Abstract
Background/Aims
Primary biliary cirrhosis (PBC) is a slowly progressing autoimmune disease of the liver that is characterized by portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. Serum total bilirubin is one of the various prognostic factors that have been proposed. A recent study found that PBC with accompanying autoimmune hepatitis (AIH) carries a negative prognosis. This study examined the clinical characteristics of PBC and analyzed the factors that affect its prognosis.
Methods
Patients diagnosed with PBC between January 1998 and December 2010 based on clinical and histopathological findings were compiled and analyzed retrospectively.
Results
Among 27 patients, 24 (1 male and 23 females, ages 50.0±9.3 years) were followed up. The follow-up period was 8.6±0.9 years. Of the 24 patients, 9 patients progressed to liver cirrhosis (LC). Comparison between patients who did and did not progress to LC revealed statistically significant differences in the patients' serum total bilirubin (2.7±1.8 vs. 0.8±0.4, P=0.012), the Mayo risk score (5.1±0.7 vs. 3.9±0.6, P=0.001), the revised IAHG (International Autoimmune Hepatitis Group) score (9.2±2.3 vs. 5.4±3.0, P=0.004) and frequency of AIH overlap (5/9 [55.6%] vs. 0/15 [0%], P=0.001) at the time of diagnosis.
Conclusions
We propose that serum total bilirubin, the Mayo risk score, and the revised IAHG score at the time of diagnosis are helpful for predicting PBC prognosis. In particular, since all of the patients with accompanying AIH progressed to LC, the presence of overlap syndrome at the time of diagnosis is helpful for predicting PBC prognosis and providing an adequate treatment.
Keywords: Primary biliary cirrhosis, Prognosis, Overlap syndrome
INTRODUCTION
Primary biliary cirrhosis (PBC) is a slowly progressive autoimmune disease of the liver that primarily affects women. In terms of pathology, PBC is characterized by portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. Fifty to 60 percent of patients are asymptomatic at diagnosis.1-3 However, if present, fatigue and pruritus are the most commonly presenting symptoms. Histologically, destruction and loss of bile duct epithelial cells occurs, and reactions with T cells, B cells, macrophages, eosinophiles, and natural killer cells accompany severe inflammation of intrahepatic bile ducts, which progresses to liver cirrhosis (LC) and hepatic failure.4,5 PBC is mostly found in patients in Northern Europe, the United Kingdom, and the northern United States; the global prevalence is reported to be 6.7-400 in one million people.1,6-8 In Asia, Japan is the only country with a known prevalence of PBC, at 27-54 per million.8,9 As of yet, no study has been conducted on the prevalence of PBC in Korea. In 2004, the Korean Association for the Study of the Liver led a nationwide multi-center autoimmune liver disease study, in which 228 cases were examined to survey clinical characteristics.8
A study conducted in 1979 claimed that when total bilirubin, a prognostic factor of PBC, is 10 mg/dL or greater, the average survival period is 1.4 years.10 The Mayo Clinic published a study that excluded the invasive technique of biopsy, and predicted prognosis based only on clinical and biochemical factors, including age, serum bilirubin level, serum albumin level, prothrombin time, and existence of peripheral edema.11 In Korea, Kim et al reported that factors such as total bilirubin, the age at the time of diagnosis, and the Mayo score affect the survival rate.8 In recent studies, it was shown that concurrent autoimmune hepatitis (AIH) can result in rapid progression toward cirrhosis and liver failure in PBC patients. 12,13 The prevalence of typical PBC possessing features of AIH has been reported to range from 5% to 19%.14-16 A scoring system has been proposed by the International Autoimmune Hepatitis Group (IAHG) for the diagnosis of AIH.17,18 The use of a revised IAHG scoring system as a diagnostic tool for identifying overlap of AIH and PBC has been evaluated.11,12
The aim of this study was to analyze the factors that affect the clinical characteristics and the prognosis of patients diagnosed with PBC at a single center.
PATIENTS AND METHODS
Patients
The subjects of this study were 27 patients diagnosed with PBC at Soonchunhyang University Hospital, Seoul, from January 1998 to December 2010. PBC was diagnosed based on liver function tests, presence of serum antimitochondrial antibody (AMA), and histopathological findings. The diagnosis of PBC was defined as having chronic cholestatic liver disease for greater than 6 months, possessing a positive AMA titer ≥1:40, having liver histology with features consistent with, or diagnostic of PBC, and the exclusion of other etiologies for chronic liver disease, including viral hepatitis, biliary obstruction, drug-induced cholestatic disease, nonalcoholic fatty liver disease, hemochromatosis, Wilson's disease, and α1-antitrypsin deficiency. Patients having viral hepatitis B and C were excluded. Among 27 patients, 24 have had follow-up monitoring to the present day. Finally, 24 patients were enrolled in this study.
