Abstract
The most common finding related to nonalcoholic steatohepatitis is obesity, but a status of severe malnutrition can also induce the steatohepatitis. The authors report a rare case of steatohepatitis leading to hepatic decompensation caused by malnutrition after pancreaticoduodenectomy. A 68-year-old female patient who had been previously diagnosed with pancreatic cancer and had undergone pancreaticoduodenectomy 5 months previously presented with abdominal distension. Routine CT performed 3 months after the surgery revealed severe fatty liver without evidence of tumor recurrence. After undergoing pancreaticoduodenectomy her food intake had reduced, and as a result she had lost 7 kg of body weight over 2 months. At this admission, CT revealed moderate amounts of ascites without tumor recurrence. Furthermore, her albumin and lipid profile levels were markedly decreased, and she had a flapping tremor and slurred speech suggestive of hepatic encephalopathy. Her liver biopsy findings were consistent with steatohepatitis and disclosed macrovesicular steatosis without definite fibrosis. After careful nutritional control, her symptoms disappeared and her laboratory findings improved.
Keywords: Nonalcoholic steatohepatitis, Malnutrition, Pancreaticoduodenectomy, Pancreatic cancer, Hepatic decompensation
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is increasingly being recognized as the hepatic manifestation of insulin resistance and the systemic complex known as metabolic syndrome.1,2 Nonalcoholic steatohepatitis (NASH) is a progressive form of NAFLD, which has been documented to have the potential to progress to cirrhosis and hepatocellular carcinoma.1,3 A diagnosis of steatosis is made when lipid deposition exceeds 5% of hepatocytes, whereas involvement of more than 50% is called fatty liver. Steatohepatitis is diagnosed when both inflammatory infiltrates of mixed cells and liver cell ballooning are detected.4 The majority of NASH cases arise in association with obesity, insulin resistance, hypertension, and diabetes, and these factors have also been associated with a high risk of developing cirrhosis.5
However, a poor nutritional status, such as, in patients with an eating disorder, postoperative gastrointestinal cancer patients, and children with kwashiorkor, could induce hepatocellular injury.6-8 Furthermore, under these heterogeneous clinical circumstances, an increase in liver enzymes and a fatty liver change are common6-8 and severe acute liver damage can develop.9-11 It has been reported that more than 10% of patients with anorexia nervosa has an elevated aminotransferase, and several case reports have described severe hepatic failure.6,9-11 In addition, malnutrition is prevalent among surgical gastrointestinal cancer patients and prolonged total parenteral nutrition can induce steatohepatitis.7,12 In particular, patients that have undergone pancreaticoduodenectomy have a high risk of de novo NAFLD development.13-15 Here, we report an unusual case of malnutrition induced steatohepatitis with hepatic decompensation after pancreaticoduodenectomy for pancreas cancer.
CASE REPORT
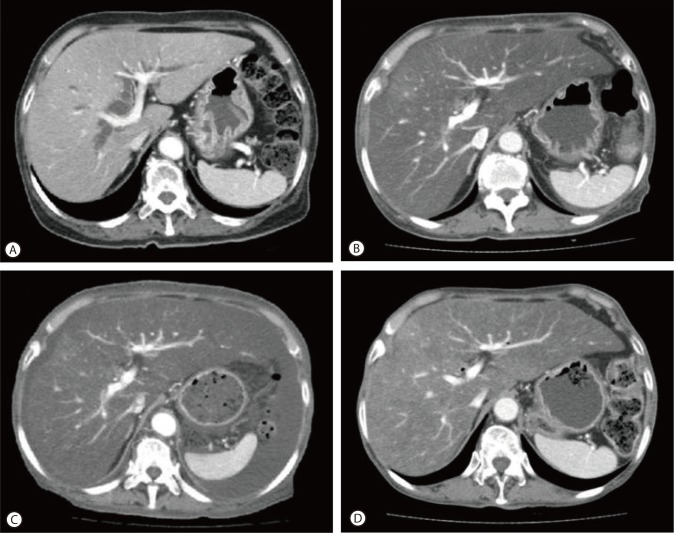
A 68-year-old female patient presented with abdominal distension of one month duration and slurred speech for one day. She had been diagnosed with pancreatic cancer 5 months previously, and had subsequently undergone pancreaticoduodenectomy. In the operation field, the pancreatic consistency was intermediate and pancreatic resection line was conducted on, but not including, the portal vein. During operation, no hypotension or massive bleeding was encountered. The surgical pathologic finding was ductal adenocarcinoma and complete tumor resection (safety margins of 2 and 3 cm from the duodenum and common bile duct, respectively). However, since pancreaticoduodenectomy, she suffered a loss of appetite and consequently her body weight reduced from 51 kg to 44 kg over 3 months (body mass index from 22.1 to 19.0 kg/m2), even though she had experienced no postoperative diarrhea or obstructive gastrointestinal symptoms. A routine check of pancreas by dynamic CT at three months after surgery revealed severe fatty liver but no tumor recurrence (Fig. 1B). At that time, the CT value of liver parenchyma had decreased from 50 Hounsfield units (HU) by preoperative CT to -30 HU.
