Abstract
Background
Tumor hypoxia is associated with malignant progression and treatment resistance. Hypoxia-related factors, such as carbonic anhydrase IX (CA IX), glucose transporter-1 (GLUT-1), and vascular endothelial growth factor (VEGF) permit tumor cell adaptation to hypoxia. We attempted to elucidate the correlation of these markers with variable clinicopathological factors and overall prognosis.
Methods
Immunohistochemistry for CA IX, GLUT-1, and VEGF was performed on formalin-fixed, paraffin-embedded tissues from 125 cases of ovarian epithelial cancer (OEC).
Results
CA IX expression was significantly associated with an endometrioid and mucinous histology, nuclear grade, tumor necrosis, and mitosis. GLUT-1 expression was associated with tumor necrosis and mitosis. VEGF expression was correlated only with disease recurrence. Expression of each marker was not significant in terms of overall survival in OECs; however, there was a significant correlation between poor overall survival rate and high coexpression of these markers.
Conclusions
The present study suggests that it is questionable whether CA IX, GLUT-1, or VEGF can be used alone as independent prognostic factors in OECs. Using at least two markers helps to predict patient outcomes in total OECs. Moreover, the inhibition of two target gene combinations might prove to be a novel anticancer therapy.
Keywords: Ovarian epithelial cancer, Carbonic anhydrase IX, GLUT-1, Vascular endothelial growth factor A
Ovarian cancer accounts for 3% of all cancers in women, and it is the fifth most common cause of cancer-related death among women in the United States.1 It is also the second most common type of malignant tumor of the female genital tract in Korea, and the trend has been increasing steadily.2 Ovarian cancer still has a very high mortality rate, despite standardized therapeutic procedures. This is because many ovarian cancers are asymptomatic at an early stage, and often manifest at an advanced stage, with peritoneal dissemination at the time of diagnosis.3 Additional prognostic factors may help determine the need for adjuvant treatment and identify new treatment strategies.
Many solid tumors, including ovarian tumors, outgrow their own vasculatures beyond the size of several cubic millimeters, which results in hypoxia. In general, hypoxia within the tumor microenvironment is an important event in carcinogenesis because it can lead to the development of a more aggressive phenotype with increased invasiveness and proliferation, formation of metastasis, and poorer prognosis.4,5 Besides, hypoxic malignant cells are more resistant to chemotherapy and radiotherapy.6 For these reasons, hypoxia has been suggested to be an adverse prognostic factor for patient outcome. Cellular adaptation to hypoxia represents an essential step in tumor progression. All normal and neoplastic tissues are thought to possess a mechanism that helps survival in hypoxic conditions by modulating certain crucial genes. One of the key factors regulating cellular responses to hypoxia via transcription is hypoxia-inducible factor 1 (HIF-1). Thus, throughout the HIF-1-mediated pathway, various hypoxia-related factors (HRFs), such as carbonic anhydrase IX (CA IX), glucose transporter-1 (GLUT-1), and vascular endothelial growth factor (VEGF), are activated.7-9 HRFs help cells to survive from hypoxic stress by switching to anaerobic glycolysis and by increasing oxygen delivery (via angiogenesis).10,11
CA IX is a membrane-associated carbonic anhydrase that plays a role in pH regulation. The induction of CA IX allows cancer cells to maintain an alkaline intracellular pH and an acidic extracellular pH, which are critical for cell proliferation and invasion, respectively.12 Elevated levels of CA IX are predictive of hypoxia in various types of cancer and are related to poor prognosis.13,14
GLUT-1 is a representative member of the GLUT family, and is widely distributed in normal tissues such as erythrocytes and endothelial cells of the blood-brain barrier.15 It is known to be a transmembrane glucose transporter that operates as a passive carrier for transporting glucose down a concentration gradient, and it is activated by an HIF-1-mediated pathway.8 This increase in glucose transporter proteins is accompanied by a corresponding increase in glucose uptake across the plasma membrane, eventually supporting the metabolic needs of rapidly dividing tumor cells. A report on GLUT-1 expression in ovarian carcinoma suggested that overexpression of this glucose transporter is correlated with aggressive biological tumor behavior.16
Angiogenesis is an essential component of solid tumor growth and metastasis. VEGF acts through its tyrosine kinase receptors to modulate motility and the proliferation of endothelial cells and vascular permeability. VEGF has been shown to have an important role in neovascular formation in tumors, as it provides nutrition to the highly metabolic tumor cells, as well as access to the host vasculature.17 Higher levels of VEGF are shown in ovarian carcinomas than in normal ovaries.18 Taken together this background information shows that targeting HRFs can be an attractive therapeutic strategy with the potential for disrupting multiple pathways that are crucial for tumor growth. The aim of this study, therefore, was to assess the expression of HRFs, including CA IX, GLUT-1, and VEGF in ovarian epithelial cancers (OECs) and determine their associations with variable clinicopathological factors and patients' overall outcomes.
MATERIALS AND METHODS
Patients and tissue samples
The tissue samples of OECs were obtained during surgical operations performed at Pusan National University Hospital, Korea, from 1999 to 2008. A total of 125 patients (mean age, 49.5 years; range, 15 to 82 years) who underwent surgical resection for OECs were examined. All patients, except those with grade 1, stage IA, were given adjuvant chemotherapy of a platinum/taxol containing multiple drugs. The patients were followed up from the date of surgery until death or the last visit to the outpatient department. The follow-up period ranged from 1 to 140 months (median, 38 months). Pathological data, such as pathologic stage, tumor grading, tumor size, mitosis, and histological tumor type, were obtained from the primary pathology reports and patient medical record was reviewed retrospectively. Surgical staging was determined based on the criteria recommended by the International Federation of Gynecology and Obstetrics (FIGO). Histologic tumor type and grade were determined according to the World Health Organization criteria. Table 1 provides an overview of the main clinicopathologic features.19
Table 1.