Methods
Subject patients' data were retrospectively collected for analysis, including age, gender, AMA, ANA (antinuclear antibody), SMA (smooth muscle antibody), IgM (immunoglobulin M), IgG (immunoglobulin G), AST (aspartate transaminase), ALT (alanine transaminase), ALP (alkaline phosphatase), r-GT (gamma glutamyl transpeptidase), total bilirubin, PT (prothrombin time), HBsAg (hepatitis B surface antigen), anti-HCV (antihepatitis C virus) and histopathological findings. ANA and AMA titers of 1:40 or greater were deemed positive. All patients had liver biopsy at the time of first evaluation.
The histological stages were determined according to Ludwig's classiflcation.19 Briefly, stage 1 was characterized by inflammatory destruction of intrahepatic small bile ducts, stage 2 by proliferation of bile ductules and/or piecemeal necrosis, stage 3 by flbrosis and/or bridging necrosis, and stage 4 by cirrhosis.
Based on the test results at the time of diagnosis, the Mayo risk score and the revised IAHG score were calculated. The Mayo risk score is designed to overcome the limitations of liver biopsy by taking into account only clinical and biochemical variables. The score is calculated using age, total bilirubin, albumin, PT and existence of peripheral edema. The revised IAHG score highlights the importance of the ALP/AST ratio and histological findings. The score is calculated by evaluating 15 items, including ANA, AMA, IgG level, hepatitis viral