Figure 1.
CT findings. (A) Preoperative CT scan showed normal liver parenchyma at the time of pancreatic cancer diagnosis (CT value of the liver parenchyma: 50 HU). (B) Follow-up CT scan obtained 3 months after pancreaticoduodenectomy, showed marked fatty liver (CT value of the liver parenchyma: -30 HU) but no evidence of tumor recurrence. (C) CT scan of the liver obtained at admission, revealed marked fatty liver and newly developed moderate amounts of ascites. (D) After 3 months of nutritional support, a follow-up CT showed some regression of the fatty liver (CT value of the liver parenchyma: 10 HU) and near complete regression of the ascites.
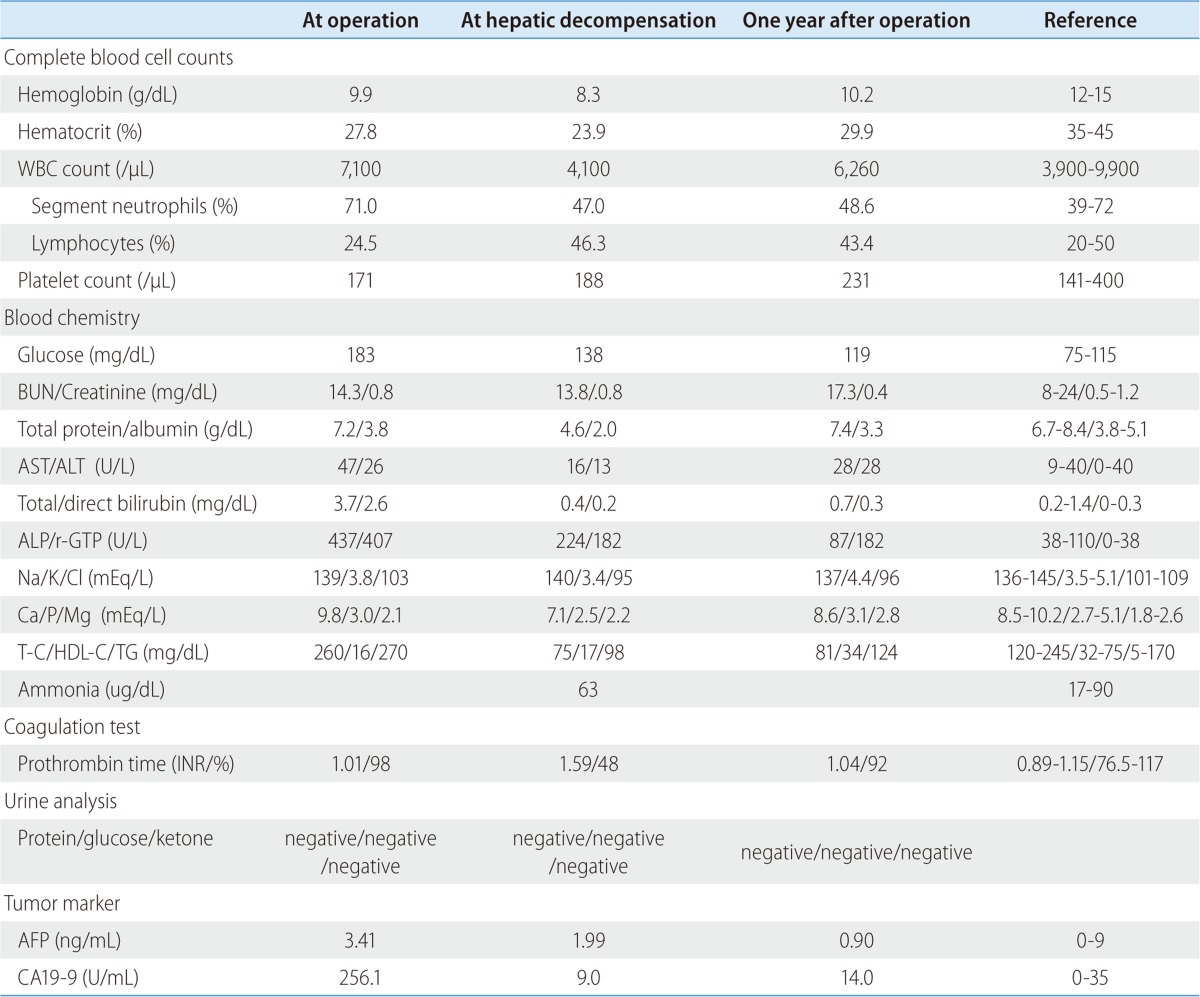
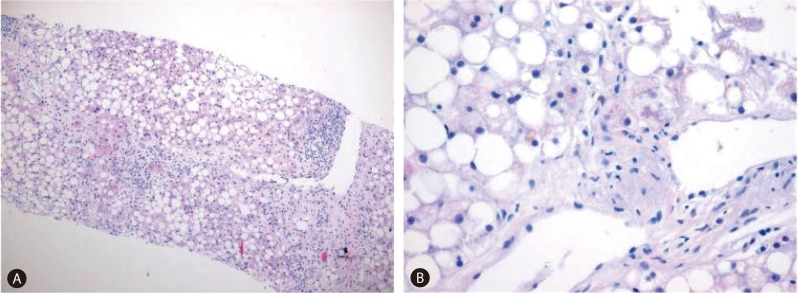
The patient began to develop abdominal distension one month before presentation, and as a result, her body weight increased from 44 to 55 kg at presentation. At admission, vital signs were as follows; blood pressure 110/60 mmHg, heart rate 90/min, and body temperature 36.0℃. In addition, she complained of cold sensation. Pertinent physical findings included shifting dullness with abdominal distension, anemic conjunctivae, pretibial pitting edema, and flapping tremor with slurred speech. Table 1 summarizes laboratory findings, which showed anemia with a chronic illness pattern, prolonged prothrombin time, hypoalbuminemia, markedly depressed lipid profiles, and slight decreases in level of calcium and phosphorus. To evaluate the presence of steatorrhea, a stool study was performed, and did not show stool fat. Hemoglobin A1c was 6.0% without anti-diabetics, although she had been diagnosed with diabetes two years prior to the pancreaticoduodenectomy. Viral