Clinicopathological features of the group of ovarian epitheilal cancer patients (n=125)
FIGO, Federation of Gynecology and Obstetrics; HPF, high power field.
aSilverberg tumor grade system.19
Immunohistochemistry
Immunohistochemistry was performed on serial 4 µm-thick paraffin sections. The sections were deparaffinized in xylene and rehydrated in four decreasing grades of ethanol (100%, 95%, 80%, and then 70%) for 2 minutes each. Endogenous peroxidase activity was blocked by immersing the slides in 3% hydrogen peroxide in methanol for 15 minutes at room temperature. Heat-induced antigen retrieval was performed using a microwave. The slides were immersed in 1× citrate buffer (pH 6.3) and treated for 5 minutes and then cooled for 5 minutes. This process was performed three times. Slides were then cooled down under running tap water for 30 seconds and washed with phosphate-buffered saline (PBS) for 10 minutes. In order to reduce nonspecific absorption of antibodies to the tissues, the slides were pre-incubated with blocking serum in 1% bovine serum albumin for 30 minutes at room temperature. The slides were then left to incubate with rabbit polyclonal anti-CA IX antibody (1:1,000, Abcam, Cambridge, UK), rabbit polyclonal anti-GLUT-1 antibody (1:200, Dako, Carpinteria, CA, USA), and mouse monoclonal anti-VEGF antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4℃. After incubation with primary antibodies, the slides were rinsed with PBS for 10 minutes. Thereafter, for detection of the antibody reactions, EnVision Detection System (Dako) was used at room temperature according to the manufacturer's recommended protocol. Finally, the color was developed using diaminobenzidine and the sections were then counterstained with Mayer's hematoxylin solution for 1 minute, and dehydrated with graded alcohols, dipped in 2 changes of xylene, and mounted. The following appropriate positive controls were used: renal cell carcinoma specimen for CA IX, erythrocytes for GLUT-1, and endothelial cells for VEGF. As negative controls for each antibody, adjacent sections were incubated in parallel using non-immune serum instead of primary antibodies.
Assessment of immunohistochemical staining
Results of immunohistochemical staining were assessed using a light microscope in a blinded fashion. Stainings were scored blinded to clinicopathologic data and the results of other stainings. For CA IX, only a membranous staining was regarded as positive. GLUT-1 and VEGF were considered positive when a membranous or cytoplasmic staining was identified. The extent of their expression was semi-quantitatively evaluated according to the following scoring system: 0, negative staining (0%); 1, weak positive (<10%); 2, moderate positive (10-50%); and 3, strong positive (>50%).16.20 Erythrocytes in each section were used as positive internal controls for GLUT-1. Finally, to perform a statistical analysis, we grouped various patterns of staining into two categories: low expression (score 0 and 1) and high expression (score 2 and 3).
Statistical analysis
All statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Pearson's chi-squared test and Fisher's exact test were used to study the associations between clinicopathological factors and immunohistochemical markers. Overall survival (OS) was calculated from the day of surgery until the death of the patient. Data on patients who had survived until the end of the observation period were censored at their last follow-up visit. Death from a cause other than ovarian cancer or cases lost to follow-up was considered a censoring event. The effect of immunohistochemical markers on survival was assessed using Kaplan-Meier survival curves and a log rank test. For all of the tests, a p≤0.05 was considered to be significant.
RESULTS
The expression of CA IX, GLUT-1, and VEGF
A total of 125 OEC samples were used for the determination of CA IX, GLUT-1, and VEGF expression. CA IX expression is shown in Fig. 1. There was negative staining in 26 specimens (score 0, 20.8%). CA IX was detectable in 99 (79.2%) of the 125 specimens. CA IX expression was scored as 1 in 47 specimens (37.6%), scored as 2 in 41 specimens (32.8%), and scored as 3 in 11 specimens (8.8%). High CA IX expression was noted in 52 (41.6%) among all of the 125 cases.