marker, drug history, alcohol intake and gender (specifically, female). The identification of definite AIH overlap is based on an aggregate score of >15 points. A score between 10 and 15 denotes the presence of probable AIH overlap.
Patients were evaluated laboratory study per three months, abdominal ultrasonography (USG) per six months and esophagogastroduodenoscopy (EGD) per 1 year during follow-up. All patients were treated with Ursodeoxycholic acid (UDCA) in doses of 13-15 mg/kg per day and seven patients also were treated with prednisolon once daily with or without azathioprine 50 mg once daily.
Presence of cirrhosis was established by clinical criteria (ascites, esophageal varices, hepatic encephalopathy, prolonged PT, thrombocytopenia, and hypoalbuminemia) and morphologic criteria (computed tomography or USG). The USG findings for cirrhosis are nodularity and irregular surface of the liver. There is also coarse echo-texture of the liver with increased echogenicity.
Statistical analysis
SPSS 18.0 version was used for statistical analysis, and quantitative variables were expressed in terms of average and standard deviation. Results were elicited using technical statistics and cross tabulation. Group comparisons for continuous and non-continuous variables were performed using the Mann-Whitney U-test, and the Fisher's exact test, respectively. Cox proportional hazards regression was used to estimate multivariable HR. Statistical significance was determined for cases whose p value was 0.05 or less.
