Abstract
Studies have shown that dentin matrices contain reservoirs of bioactive molecules capable of directing tissue repair. Elucidating the release mechanisms of such endogenous growth factors will enhance our understanding of dentinpulp regeneration and support the development of novel treatment modalities to enhance dentin repair following trauma and disease. Current clinical practice using new materials which are perceived to maintain pulpal viability require biological evidence to assess their therapeutic benefit and there is a need for better effective methods of assessing therapeutic approaches to improving dentin regeneration at the cellular and tissue level. Experimental modelling of dentin regeneration is hampered by the lack of suitable models. In vivo and in vitro studies have yielded considerable information on the processes taking place, but are limited, due to the cost, ethics and lack of cell/matrix interactions. Novel organotypic models, whereby cells and tissues are cultured in situ may provide a more suitable model system to facilitate dental tissue engineering and regeneration.
Keywords: Dentin-pulp complex, tissue regeneration, ex vivo, inflammation.
INTRODUCTION
The development of alternative therapies in dental tissue diseases or traumas, including caries, dentinal fractures, periodontal disease or obtaining biological evidence to support current clinical treatment regimes is hampered by the research model systems currently available for the study of dental tissue repair. Currently, animal or human in vivo, in vitro cell culture systems are utilised to investigate the bone repair process. In vitro models use a single cell system, or at the most two in the case of organotypical models. However, these are unable to recapitulate the specific 3D spatial arrangement of cells in vivo and the potential altered cell behaviour is limiting. In vivo experimentation has provided the gold standard in elucidating the processes which occur during dental tissue and bone repair, but models of this kind have significant cost implications and require large numbers of animals impacting on the ethical standing of such studies. It can be difficult to obtain clear data in such models due to systemic influences which make investigation of specific cellular and tissue responses difficult. Ex vivo culture models have been developed to study a wide variety of developmental, physiological and pathological conditions. Such ex-vivo models have significant advantages, as the cells and tissue are able to be cultured in the same spatial arrangement as would be found in the in vivo situation, while systemic influences, which often hinder in vivo work, are removed. Such systems are not uncommon and find use within the tissue engineering and repair community. The organ culture of cartilage has facilitated the understanding of cartilage repair [1] and with respect to mineralised tissues the development of a tooth slice ex vivo culture system [2-4] has greatly enhanced the understanding of dental tissue repair processes.
An important application of such models is in the development and understanding of clinical dental techniques. The management of teeth damaged by dental caries or trauma is of great significance when avoiding pain and infection, while returning the affected tooth to function and a suitable aesthetic appearance. Despite an increased focus on preventive dentistry, the management of dental caries accounts for a significant amount of work for dentists, either in the management of new lesions of caries, involving tooth restoration, or in the subsequent management, treatment or replacement of existing restorations. Current operative treatments for managing teeth affected by caries include the placement of silver amalgam or resin composite materials. Silver amalgam has long been the treatment of choice for restoring posterior teeth and has been regarded as straightforward, satisfactory and relatively inexpensive. However, this material suffers mechanical limitations in that it is not adherent of tooth tissue and often requires sacrifice of disease-free adjacent tooth tissue with the aim of creating a mechanical undercut to optimise retention. Resin composites are polymeric resins reinforced with fillers. These materials offer significant advantages to amalgam, including being capable of being micromechanically "bonded" to tooth tissue, thereby not requiring the same removal of intact tooth tissue for retention as amalgam. Significantly, while dental caries is still a challenge for dentists, there has, in recent years, been a shift in the pattern/ presentation of lesions of caries due an increased emphasis on prevention, the incorporation of fluoride into toothpastes and diets, and regular check-ups. In contrast to large cavities of yesteryear, lesions of caries are now detected at a much earlier stage - much smaller than that which will allow a satisfactory silver amalgam restoration to be replaced without removal of disease-free tooth substance. This has been driven by a demand for a more minimally invasive form of cavity preparation, which has been facilitated by adhesive resin composites.
Resin based composites are of increasing popularity: dental students of today receive more training in the placement of these materials than those of fifteen years ago. This drive towards the use of resin composites has been backed up by long term clinical data to support their longevity. There has been a veritable explosion in the range and number of products available to facilitate adhesive dentistry; these have been mainly backed up by mechanical based studies of materials strength and other properties. However, little, if no evidence exists on the "biological" effects of such materials. Dental restorative materials are placed in intimate contact with operatively exposed dentin - an important consideration when avoiding subsequent problems such as sensitivity, pulpal damage and restoration failure. Mishandling of operatively exposed dentin could cause the need for a root canal treatment - an expensive form of dental treatment, often difficult to access, with an increased risk of ultimate tooth loss.
Many popular composite placement techniques, in particular, in relation to operatively exposed dentin are not evidence-based and defy common sense. These are mainly "hang overs" from previous amalgam placement techniques. An important example of such confusion in contemporary clinical dentistry is the dilemma of "bonding" or "basing" composite restorations. Following a technique used with amalgam placement, many practitioners choose to place a "base" cement under a composite restoration to "protect" operatively exposed dentin at the expense of the mechanical properties of the completed restoration. However, a growing number of practitioners choose not to use a "base" cement and instead simply "bond" the composite in place without using a cement base. There is no real reliable evidence - biological or clinical - to support the merit of either technique. As such, patients are potentially exposed to risk. Clear elucidation of the biological effects of such materials via appropriate model systems is required for proper investigation. Let alone this, opportunity exists to explore the development/ selection of materials which can drive dental tissue repair via studying materials/ dentin interactions in appropriate dentin-pulp models. The ultimate aim of such an approach is to improve treatment outcomes for patients, retain tooth viability, and avoid costly, expensive and complex care.