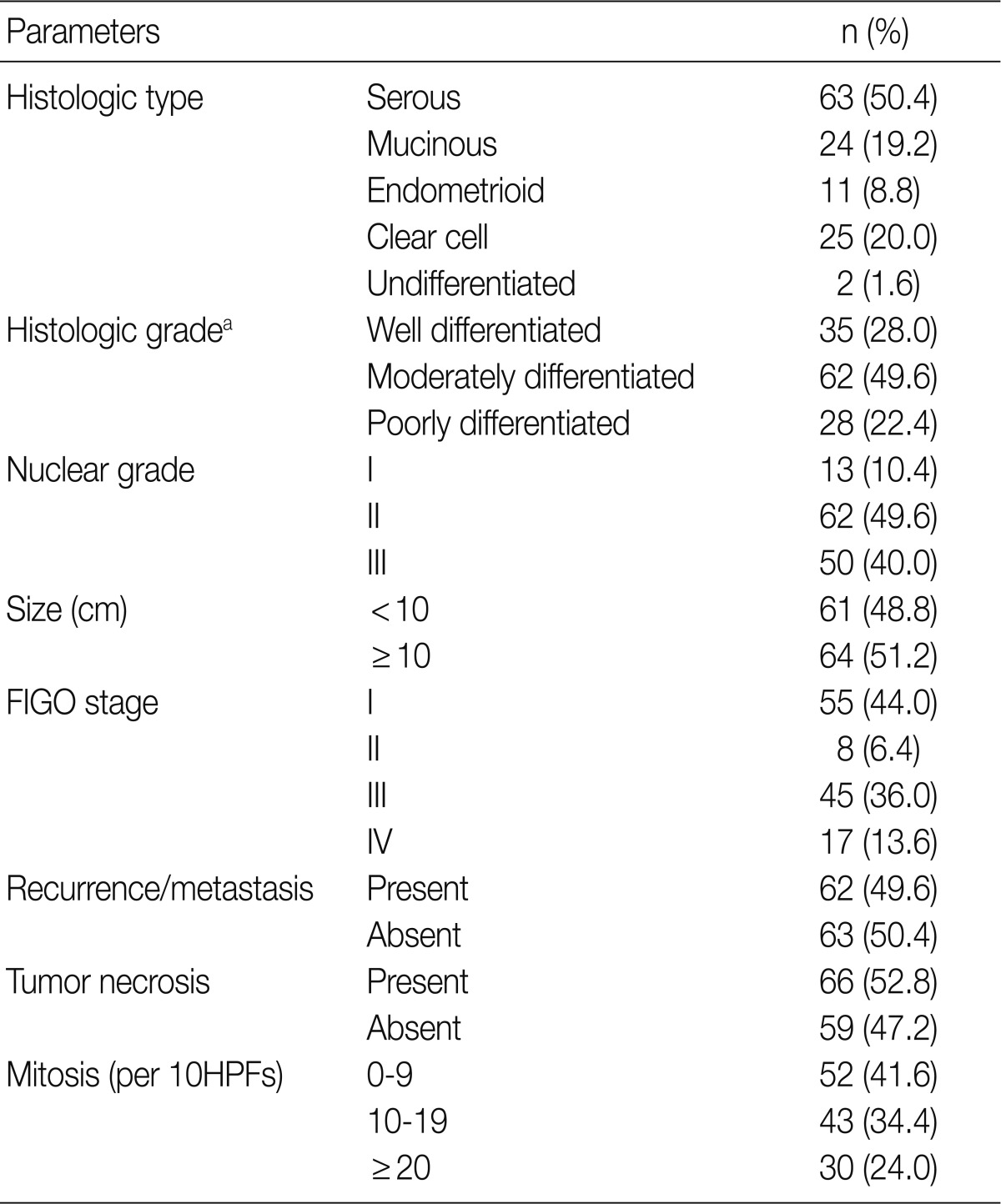
Fig. 1.
Immunohistochemical staining for carbonic anhydrase IX in ovarian epithelial carcinoma. Highlighted are the perinecrotic patterns in mucinous (A) and endometrioid carcinoma (B).
GLUT-1 expression showed a membranous or cytoplasmic staining pattern. Of 125 OEC cases, 77 (61.6%) showed positive GLUT-1 immunostaining. Weak staining was detected in 13 cases (score 1, 10.4%), moderate staining in 46 cases (score 2, 36.8%), and strong staining in 18 cases (score 3, 14.4%) (Fig. 2). High GLUT-1 expression was noted in 64 (51.2%) of the total of 125 OEC cases, whereas, negative staining was observed in 48 cases (score 0, 38.4%).
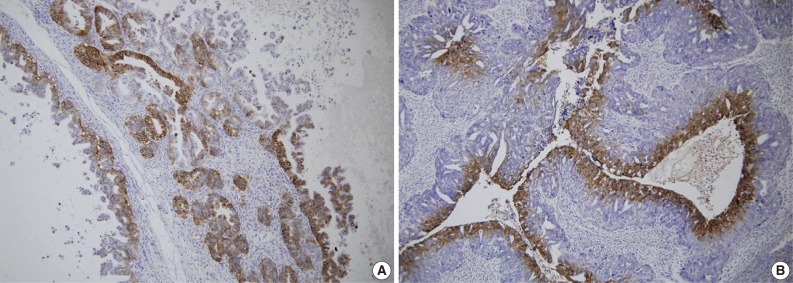
Fig. 2.
Immunohistochemical staining for glucose transporter-1 in ovarian epithelial carcinoma. Highlighted are the perinecrotic patterns in clear cell (A) and serous carcinoma (B).
An immunohistochemical study of VEGF expression in the 125 OEC cases revealed no VEGF expression in 10 cases (score 0, 8.0%), a score of 1 in 24 cases (19.2%), a score of 2 in 60 cases (48.0%), and a score of 3 in 31 cases (24.8%) (Fig. 3). Thus high VEGF expression was observed in 91 (72.8%) of all of the 125 OEC cases.
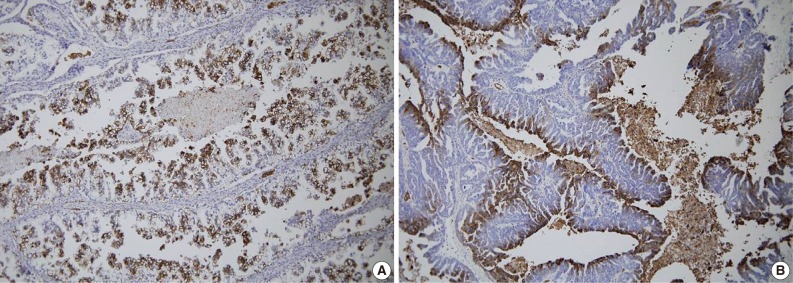
Fig. 3.