markers for hepatitis B and C were negative. Carbohydrate antigen (CA) 19-9 had been maintained within the normal range post-pancreaticoduodenectomy. Infectious and autoimmune causes of liver disease and functional abnormalities of the thyroid and adrenal glands were excluded by laboratory testing. She denied alcohol intake and taking any other medicine, including herbal medicine, except supportive pancreatic enzyme. Follow-up liver dynamic CT at this presentation also revealed marked fatty liver and newly developed moderate amounts of ascites (Fig. 1C). There was no evidence of local tumor recurrence or lymph node enlargement. The ascites was tapped and proved to be transudate (serum-ascites albumin gradient 1.5) free of malignant cells. It was then controlled with diuretic agents. Her slurred speech, disorientation, and flapping tremor improved within 1-2 days of frequent laxative administration (lactulose). Although her laboratory findings showed mild metabolic alkalosis (pH 7.52, PCO2 33 mmHg, PO2 66 mmHg, HCO3 26.9 mmol/L, O2 saturation 95.0%) in blood gas analysis, mild electrolyte imbalances and no electroencephalographic study was performed, the neurologist checked our patient and there was no organic problem explaining her mental state except hepatic encephalopathy. A liver biopsy was performed to exclude other causes of liver parenchymal disease, and its findings were consistent with severe steatohepatitis which of NAFLD activity score 7 (NAS; macrovesicular steatosis (3), lobular inflammation (2) and hepatocellular ballooning (2)) and a fibrosis score of 1 (Fig. 2A). In addition, a Mallory's body was observed in the high power field (Fig. 2B).
Table 1.
