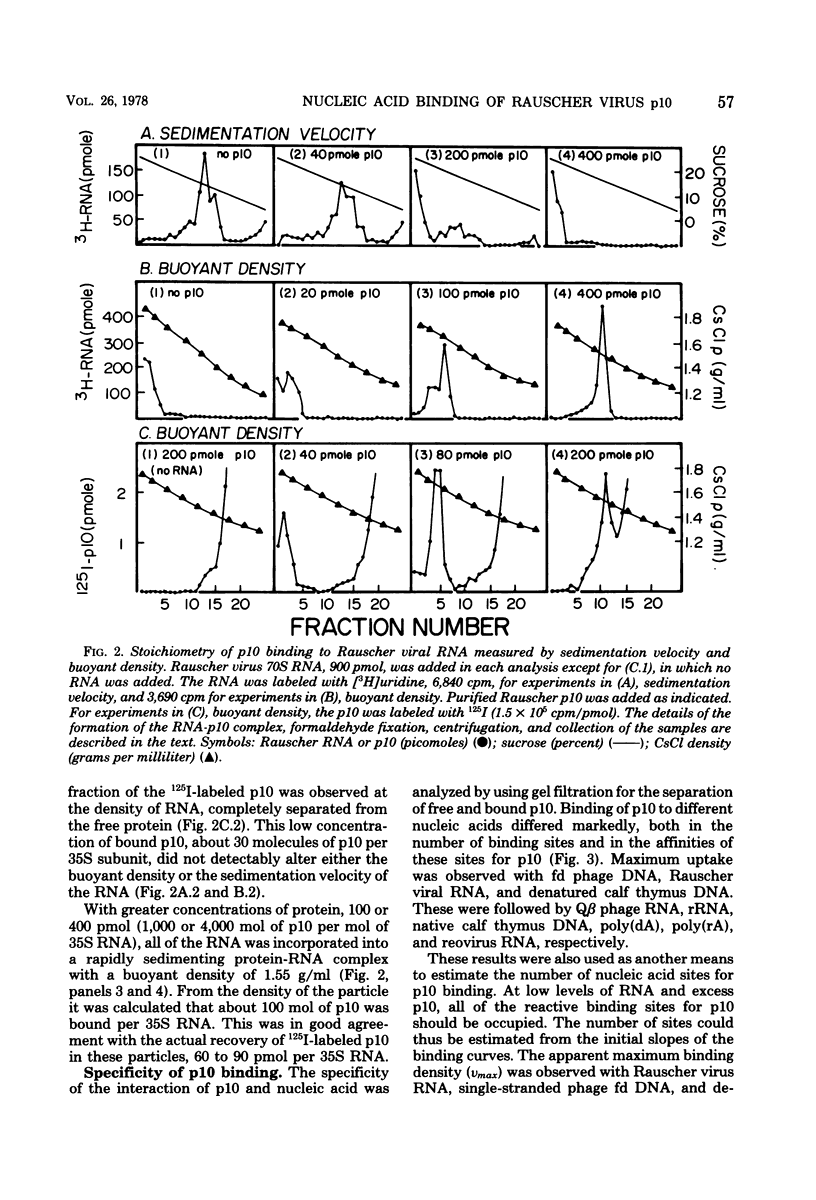
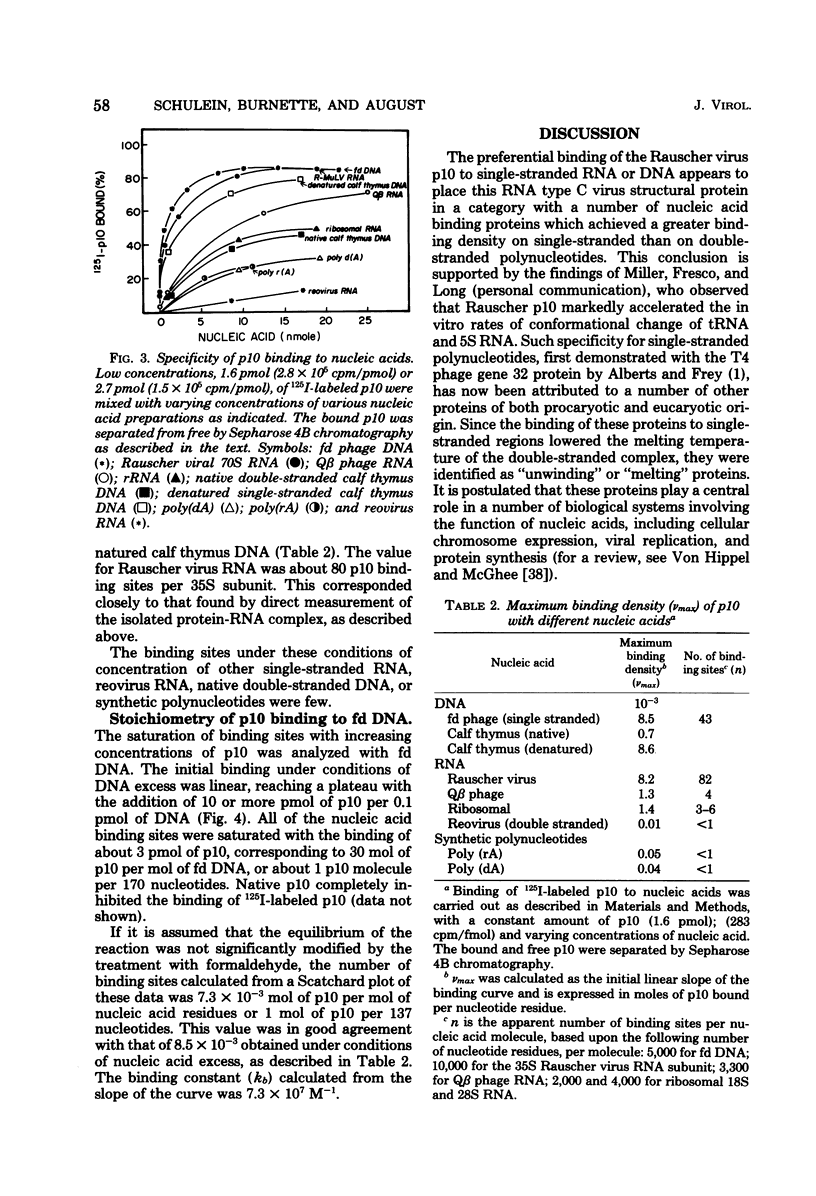
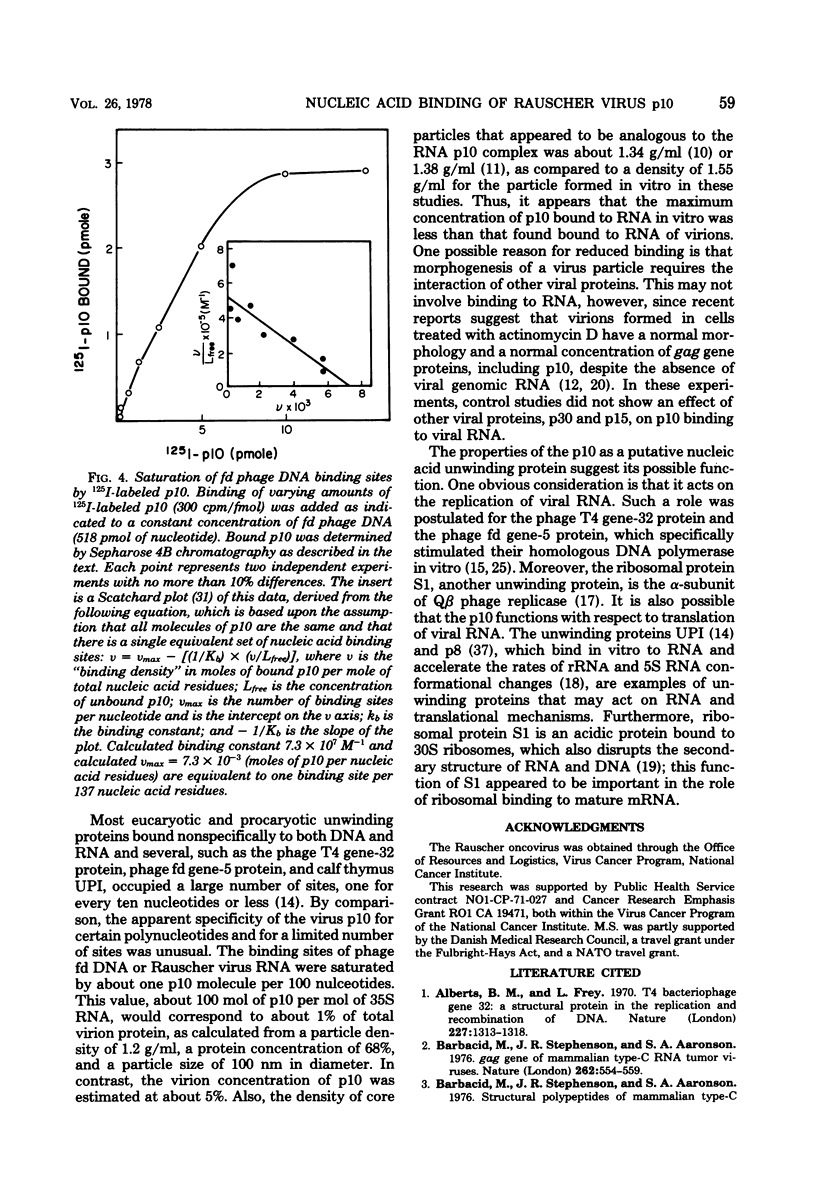
A structural protein of Rauscher oncovirus of about 8,000 to 10,000 daltons (p10), encoded by the gag gene, has been purified in high yield to apparent homogeneity by a simple three-step procedure. The purified protein was highly basic, with an isoelectric point of more than 9.0, and its immunological antigenicity was chiefly group specific. A distinctive property of the protein was the binding to nucleic acids. The stoichiometry of p10 binding to Rauscher virus RNA was analyzed using both 125I-labeled p10 and 3H-labeled RNA. The protein-RNA complex, cross-linked by formaldehyde, was separated from free RNA and free protein by velocity sedimentation and density gradient centrifugation. A maximum of about 140 mol of p10 was bound per mol of 35S RNA, or about one molecule of p10 per 70 nucleotides. This protein-RNA complex banded at a density of about 1.55 g/ml. The number of nucleic acid sites bound and the affinity of p10 binding differed significantly among the other polynucleotides tested. The protein bound to both RNA and DNA with a preference for single-stranded molecules. Rauscher virus RNA and single-stranded phage fd DNA contained the highest number of binding sites. Binding to fd DNA was saturated with about 30 mol of p10 per mol of fd DNA, an average of about one p10 molecule per 180 nucleotides. The apparent binding constant was 7.3 X 10(7) M(-1). The