RESULTS
Clinical characteristics of the patients
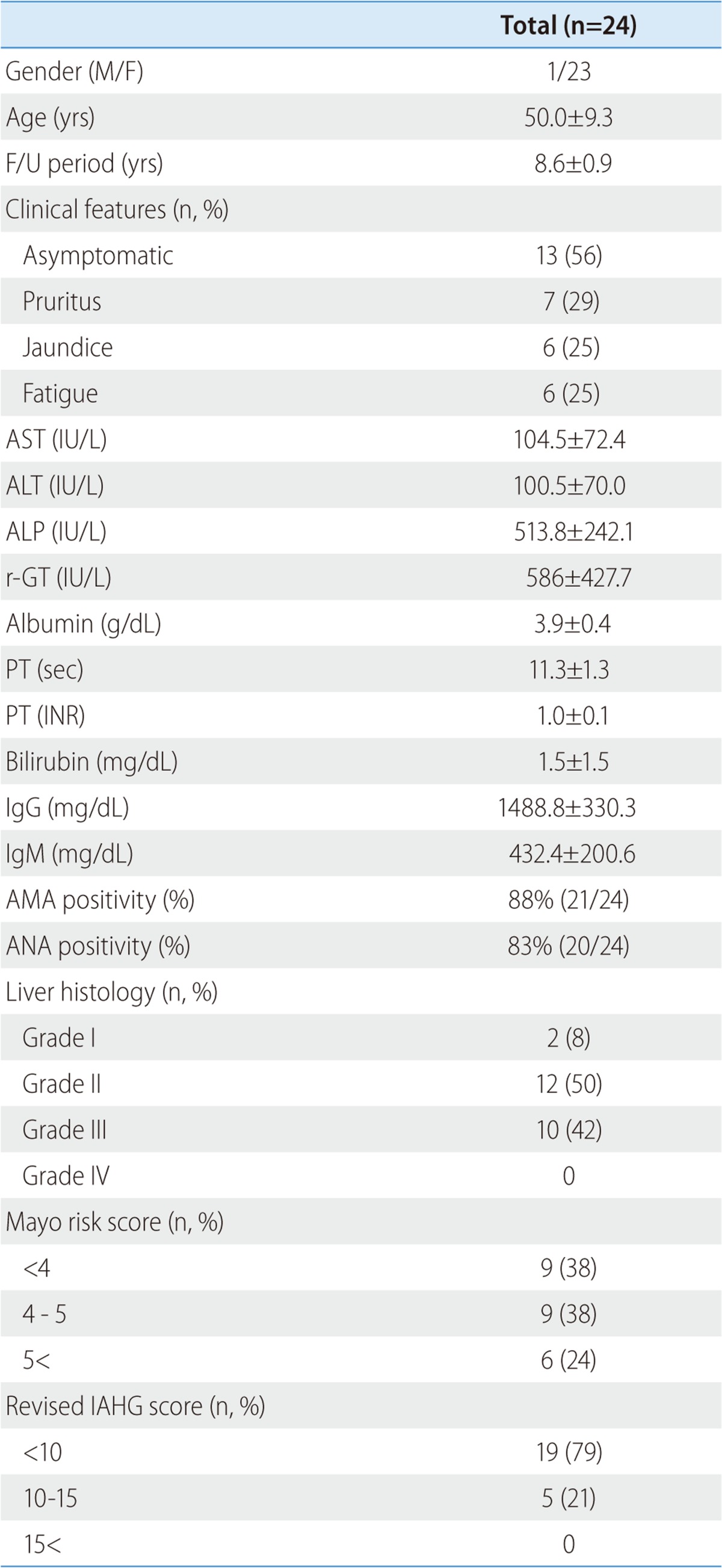
Of the 27 patients who participated in this study, 24 (1 male and 23 female with mean age of 50.0±9.3) have been followed and monitored thus far. The average follow-up period is 8.6±0.9 years. At the time of diagnosis, 13 (56%) were asymptomatic, 7 (29%) suffered from pruritus, 6 (25%) showed signs of jaundice, and 6 (25%) complained of fatigue among 24 patients. The levels of AST, ALT, ALP, and r-GT were 104.5±72.4 IU/L, 100.5±70.0 IU/L, 513.8±242.1 IU/L, and 586±427.7 IU/L, respectively. The levels of albumin, PT, INR (international normalized ratio), and total bilirubin were 4.1±0.4 g/dL, 11.2±1.0 sec, 1.0±0.1 unit, and 1.5±1.5 mg/dL, respectively. Average IgG and IgM were 1488.8±330.3 mg/dL (normal range 870-1700) and 432.4±200.6 mg/dL (35-220), respectively. AMA and ANA positivity was 88% and 83%, respectively (Table 1).
Table 1.
Clinical characteristics of the patients
Data are presented as n (%) or mean±SD.
AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkalaine phosphatase; r-GT, gamma glutamyl transpeptidase; PT, prothrombin time; INR, international normalized ratio; IgG, immunoglobulin G; IgM, immunoglobulin M; AMA, antimitochondrial antibody; ANA, antinuclear antibody; IAHG, International Autoimmune Hepatitis Group.
Factors related to progression of PBC
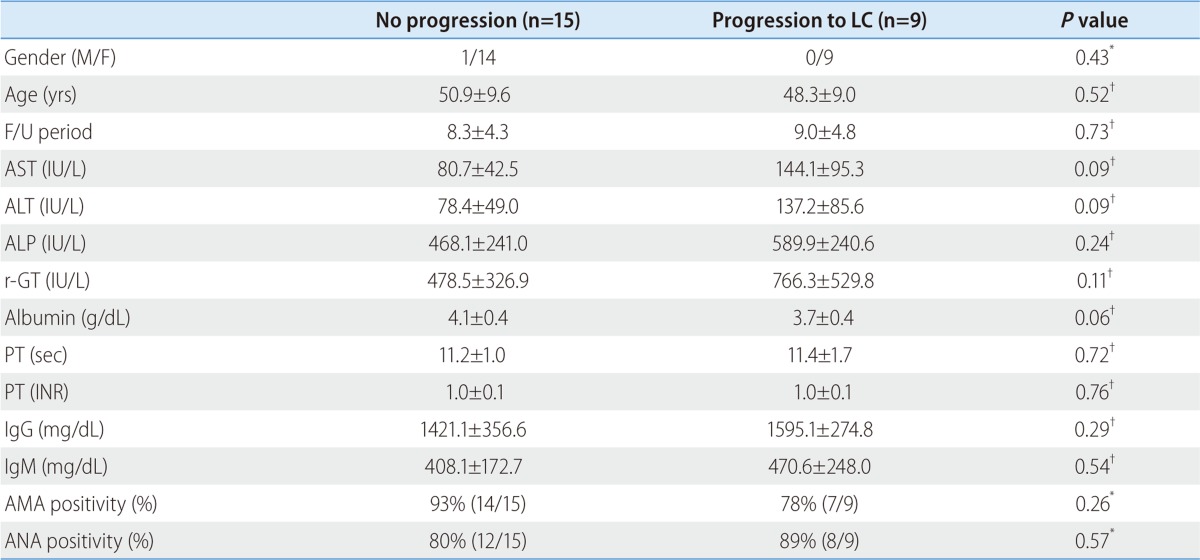