Serial laboratory findings at surgery, during hepatic decompensation, and 1 year after operation
BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-C, total cholesterol; HDL-C, HDL cholesterol; TG, triglyceride; INR, international normalized ratio.
Figure 2.
Pathologic findings. (A) The liver biopsy findings were consistent with severe steatohepatitis, which yielded a nonalcoholic fatty liver disease activity score of 7 (macrovesicular steatosis, 3; lobular inflammation, 2; and hepatocellular ballooning, 2) and a fibrosis score of 1 (hematoxylin-eosin stain, ×40). (B) A Mallory's body in a high-power field (hematoxylin-eosin stain, original magnification ×400).
The patient was hydrated with saline at 20-30 mL/kg/day and then parenteral and enteral feeding was started at 10 kcal/kg/day for 3 days and then increased to 20-25 kcal/kg/day, in view of the risk of refeeding syndrome. In addition, she was administered multi-vitamins including folic acid, thiamine, and zinc and selenium. Her electrolyte levels, body weight, and urine output were checked daily. After 3 months of nutritional support, follow-up CT showed some regression of fatty liver (CT value 10 HU) and near complete regression of the ascites (no diuretic was administered; Fig. 1D). After 7 months of controlled oral nutritional intake, she was free of symptoms of ascites and encephalopathy and her liver function tests and lipid profiles were improved without evidence of tumor recurrence (Table 1).
DISCUSSION
The present study showed an unusual case of malnutrition-induced severe hepatic steatohepatitis and hepatic decompensation after pancreaticoduodenectomy for pancreas cancer. NASH is usually encountered in patients with metabolic syndrome, although a few reports of malnutrition-induced hepatocellular injury have been released predominantly in anorexia nervosa patients.6,9-11 However, patients that undergo surgery for gastrointestinal cancer are also at risk of malnutrition7 and some that have undergone pancreaticoduodenectomy develop de novo NAFLD.13,15 However, few reports have been issued regarding post-pancreaticoduodenectomy-induced hepatic steatohepatitis leading to hepatic decompensation.16
The pathogenesis of NAFLD is described by the 'two hit' model.13 That is initially triglyceride (TG) deposits in the cytoplasm of hepatocytes (hepatic steatosis), and this is followed by inflammation, cell death, and fibrosis (steatohepatitis).17 Although the mechanisms and pathogenesis of hepatocellular steatosis in patients with malnutrition remain unclear, the liver represents the major reservoir for fatty acids in the form of TGs during starvation.18,19 In the nourished state, TGs stored in adipose tissue are hydrolyzed to free fatty acids (FFAs) which play a major role in energy production in the liver.19 When not subjected to oxidation in mitochondria to form ketone bodies, FFAs can undergo reesterification into TGs, that may be subsequently deposited in the cytoplasm of hepatocytes.18 In addition, it has been reported that the liver specimens of 12 patients with acute liver injury due to anorexia nervosa showed hepatocyte glycogen depletion and numerous autophagosomes by electron microscopy.20 Furthermore, in this study, it was suggested that starvation induced autophagy in the human liver may be involved in liver cell death.
In addition, the relationship between NAFLD and pancreatic dysfunction has not been resolved. According to the clinical features of NAFLD associated with pancreatic dysfunction, it is characterized by nonobesity, the absence of metabolic syndrome, and a severe nutrition disorder, as was observed in our case.13-15 Furthermore, 23-37% of patients that undergo pancreaticoduodenectomy develop fatty liver.13,15 In these reports, inadequate pancreatic exocrine function was suggested to be the main cause of de novo NAFLD after pancreaticoduodenectomy.13,15 However, others have suggested that the development of NAFLD after pancreaticoduodenectomy is caused by markedly impaired intestinal absorption, a gut barrier dysfunction with zinc deficiency,15,21,22 and a reduced serum apoB concentration, the latter of which impairs very-low-density lipoprotein secretion and leads to steatogenesis.15 Although, we did not check serum zinc levels and our case did not show steatorrhea or uncontrolled diabetes, we did administer multi-vitamins and the trace elements such as zinc and selenium to counterbalance any trace element deficiency and to maintain pancreatic enzyme levels from just after pancreaticoduodenectomy.