Immunohistochemical staining for vascular endothelial growth factor (VEGF) in ovarian epithelial carcinoma. VEGF expression is seen in clear cell (A) and mucinous carcinoma (B).
Correlation between high expression of HRFs and clinicopathological characteristics in OECs
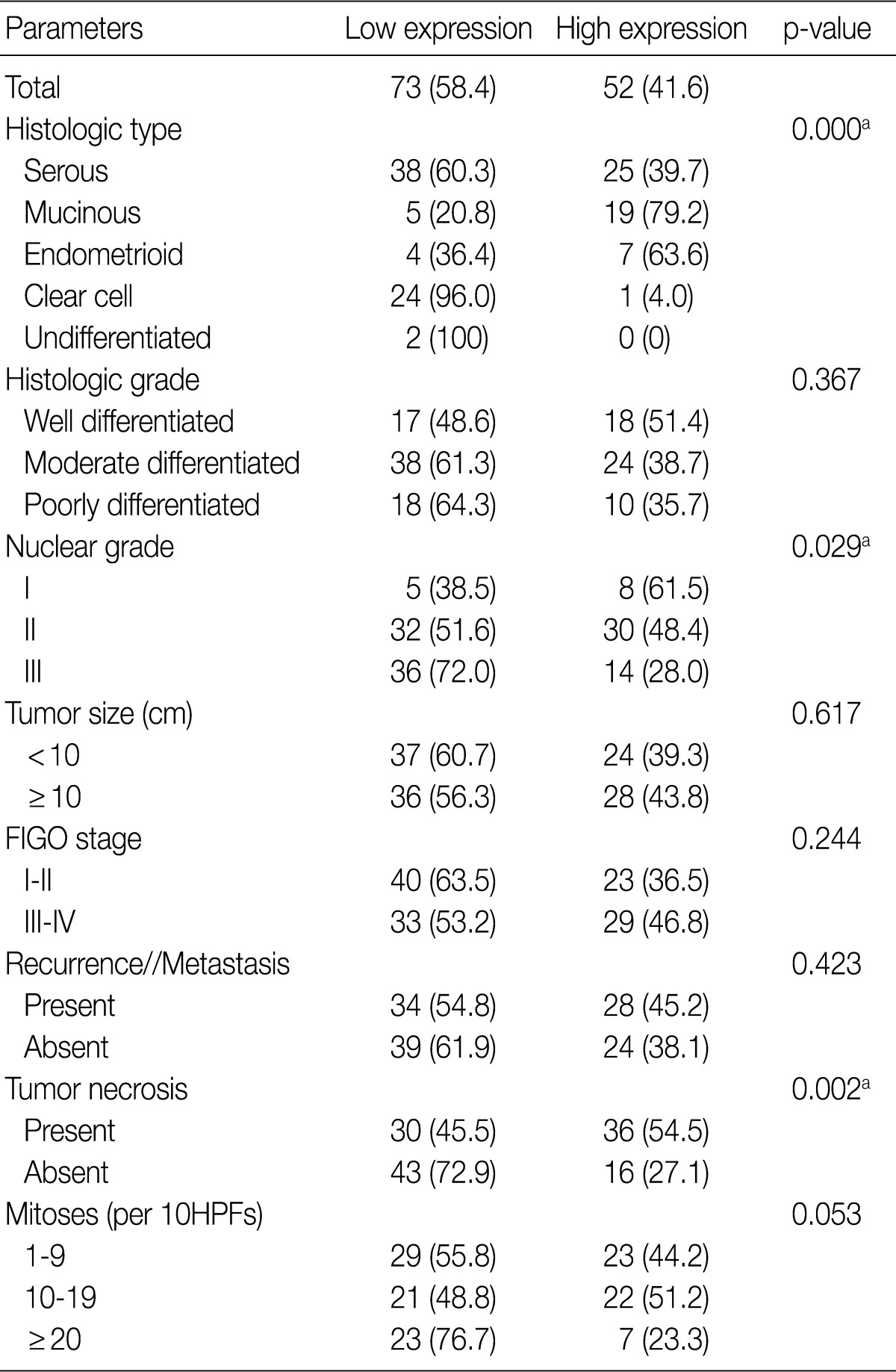
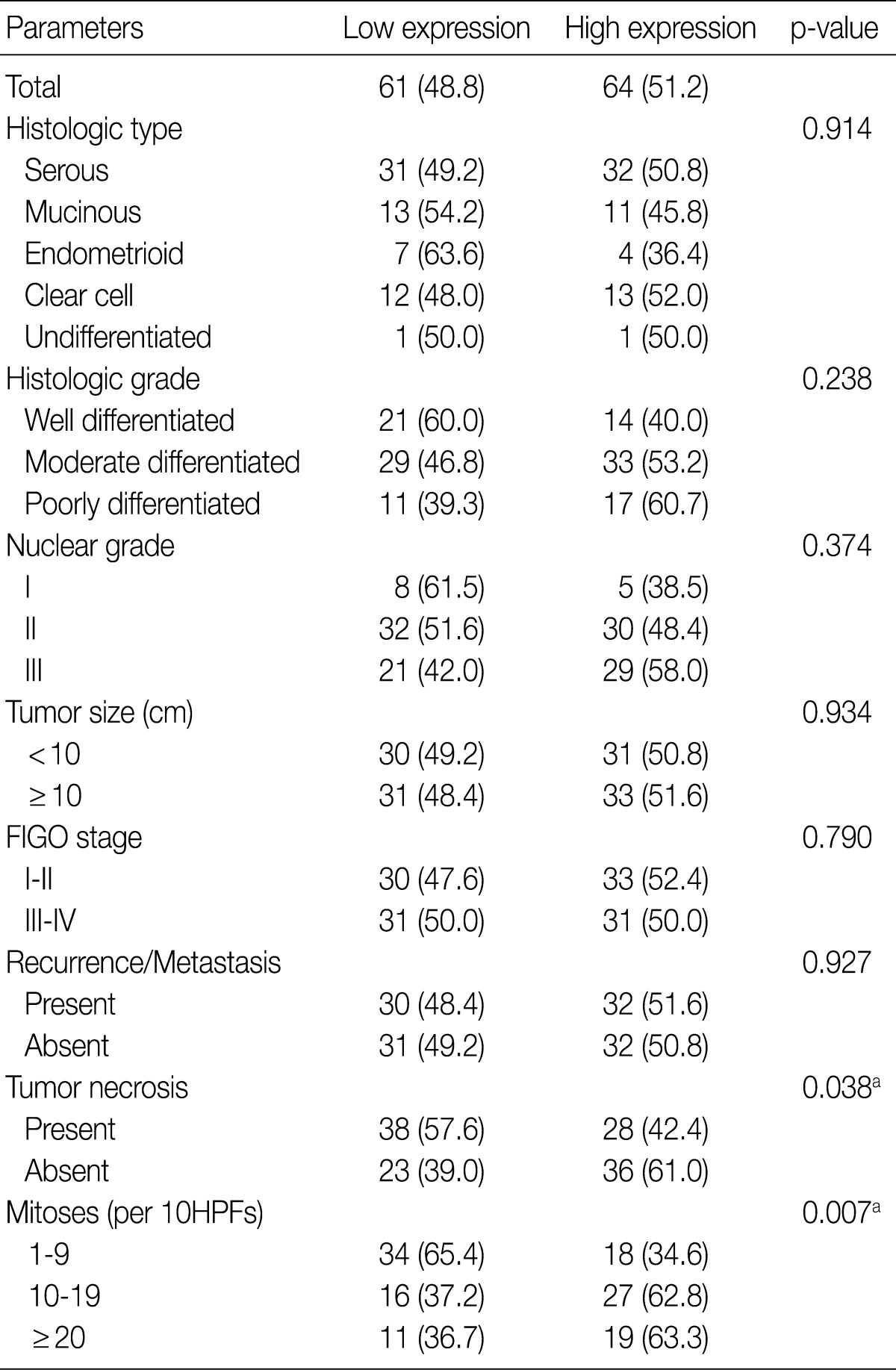
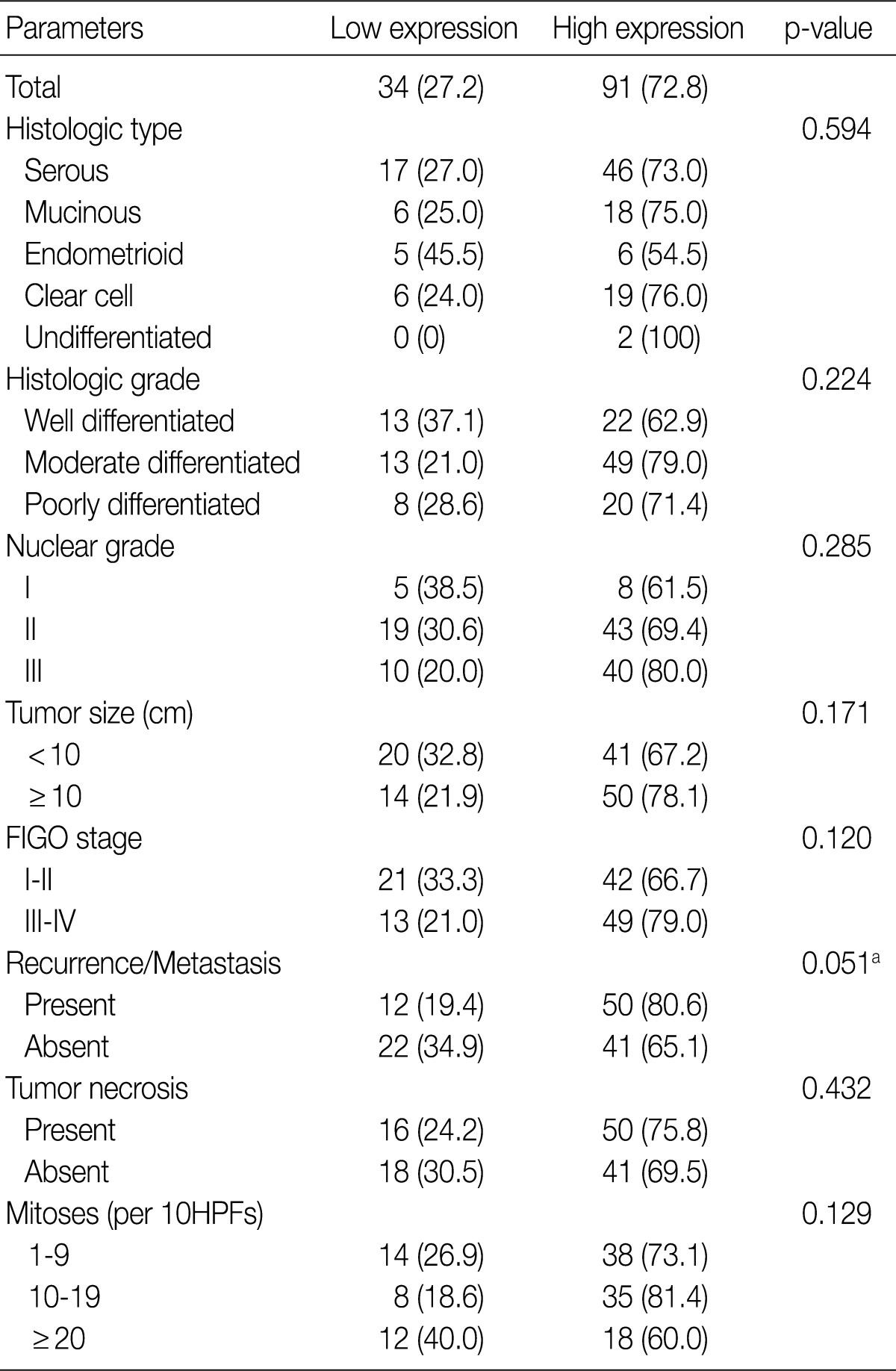
Table 2 summarizes the association of CA IX expression in OECs with clinicopathological parameters. High CA IX expression was associated with the histological tumor type, nuclear grade, and tumor necrosis. Mucinous and endometrioid carcinomas exhibited higher CA IX expression levels more frequently than serous and clear cell carcinomas (p=0.000). The expression of CA IX was lower when the nuclear grade was higher (p=0.029). Table 3 summarizes the association of GLUT-1 expression in OECs with clinicopathological parameters. GLUT-1 showed a positive correlation with tumor necrosis and mitosis. Correlation of immunohistochemical results of VEGF with clinicopathological parameters is summarized in Table 4. VEGF expression showed a positive correlation only with disease recurrence or metastasis. No statistical significance was observed between VEGF expression and the histologic type, histologic grade, nuclear grade, tumor size, FIGO stage, tumor necrosis, and mitosis (p>0.05).
Table 2.
Correlation between CA IX expression and clinicopathological characteristics in ovarian epithelial carcinomas
Values are presented as number (%).
Low expression, less than 10% positive staining of tumor cells; high expression, more than 10% positive staining of tumor cells.