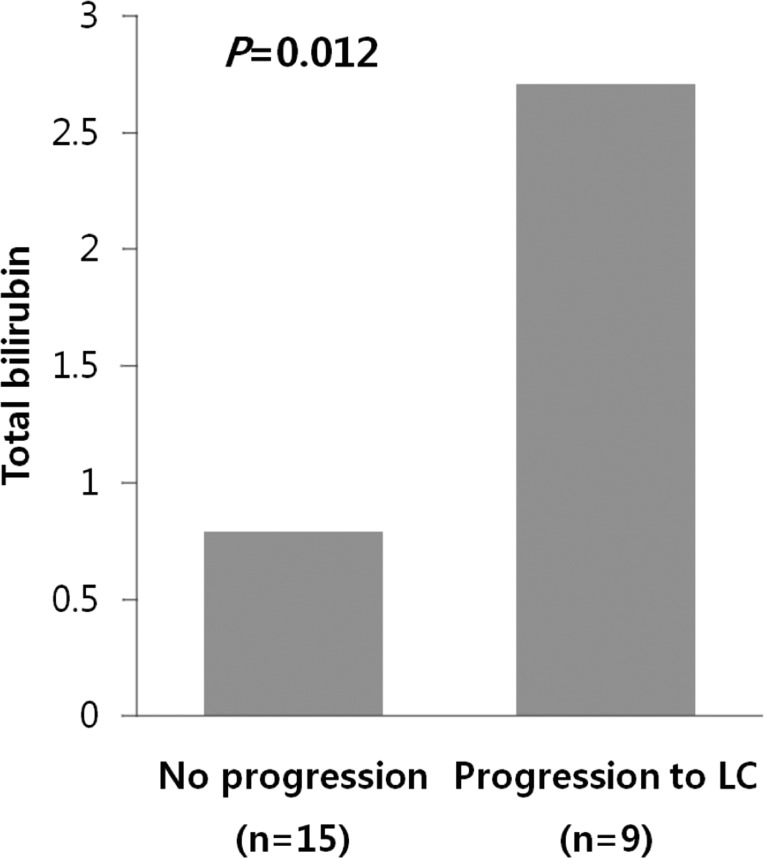
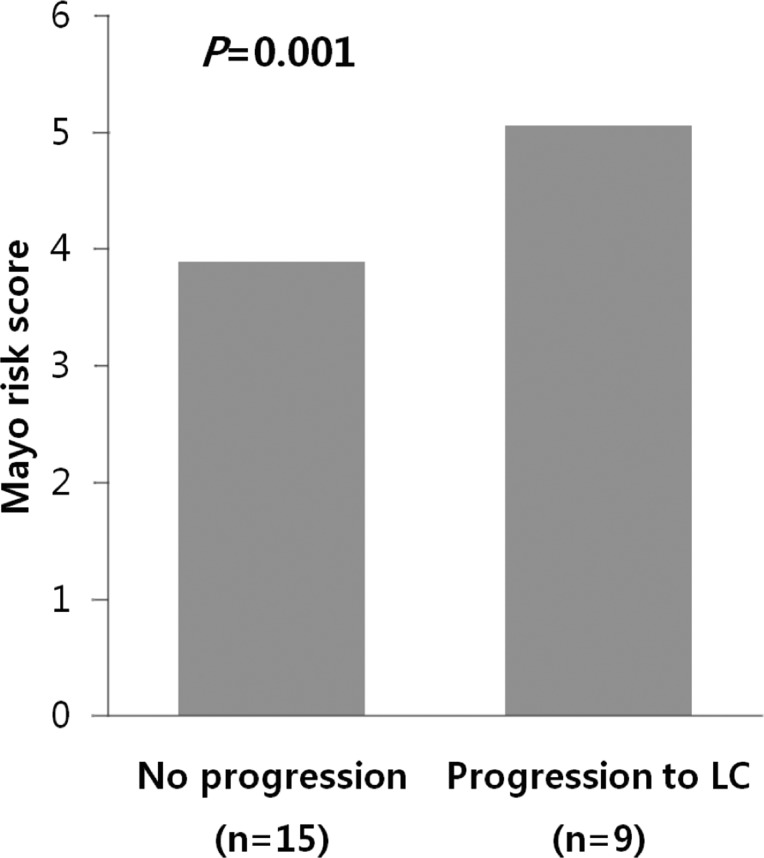
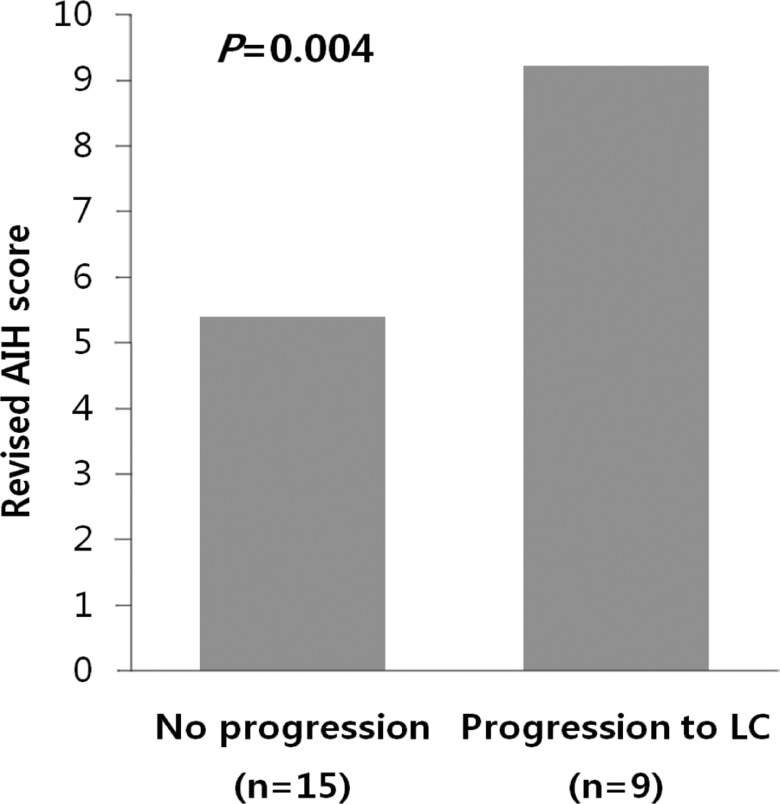
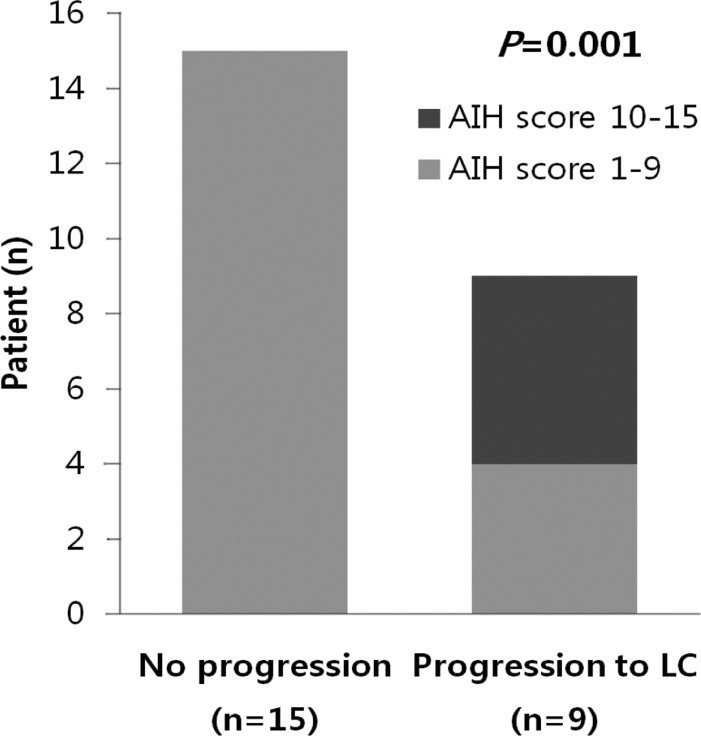
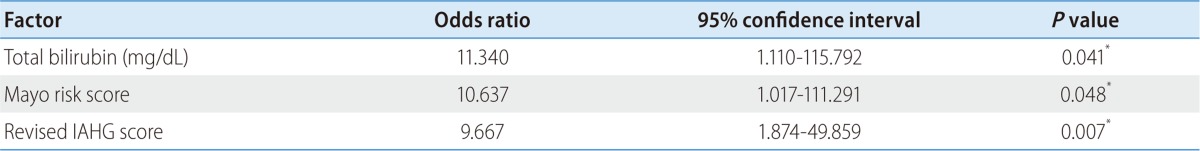
Among 24 patients, nine progressed to LC. There were no differences in clinical traits, such as gender, age, AST, ALT, ALP, r-GT, albumin, PT, IgG, and IgM, between the progressed and non-progressed groups (Table 2). In terms of total bilirubin, the progressed group (2.7±1.8) was significantly higher than the non-progressed group (0.8±0.4) (P=0.012) (Fig. 1). The Mayo risk score for the non-progressed group was 3.9±0.6, and that of the progressed group was significantly higher at 5.1±0.7 (P=0.001) (Fig. 2). The revised IAHG score of the non-progressed group was 5.4±3.0, and that of the progressed group was significantly higher at 9.2±2.3 (p=0.004) (Fig. 3). Among nine PBC patients whose case progressed to LC, five showed AIH overlap, and the frequency of AIH occurrence was significantly higher than in the non-progressed group, in which no patients had accompanying AIH (progressed group vs. non-progressed group: 5/9 [55.6%] vs. 0/15 [0%], P=0.001) (Fig. 4). All variables showing statistical significance in univariate analysis were introduced into multivariate analysis. Total bilirubin ≥1.3 mg/dL (P=0.041), the Mayo risk score ≥4.5 (P=0.048), and the revised IAHG score ≥10 (P=0.007) were considered as significant predictors (Table 3).