Of the putative operative and post-operative factors, ductal adenocarcinoma, a pancreatic resection line on the left side of the superior mesenteric artery (SMA), and post operative diarrhea have been identified to be the most influential risk factors of NAFLD development after pancreaticoduodenectomy,13 since pancreatic cancer originating from the pancreatic duct is characterized by occlusion, resulting in obstructive pancreatitis and distal pancreatic atrophy. Furthermore, the pancreatic resection line directly affects pancreatic function and reflects dissection of the nerve plexus around the SMA leading to diarrhea after pancreaticoduodenectomy. However, although the pathology in our case was ductal adenocarcinoma, the resection line was favorable at the portal vein level, and after pancreaticoduodenectomy though the patient had a poor appetite she did not have postoperative diarrhea. In addition, delayed gastric emptying and digestive disturbance due to complicated reconstruction, has been reported to lead to the development of NAFLD.13 Therefore, several mechanisms are believed to contribute to the development of NAFLD after pancreaticoduodenectomy.
The present case has several unique findings. Hepatic decompensation signs, such as, ascites and hepatic encephalopathy developed several months postoperatively and improved after the initiation of nutritional treatment. In the majority of cases with anorexia nervosa and in cases of de novo NAFLD after pancreaticoduodenectomy, hepatocellular injuries are usually mild and improve to the normal range after the provision of nutritional and pancreatic enzyme support.6,9-11,15 In a previous case report, hepatic encephalopathy was developed for 15 years after pancreaticoduodenectomy for pancreas cancer.16 However, the patient was believed to progress from NAFLD and NASH to liver cirrhosis over this protracted period. Also, several case reports of anorexia nervosa have described severe hepatic failure leading to a fatal outcome.6,9-11 In these studies,6,9-11,20 ascites was found in 4 of 12 patients with severe acute liver insufficiency and no patient developed encephalopathy. Our patient showed similar laboratory findings, due to a rapid decrease in liver function (though with normal liver enzymes) and clinical symptoms to patients with anorexia nervosa. These patients show decreases in hemoglobin, albumin, platelet count, and potassium and increases in transaminases and alkaline phosphatases, and present with hypotension, bradycardia, hyperthermia, and dehydration. Regarding these clinical symptoms, some authors have suggested that acute liver injury may be secondary to acute liver hypoperfusion, microcirculatory failure, and oxidative stress rather than to a chronic underlying liver insufficiency.10,23,24 Safe and rapid recovery after therapeutic intervention based on hydration with plasma volume support appears to confirm this hypothesis. Moreover, a number of patients with normal aminotransferase levels may also have NAFLD and even advanced fibrosis, although elevated liver enzymes are generally associated with histological NASH.25 Therefore, aminotransferase activity alone cannot be used to rule out significant liver disease in patients with NAFLD, especially those with type II diatbetes.25,26 Mofrad et al have found that histological features of NAFLD may progress in persons with normal aminotransferase values and the liver histology in these persons is not very different from that in patients with high aminotransferase levels.27 The age of our patient (68 years) may have created a status comparable to anorexia nervosa and her post-pancreaticoduodenectomy status for pancreas cancer may have exacerbated symptoms as compared with those who with general NAFLD and/or NASH associated metabolic syndrome.
We should be careful for refeeding syndrome when treating malnourished patients. Refeeding syndrome represents a set of symptoms caused by fluid and electrolyte imbalances associated with nutritional supplementation via oral, enteral, or parenteral routes following a period of adaption to prolonged starvation.28 Careful monitoring of body weight, urine output, and electrolytes is necessarily, and fluid overload and pulmonary edema should be prevented.
In summary, we report an unusual case that showed severe hepatic steatohepatitis with signs of hepatic decompensation resulting from malnutrition after pancreaticoduodenectomy for pancreatic cancer. This case suggests poor nutritional status after pancreaticoduodenectomy in pancreatic cancer patients can induce severe liver steatosis, and that there is a need to monitor the image study and liver function test, and support nutritional status in these patients.
Abbreviations
- CA19-9
carbohydrate antigen 19-9
- CT
computed tomography
- FFA
free fatty acid
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TG
triglyceride
Footnotes
The authors have no conflicts to disclose.