CA IX, carbonic anhydrase IX; FIGO, Federation of Gynecology and Obstetrics; HPF, high power field.
aStatistically significance (Pearson's chi-squared test).
Table 3.
Correlation between GLUT-1 expression and clinicopathological characteristics in ovarian epithelial carcinomas
Values are presented as number (%).
Low expression, less than 10% positive staining of tumor cells; high expression, more than 10% positive staining of tumor cells.
GLUT-1, glucose transporter-1; FIGO, Federation of Gynecology and Obstetrics; HPF, high power field.
aStatistically significance (Pearson's chi-squared test).
Table 4.
Correlation between VEGF expression and clinicopathological characteristics in ovarian epithelial carcinomas
Low expression, less than 10% positive staining of tumor cells; high expression, more than 10% positive staining of tumor cells.
VEGF, vascular endothelial growth factor; FIGO, Federation of Gynecology and Obstetrics; HPF, high power field.
aStatistically significance (Pearson's chi-squared test).
Correlation between high expression of HRFs and OS
Follow-up was available for 125 patients (median, 38 months; range, 1 to 140 months). Thirty patients (24.0% of a total of 125 patients) died from the disease during the follow-up period. In univariate analyses, there were no significant correlations between high expressions of CA IX, GLUT-1, and VEGF and patient survival. Statistical significance was not seen (p>0.05), but a trend towards shorter OS in the high-expression groups was demonstrated. With respect to CA IX expression, the high-expression group showed a lower 5-year survival rate (YSR) compared to the low-expression group (61.0% vs. 69.0%, p=0.16). In the case of GLUT-1 expression, the