Table 2.
Clinical characteristics of the patients relative to the progression of PBC
Data are presented as n (%) or mean±SD.
*Statistical significance was calculated by the Fisher's exact test.
†Statistical significance was calculated by the Mann-Whitney U-test.
AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkalaine phosphatase; r-GT, gamma glutamyl transpeptidase; PT, prothrombin time; INR, international normalized ratio; IgG, immunoglobulin G; IgM, immunoglobulin M; AMA, antimitochondrial antibody; ANA, antinuclear antibody.
Figure 1.
Serum bilirubin relative to the progression of PBC. Serum total bilirubin was significantly higher in the progressed group (2.7±1.8) than in the unprogressed group (0.8±0.4; P=0.012, Mann-Whitney U test).
Figure 2.
The risk score of the Mayo clinic model relative to the progression of PBC. The Mayo score was significantly lower in the unprogressed group (3.9±0.6) than in the progressed group (5.1±0.7; P=0.001, Mann-Whitney U test).
Figure 3.
AIH score relative to the progression of PBC. The revised IAHG score was significantly lower in the unprogressed group (5.4±3.0) than in the progressed group (9.2±2.3; P=0.004, Mann-Whitney U test).
Figure 4.
Frequency of overlapping AIH relative to the progression of PBC. Among nine PBC patients whose case progressed to LC, five exhibited overlap with AIH, and the prevalence of AIH was significantly higher among these progressed patients than among the unprogressed group, in which no patients had accompanying AIH (P=0.001, Fisher's exact test).
Table 3.
Multivariate analysis of the prognostic factors
*Statistical significance was calculated by the Cox proportional hazards regression.
IAHG, International Autoimmune Hepatitis Group.
In this study, there was one case of liver transplantation because the patient showed progress to LC and hepatic failure after being diagnosed with PBC. The case involved a 38-year-old female patient who visited our hospital because of jaundice. At the time of the initial visit, the total bilirubin, Mayo score, and AIH scores were 4.0 mg/dL, 6.5, and 12, respectively. The results of the liver biopsy performed in November 2004 revealed stage 3 PBC. As the patient was administered UDCA 900 mg daily and was being followed up as outpatient, symptoms of jaundice became increasingly more severe. A follow-up liver biopsy was performed in May 2007, and the findings showed that the disease had progressed to stage 4 PBC. On multiple occasions, the patient suffered from hepatic encephalopathy, a complication of LC, and received liver transplantation in May 2010.
DISCUSSION
Owing to easy administration of biochemical tests such as quantification of AST, ALT, total bilirubin, ALP, and r-GT, as well as other forms of tests, including AMA, IgM, and IgG, PBC is often diagnosed in its early stages. Common symptoms of PBC include fatigue and pruritus; however, more than 50% of cases are asymptomatic, and frequently occur in middle-aged women.1,11 In our study, 54% of patients did not display any symptoms, and 29% complained of pruritus. Ninety-six percent of the patients were female and the mean age was 50.0±9.3, which were similar to previous studies. In earlier studies, AMA and ANA were reported to be 88-90% and 50-63%, respectively.1,8,20 In this study, AMA was 88%, which was similar to earlier studies, and ANA was 83%, somewhat higher than in earlier studies.
The importance of this study is that total bilirubin, the Mayo risk score, and the revised IAHG score were significantly important as prognostic factors of PBC.
In terms of prognostic indicators, the level of serum bilirubin concentration is the best indicator for prognostic purposes. Pretreatment levels of serum bilirubin, bilirubin levels during follow-up, and the occurrence of normal levels of serum bilirubin were significantly associated with prognosis.21 Previous studies reported the duration of increased initial serum bilirubin, as well as the level of bilirubin, to be important prognostic factors. When serum bilirubin is 10 mg/dL or greater, the average survival period is reported to be 1.4 years.10 Consistent with previous studies, our study found that the total bilirubin in the patient group who had progressed to LC was 2.7±1.8 years, which was significantly higher than that of the non-progressed group at 0.8±0.4 (P=0.012).