References
- 1.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 3.McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255–262. doi: 10.1097/00004836-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Tannapfel A, Denk H, Dienes HP, Langner C, Schirmacher P, Trauner M, et al. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. 2011;458:511–523. doi: 10.1007/s00428-011-1066-1. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 6.Tsukamoto M, Tanaka A, Arai M, Ishii N, Ohta D, Horiki N, et al. Hepatocellular injuries observed in patients with an eating disorder prior to nutritional treatment. Intern Med. 2008;47:1447–1450. doi: 10.2169/internalmedicine.47.0824. [DOI] [PubMed] [Google Scholar]
- 7.Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23:393–401. doi: 10.1111/j.1365-277X.2010.01058.x. [DOI] [PubMed] [Google Scholar]
- 8.Graham GG. Starvation in the modern world. N Engl J Med. 1993;328:1058–1061. doi: 10.1056/NEJM199304083281429. [DOI] [PubMed] [Google Scholar]
- 9.Sakada M, Tanaka A, Ohta D, Takayanagi M, Kodama T, Suzuki K, et al. Severe steatosis resulted from anorexia nervosa leading to fatal hepatic failure. J Gastroenterol. 2006;41:714–715. doi: 10.1007/s00535-006-1845-7. [DOI] [PubMed] [Google Scholar]
- 10.De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition. 2006;22:572–575. doi: 10.1016/j.nut.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Lee TH, Cheon SL, Sun JH, Choi IK, Kim YS, et al. Severe acute liver and pancreas damage in anorexia nervosa. Korean J Gastroenterol. 2009;54:257–260. doi: 10.4166/kjg.2009.54.4.257. [DOI] [PubMed] [Google Scholar]
- 12.Fauci AS, Kasper DL, Braunwald E, Hauser SL, Longo DL, Jameson JL, et al. Harrison's principles of internal medicine. 17th ed. New York: McGra Hill; 2008. p. 1982. [Google Scholar]
- 13.Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, et al. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296–304. doi: 10.1007/s00534-009-0187-2. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka N, Horiuchi A, Yokoyama T, Kawa S, Kiyosawa K. Pancreatic exocrine insufficiency: a rare cause of nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:245–246. doi: 10.1111/j.1572-0241.2007.01562_7.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, et al. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758–768. doi: 10.1007/s00535-011-0370-5. [DOI] [PubMed] [Google Scholar]
- 16.van Galen KP, Stam F, van Oijen AH. Hepatic encephalopathy following a Whipple operation. Ned Tijdschr Geneeskd. 2009;153:A463. [PubMed] [Google Scholar]
- 17.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ginneken V, Verhey E, Poelmann R, Ramakers R, van Dijk KW, Ham L, et al. Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study of hepatic steatosis. Biochim Biophys Acta. 2007;1771:1263–1270. doi: 10.1016/j.bbalip.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 20.Rautou PE, Cazals-Hatem D, Moreau R, Francoz C, Feldmann G, Lebrec D, et al. Acute liver cell damage in patients with anorexia nervosa: a possible role of starvation-induced hepatocyte autophagy. Gastroenterology. 2008;135:840–848. 848.e1–848.e3. doi: 10.1053/j.gastro.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Kato K, Isaji S, Kawarada Y, Hibasami H, Nakashima K. Effect of zinc administration on pancreatic regeneration after 80% pancreatectomy. Pancreas. 1997;14:158–165. doi: 10.1097/00006676-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mizumoto R, Kawarada Y, Goshima H, Tamaki H, Sekoguchi T, Tomikawa I. Carbohydrate metabolism and endocrine function in the pancreas remnant after major pancreatic resection. Am J Surg. 1982;143:237–243. doi: 10.1016/0002-9610(82)90077-0. [DOI] [PubMed] [Google Scholar]
- 23.Mine T, Ogata E, Kumano H, Kuboki T, Suematsu H. An analysis of liver injury in patients with AN. Research reports from investigational group for anorexia nervosa. Ministry of Health and Welfare; 1992. (in Japanese) [Google Scholar]
- 24.Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. A case report of oxidative stress in a patient with anorexia nervosa. Int J Eat Disord. 2006;39:616–618. doi: 10.1002/eat.20326. [DOI] [PubMed] [Google Scholar]
- 25.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 26.Amarapurkar DN, Patel ND. Clinical spectrum and natural history of non-alcoholic steatohepatitis with normal alanine aminotransferase values. Trop Gastroenterol. 2004;25:130–134. [PubMed] [Google Scholar]
- 27.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 28.Boateng AA, Sriram K, Meguid MM, Crook M. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. 2010;26:156–167. doi: 10.1016/j.nut.2009.11.017. [DOI] [PubMed] [Google Scholar]