properties of the p10 place it in a category with other nucleic acid binding proteins that achieve a greater binding density on single-stranded than on double-stranded molecules and appear to act by facilitating changes in polynucleotide conformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Buchhagen D. L., Stutman O., Fleissner E. Chromatographic Separation and Antigenic Analysis of Proteins of the Oncornaviruses IV. Biochemical Typing of Murine Viral Proteins. J Virol. 1975 May;15(5):1148–1157. doi: 10.1128/jvi.15.5.1148-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Scherer M., Tsai W. P., Long C. Low-molecular- weight Rauscher leukemia virus protein with preferential binding for single-stranded RNA and DNA. J Virol. 1976 May;18(2):709–718. doi: 10.1128/jvi.18.2.709-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., von Hippel P. H. DNA "melting" proteins. I. Effects of bovine pancreatic ribonuclease binding on the conformation and stability of DNA. J Biol Chem. 1976 Nov 25;251(22):7198–7214. [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Kolb A., Hermoso J. M., Thomas J. O., Szer W. Nucleic acid helix-unwinding properties of ribosomal protein S1 and the role of S1 in mRNA binding to ribosomes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2379–2383. doi: 10.1073/pnas.74.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M. K., Long C. W., Robey W. G., Scherer M. A., Vande Woude G. F. Phosphorylation and nucleic acid binding properties of m1 Moloney murine sarcoma virus-specific pP60gag. J Virol. 