A number of studies have reported that the Mayo risk score is an effective means of predicting prognoses of PBC patients. Some have claimed that cases with Mayo risk scores of 4.5 or lower reacted favorably to treatment,22 while others reported that a high Mayo risk score has negative consequences for the survival rate.23 In our study, the Mayo score of the progressed group was 5.1±0.7, which was significantly higher than the non-progressed group's score of 3.9±0.6 (P=0.001).
The simultaneous coexistence of PBC and AIH in the same patient has been described as overlap syndrome.24,25 On account of this, the diagnosis of PBC-AIH overlap syndrome remains challenging, especially with no current consensus on diagnositic criteria.26 A scoring system has been proposed by the IAHG for the diagnosis of definite AIH,17 and the use of a revised IAHG scoring system as a diagnostic tool for identifying overlap of PBC and AIH was evaluated recently.12,15,18 Papamichalis et al demonstrated that the specificity of the revised IAHG score was very high at 98.1%.27 Lee et al applied the revised IAHG score to AIH patients in Korea and reported high sensitivity and specificity of 90.9% and 96.2%, respectively.28 In our study, the revised IAHG score of the group that had progressed to LC was 9.2±2.3, significantly higher than the non-progressed group's score of 5.4±3.0 (P=0.004). Since all five AIH-overlapping cases progressed to LC, existence of the overlap syndrome during diagnosis is likely to help predict the prognosis of PBC and provide adequate means of treatment. Patients with features of PBC-AIH overlap had greater risk of cirrhosis, varices, ascites, portal hypertension, transplantation, therapy death.12
UDCA is a safe and life-extending therapye for most patients with PBC,29 whereas immunosuppressive therapy markedly improves survival in patients with AIH.29,30 To date, it is generally accepted that patients with PBC and AIH overlap should receive UDCA, but it is unclear if the degree of AIH overlap in these cases can justify the addition of immunosuppressive therapy.12
All 24 patients in our study received UDCA treatment, and seven also received immunosuppressive therapy. Despite immunosuppressive therapy, the seven patients progressed to LC. We attribute this to the fact that immunosuppressive therapy did not begin immediately upon diagnosis of PBC-AIH overlap syndrome, but after its progression. However, prospective research is necessary to investigate this issue.
Thanks to advances in testing methodology, many cases of PBC are diagnosed early in the asymptomatic phase, and early diagnosis of PBC provides a much better prognosis. UDCA is effective in reducing total bilirubin, ALP, AST, ALT, IgM, and cholesterol, and in delaying the progress of the disease. In addition, early diagnosis and treatment of PBC is important because early administration of UDCA is effective in delaying development of hepatic fibrosis and esophageal varix.1,29,31,32
Hirshfield et al recently reported that PBC is associated with the mutation of genes such as HLA, IL12A, IL12RB2 and IRF5-TNPO3.33-35 George et al also reported correlation of PBC with 12 new genes, including STAT4, DENND1B and CD80.36 Active genetic research is expected to provide significant achievements for prevention and early diagnosis of PBC.
This research had limitations in terms of data compilation and evaluating long-term prognosis and prognostic factors because it was a retrospective small-scale study conducted with 24 patients, over an average follow-up period of 8.6±0.9 years. Therefore, prospective research involving multiple facilities is necessary in the future.
In our study, total bilirubin, the Mayo risk score, and the revised IAHG score were significantly important as prognostic factors of PBC. In particular, since all cases that accompanied AIH progressed to LC, existence of the overlap syndrome at the time of diagnosis is believed to be significantly helpful for predicting the prognosis of, and providing treatment for, PBC.
Abbreviations
- AIH
autoimmune hepatitis
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AMA
antimitochondrial antibody
- ANA
antinuclear antibody
- anti-HCV
antihepatitis C virus
- AST
aspartate transaminase
- HBsAg
hepatitis B surface antigen
- IAHG
International Autoimmune Hepatitis Group
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- INR
international normalized ratio
- LC
liver cirrhosis
- PBC
primary biliary cirrhosis
- PT
prothrombin time
- r-GT
gamma glutamyl transpeptidase
- SMA
smooth muscle antibody
- UDCA
Ursodeoxycholic acid
Footnotes
The authors have no conflicts to disclose.