high-expression group showed a significantly lower 5-YSR compared to the low-expression group (30.0% vs. 73.0%, p=0.244). The 5-YSR of the high VEGF expression group was lower than that of the low VEGF expression group (60% vs. 83%, respectively, p=0.110). However, these differences were not statistically significant.
We tried to explore whether the coexpression among these markers had any prognostic value. The high coexpression of CA IX/GLUT-1 was observed in 26 cases (20.8% of a total of 125 patients). The high coexpression of CA IX/GLUT-1 was significantly correlated with histologic type (p=0.012) and mitosis (p=0.019). Mucinous and endometrioid carcinomas were the most common type of cancers (37.5% vs. 36.4%, respectively). No correlation was observed between high CA IX/GLUT-1 coexpression and other clinical factors, such as tumor grade, nuclear grade, tumor size, FIGO stage, disease recurrence, and tumor necrosis. The high coexpression of CA IX/VEGF was seen in 40 patients (32% of total 125 patients). There was a significant correlation only between CA IX/VEGF coexpression and the histologic type (p=0.000). Similarly, a high coexpression of CA IX/VEGF was noted in mucinous and endometrioid carcinomas (62.5% vs. 45.5%, respectively). The high coexpression of CA IX/VEGF was associated with tumor stage (p=0.018), disease recurrence (p=0.018), tumor necrosis (p=0.008), and mitosis (p=0.021), but not with tumor grade, nuclear grade, or tumor size (p>0.05). There were 50 patients (40% of a total of 125 patients) who showed a high expression of both GLUT-1 and VEGF. In these cases, there were no correlations with any of the clinicopathological factors (p>0.05).