1977 Jul;23(1):196–204. doi: 10.1128/jvi.23.1.196-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Specific binding of the type C viral core protein p12 with purified viral RNA. Cell. 1976 Jan;7(1):21–32. doi: 10.1016/0092-8674(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Belitsina N. V., Lerman M. I. Use of formaldehyde fixation for studies of ribonucleoprotein particles by caesium chloride density-gradient centrifugation. J Mol Biol. 1965 Dec;14(2):611–615. doi: 10.1016/s0022-2836(65)80213-3. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Strand M., Wilsnack R., August J. T. Structural proteins of mammalian oncogenic RNA viruses: immunological characterization of the p15 polypeptide of Rauscher murine virus. J Virol. 1974 Dec;14(6):1575–1583. doi: 10.1128/jvi.14.6.1575-1583.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Specific recognition of single-stranded nucleic acids. Interaction of tryptophan-containing peptides with native, denatured, and ultraviolet-irradiated DNA. J Biol Chem. 1977 Jan 10;252(1):244–249. [PubMed] [Google Scholar]
- Tsai R. L., Green H. Studies on a mammalian cell protein (P8) with affinity for DNA in vitro. J Mol Biol. 1973 Feb 19;73(3):307–316. doi: 10.1016/0022-2836(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., McGhee J. D. DNA-protein interactions. Annu Rev Biochem. 1972;41(10):231–300. doi: 10.1146/annurev.bi.41.070172.001311. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]