References
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003;362:53–61. doi: 10.1016/S0140-6736(03)13808-1. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari BM, Bayat H, Rothstein KD. Primary biliary cirrhosis. Gastroenterol Clin North Am. 2011;40:373–386. doi: 10.1016/j.gtc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Jones DE. Pathogenesis of primary biliary cirrhosis. Gut. 2007;56:1615–1624. doi: 10.1136/gut.2007.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470–475. doi: 10.1053/j.gastro.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 7.Metcalf J, James O. The geoepidemiology of primary biliary cirrhosis. Semin Liver Dis. 1997;17:13–22. doi: 10.1055/s-2007-1007179. [DOI] [PubMed] [Google Scholar]
- 8.Kim KA, Jeong SH, Lee JI, Yeon JE, Lee HJ, Kwon SY, et al. Clinical features and prognosis of primary biliary cirrhosis in Korea. Korean J Hepatol. 2010;16:139–146. doi: 10.3350/kjhep.2010.16.2.139. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K, Hirohara J, Nakano T, Seki T, Sasaki H, Higuchi K, et al. Prediction of prognosis of primary biliary cirrhosis in Japan. Liver. 1995;15:70–77. doi: 10.1111/j.1600-0676.1995.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20:137–140. doi: 10.1136/gut.20.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parés A, Rodés J. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2003;7:779–794. doi: 10.1016/s1089-3261(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 12.Silveira MG, Talwalkar JA, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long-term outcomes. Am J Gastroenterol. 2007;102:1244–1250. doi: 10.1111/j.1572-0241.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 13.Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, et al. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345–353. doi: 10.1038/ajg.2009.616. [DOI] [PubMed] [Google Scholar]
- 14.Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 15.Talwalkar JA, Keach JC, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: an evaluation of a modified scoring system. Am J Gastroenterol. 2002;97:1191–1197. doi: 10.1111/j.1572-0241.2002.05703.x. [DOI] [PubMed] [Google Scholar]
- 16.Poupon R, Chazouilleres O, Corpechot C, Chrétien Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology. 2006;44:85–90. doi: 10.1002/hep.21229. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis) Virchows Arch A Pathol Anat Histol. 1978;379:103–112. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Lindor KD, Locke GR, 3rd, Therneau TM, Homburger HA, Batts KP, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631–1636. doi: 10.1053/gast.2000.20197. [DOI] [PubMed] [Google Scholar]
- 21.van Hoogstraten HJ, Hansen BE, van Buuren HR, ten Kate FJ, van Berge-Henegouwen GP, Schalm SW Dutch Multi-Centre PBC Study Group. Prognostic factors and long-term effects of ursodeoxycholic acid on liver biochemical parameters in patients with primary biliary cirrhosis. J Hepatol. 1999;31:256–262. doi: 10.1016/s0168-8278(99)80222-0. [DOI] [PubMed] [Google Scholar]
- 22.Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 1999;19:115–121. doi: 10.1111/j.1478-3231.1999.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 23.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 24.Czaja AJ. The variant forms of autoimmune hepatitis. Ann Intern Med. 1996;125:588–598. doi: 10.7326/0003-4819-125-7-199610010-00009. [DOI] [PubMed] [Google Scholar]
- 25.Poupon R. Autoimmune overlapping syndromes. Clin Liver Dis. 2003;7:865–878. doi: 10.1016/s1089-3261(03)00092-8. [DOI] [PubMed] [Google Scholar]
- 26.Czaja AJ. Overlap syndrome of primary biliary cirrhosis and autoimmune hepatitis: a foray across diagnostic boundaries. J Hepatol. 2006;44:251–252. doi: 10.1016/j.jhep.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Papamichalis PA, Zachou K, Koukoulis GK, Veloni A, Karacosta EG, Kypri L, et al. The revised international autoimmune hepatitis score in chronic liver diseases including autoimmune hepatitis/overlap syndromes and autoimmune hepatitis with concurrent other liver disorders. J Autoimmune Dis. 2007;4:3. doi: 10.1186/1740-2557-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YN, Kim YS, Kim SG, Lim JH, Jeong SW, Jang JY, et al. Diagnostic Value and Utility of the Simplified International Autoimmune Hepatitis Group (IAIHG) Criteria for Autoimmune Hepatitis in Korea. Korean J Med. 2011;81:340–350. [Google Scholar]
- 29.Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 30.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 31.Shi J, Wu C, Lin Y, Chen YX, Zhu L, Xie WF. Long-term effects of mid-dose ursodeoxycholic acid in primary biliary cirrhosis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:1529–1538. doi: 10.1111/j.1572-0241.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- 32.Poupon RE, Bonnand AM, Chrétien Y, Poupon R The UDCA-PBC Study Group. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. Hepatology. 1999;29:1668–1671. doi: 10.1002/hep.510290603. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C, et al. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42:655–657. doi: 10.1038/ng.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschfield GM, Xie G, Lu E, Sun Y, Juran BD, Chellappa V, et al. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes Immun. 2012;13:328–335. doi: 10.1038/gene.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, et al. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329–332. doi: 10.1038/ng.789. [DOI] [PMC free article] [PubMed] [Google Scholar]