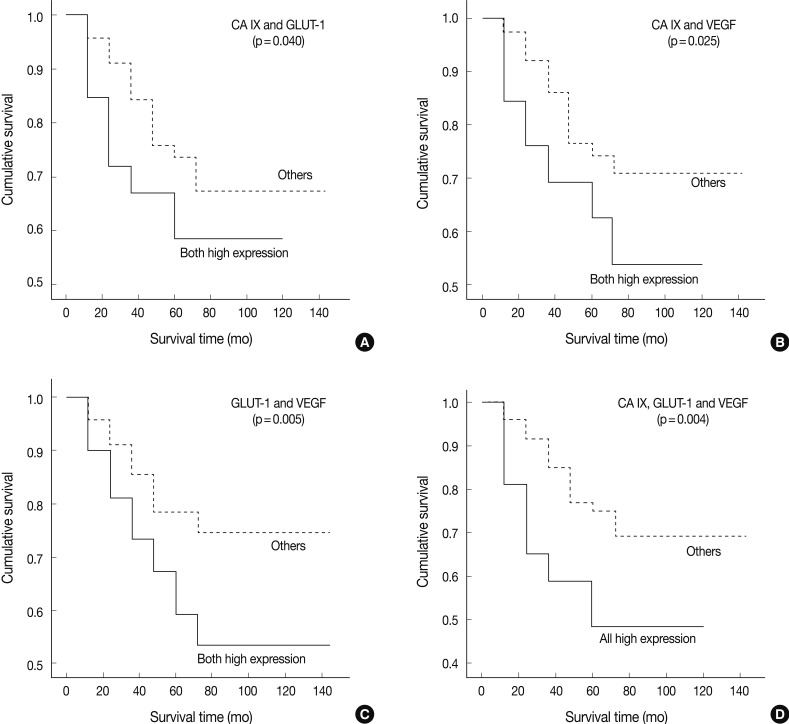
In univariate survival analyses, using the Kaplan-Meier survival curves and log rank test, all three categories showed a significantly negative prognostic impact on OS (Fig. 4). The 5-YSR according to each coexpression pattern was as follows (high expression of two factors vs. the others): CA IX/GLUT-1, 59.0% vs. 67.0%; CA IX/VEGF, 54.0% vs. 71.0%; and GLUT-1/VEGF, 53.0% vs. 74.0%, respectively.
Fig. 4.
Association of immunohistochemical staining for carbonic anhydrase IX (CA IX), glucose transporter-1 (GLUT-1), and vascular endothelial growth factor (VEGF) with overall survival of patients. (A) The Kaplan-Meier survival curves show significantly poor prognosis in patients with high expression of CA IX and GLUT-1 (p=0.040). (B) Both high expression of CA IX and VEGF (p=0.025). (C) Both high expression of GLUT-1 and VEGF (p=0.005). (D) High expression of all three: CA IX, GLUT-1, and VEGF (p=0.004).
DISCUSSION
Clinical investigations have clearly demonstrated that the prevalence of hypoxic tissue areas is a characteristic pathophysiological property of locally advanced solid tumors and a relevant factor of tumor pathophysiology. Tumor hypoxia has been found in a wide range of malignancies such as cancers of the breast, uterine cervix, vulva, head and neck, prostate, lung, and kidney.21,22 When tumors develop, they often become more malignant over time. Substantial data suggest that tumor hypoxia and the HIF-system are intensely involved in the processes conferring a growth advantage to tumor cells and the development of a more malignant phenotype.4 In addition, tumor hypoxia is classically associated with resistance to radiotherapy, and it has also been shown to decrease the efficacy of certain forms of chemotherapy. Evidence has accumulated showing that up to 50-60% of locally advanced solid tumors may exhibit hypoxic tissue areas that are heterogeneously distributed within the tumor mass. On this basis, there is convincing evidence of the clinical importance of tumor hypoxia.
HIF-1 has been known to be a key gene for adapting cells to microenvironmental conditions via the up-regulation of the transcription response to hypoxia.23 Through the HIF-1-mediated pathway, various endogenous hypoxia markers used for the estimation of the oxygenation status of total OECs are activated; among them, CA IX, GLUT-1, and VEGF have been known to play an important role.24,25 Previous studies have indicated that HIF-1α and its target genes act as positive regulators of tumor growth in many solid tumors, including OECs.26 We tried to analyze the association between the prognosis and the overexpression of HRFs, including CA IX, GLUT-1, and VEGF in various histological types of OECs.
Necrotic areas, most probably induced by hypoxia, were observed more frequently in tissue samples of poorly differentiated OECs, compared to those in tissue samples of well-differentiated OECs. The high expression of these markers was identified specifically in the perinecrotic zones that mark areas of avascularity and hypoxia and was correlated with tumor necrosis in this study. The distribution pattern of overexpression suggests that the oxygen level within the tumor regulates CA IX, GLUT-1, and VEGF expression in OECs.
Several studies evaluated the correlation of HRFs with the histological subtypes.15,27 In these reports, the patients were grouped into histologic subtypes based on the most frequent immunoreactivity: mucinous and endometrioid carcinomas expressing CA IX, clear cell and serous carcinomas expressing GLUT-1, and clear cell and mucinous carcinomas expressing VEGF. Our results showed that CA IX was overexpressed in a substantial proportion of mucinous and endometrioid type OECs and GLUT-1 overexpression was observed more frequently in serous and clear cell type OECs compared to that in the other types of OECs. One of the reasons for this is that each of these subtypes is reportedly associated with different molecular pathogenetic events and different responses.15 Therefore, it may be useful to evaluate the patient's prognosis using a single, hypoxic marker according to the histologic subtype of OECs. This suggests a specific application of each marker, namely, CA IX in mucinous and endometrioid carcinomas, and GLUT-1 in clear cell carcinomas. However, we could not establish that CA IX, GLUT-1, and VEGF were each correlated with OS in this study.
Some positive links have been drawn between the clinical outcome and the expression of these HRFs.15,16,27,28 GLUT-1 overexpression was demonstrated in OECs,15,16 and it was related to a shorter disease-free survival, while high VEGF expression predicted poor prognosis in OECs.28 Overexpression of CA IX was reported to be an unfavorable prognostic marker in endometrioid ovarian cancer in one study.27 In the present study, there were no significant associations between each of these markers and patients' outcomes. Even though the OS rate tended to decrease when each marker was highly expressed, it is questionable whether CA IX, GLUT-1, and VEGF can be used independently as prognostic markers in OECs. We also assessed the patients who were assigned to the coexpression groups according to the combination of the three markers. It was found that the survival rate of patients with combined high coexpression (of CA IX/GLUT-1, CA IX/VEGF, and GLUT-1/VEGF, respectively) was lower compared to those without. The high expression of all three markers had an increase detrimental effect on patient prognosis. Particularly, the coexpression of CA IX/GLUT-1 and CA IX/VEGF were related to shorter OS in mucinous and endometroid type OECs. CA IX/VEGF coexpression also showed a correlation with advanced tumor stage and tumor recurrence in mucinous and endometrioid type OECs. In the present study, there were no substantial, single predictors of HRF expression in OECs; instead, it appears that the coexpression of two markers may provide a better predictive value in OECs.
In conclusion, the expressions of HRFs differed based on the histologic type of OECs and HRFs, including CA IX, GLUT-1, and VEGF, which are not always independent prognostic factors in OECs when they are used individually, but the high coexpression of HRFs may provide useful prognostic information and have a significantly negative impact on patient survival. Identifying two or three HRFs and inhibiting CA IX, GLUT-1, and VEGF may be a key strategy for an attractive anticancer therapy, comprising of the combination of two or three markers.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murdoch WJ, McDonnel AC. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 2002;123:743–750. doi: 10.1530/rep.0.1230743. [DOI] [PubMed] [Google Scholar]
- 4.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 5.Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009;107:1053–1062. doi: 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- 6.Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(Suppl 5):31–40. doi: 10.1634/theoncologist.9-90005-31. [DOI] [PubMed] [Google Scholar]
- 7.Kaluz S, Kaluzová M, Liao SY, Lerman M, Stanbridge EJ. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochim Biophys Acta. 2009;1795:162–172. doi: 10.1016/j.bbcan.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrooz A, Ismail-Beigi F. Dual control of glut1 glucose transporter gene expression by hypoxia and by inhibition of oxidative phosphorylation. J Biol Chem. 1997;272:5555–5562. doi: 10.1074/jbc.272.9.5555. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW, Gao P, Dang CV. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007;26:291–298. doi: 10.1007/s10555-007-9060-4. [DOI] [PubMed] [Google Scholar]
- 10.Greijer AE, van der Groep P, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 14.Loncaster JA, Harris AL, Davidson SE, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 15.Yasuda M, Miyazawa M, Fujita M, et al. Expression of hypoxia inducible factor-1alpha (HIF-1alpha) and glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas: difference in hypoxic status depending on histological character. Oncol Rep. 2008;19:111–116. [PubMed] [Google Scholar]
- 16.Cantuaria G, Fagotti A, Ferrandina G, et al. GLUT-1 expression in ovarian carcinoma: association with survival and response to chemotherapy. Cancer. 2001;92:1144–1150. doi: 10.1002/1097-0142(20010901)92:5<1144::aid-cncr1432>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 18.Shen GH, Ghazizadeh M, Kawanami O, et al. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer. 2000;83:196–203. doi: 10.1054/bjoc.2000.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000;19:7–15. doi: 10.1097/00004347-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa M, Yasuda M, Fujita M, et al. Therapeutic strategy targeting the mTOR-HIF-1alpha-VEGF pathway in ovarian clear cell adenocarcinoma. Pathol Int. 2009;59:19–27. doi: 10.1111/j.1440-1827.2008.02320.x. [DOI] [PubMed] [Google Scholar]
- 21.Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res. 2001;21:4317–4324. [PubMed] [Google Scholar]
- 22.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- 25.Jubb AM, Pham TQ, Hanby AM, et al. Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol. 2004;57:504–512. doi: 10.1136/jcp.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 27.Choschzick M, Oosterwijk E, Müller V, et al. Overexpression of carbonic anhydrase IX (CAIX) is an independent unfavorable prognostic marker in endometrioid ovarian cancer. Virchows Arch. 2011;459:193–200. doi: 10.1007/s00428-011-1105-y. [DOI] [PubMed] [Google Scholar]
- 28.Duncan TJ, Al-Attar A, Rolland P, et al. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res. 2008;14:3030–3035. doi: 10.1158/1078-0432.CCR-07-1888. [DOI] [PubMed] [Google Scholar]