MODELS FOR DENTAL TISSUE REGENERATION
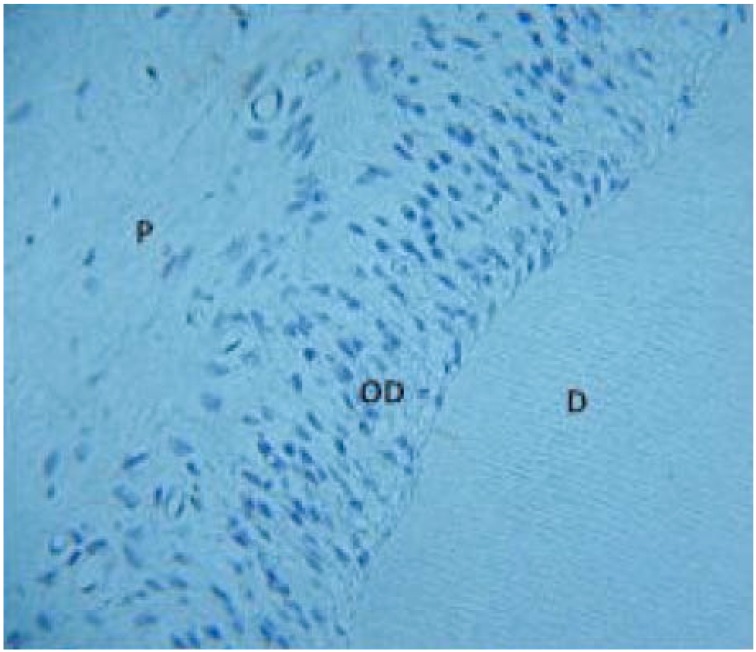
In vivo, the mineralized dentin matrix and the odontoblasts, together with the pulpal soft connective tissue are considered as a cooperative functional complex. Various investigators have attempted to culture odontoblasts and other cells of the pulp in vitro [5-7] but these and other previous attempts to culture odontoblasts in vitro have demonstrated the need to maintain contact between them and the dentine matrix to preserve their phenotypic morphology [8, 9]. The culture of the dentin - pulp complex of rat incisor teeth [2] when embedded in a semi - solid agar based medium and cultured at the liquid gas interface (Fig 1) allowed for culture of the tissue successfully for up to 14 days and tissue architecture of the entire dentin - pulp complex was maintained during the culture period. An in vitro model of human dental tissue repair has also been reported, whereby thick tooth slices have been cultured in liquid media [4]. Such culture models now facilitate the investigation of dentinogenesis and tissue repair mechanisms, as the odontoblasts can be examined within the normal environment of the dentin - pulp complex, but in the absence of normal inflammatory processes which occur in vivo. This model system has been pivotal in understanding the bioactive nature of the dentin matrix and the role of TGF-β1 and BMP-7 in directing reparative events in dental tissue repair [3, 10]. This culture system has been further developed to be used as a reproducible method for assessing biocompatibility and cytotoxicity of novel and existing dental materials [11] and has also been essential as an experimental system to investigate the effects of fluoride on dentin ECM alterations and subsequent mineralization [12-14].
Fig. (1).
Dentin-pulp complex from a 2mm transverse slice of incisor cultured for 14 days demonstrating preservation of cell and tissue architecture.
D- dentin, OD – odontoblasts, P – pulp
It is now accepted that the extracellular matrix (ECM) plays a critical role in mediating induction of odontoblast differentiation during tooth development [15] and also mediates similar processes in dental tissue repair in mature teeth. After injury, a process of reparative dentinogenesis leads to the differentiation of a new generation of odontoblast like cells, which secrete a tertiary dentin matrix and many of these events recapitulate, to a certain extent, those seen during tooth development [15]. The dentin matrix contains a reservoir of bioactive growth factors which can mediate these processes and the functional roles of members of the TGF-β super-family in reparative dentinogenesis suggests a possible role in novel therapeutic mediators or tissue engineering solutions to dental regeneration. During dental disease or trauma, tissue damage and inflammation at sites of injury may compromise the ability of the pulpal ECM to mediate reparative events, and there may be advantages to providing a suitable matrix to encourage cell migration and differentiation at such sites. Recent studies using the tooth slice ex vivo culture system have demonstrated the ability of bioactive growth factor TGF-ß1 contained within alginate hydrogels, to induce odontoblast-like cell differentiation within the dentin-pulp complex, with subsequent up-regulation of dentin matrix secretion [16].
Our knowledge of dentin regeneration has improved significantly in recent times. For example, twenty years ago, it was felt that "pulp capping (application of a calcium hydroxide based material to exposed dental pulp tissue) could stimulate Dentin Bridge" formation and new calcified tissue. The nature of this process was unclear and it was thought that "irritation" of the dental pulp by cements such as calcium hydroxide would cause recruitment of Ca2+ ions from pulpal vasculature to help this calcified barrier formation. With the benefit of contemporary knowledge and techniques we now know that the increased pH of certain pulp capping agents result in the liberation of bioactive molecules, such as TGF-β, amongst others, sequestered within dentin and/or recruitment of dental pulp stem cells, which lead to the formation of new dentin or dentin-like material. Harnessing the effect of new materials on this process of dentin repair should be studied as the best protection for a dental pulp is dentin itself and this will reduce the need for restoration maintenance and replacement, which would ultimately place the viability of the tooth at risk.
CURRENT MODELS OF PERIODONTAL DISEASE AND BONE DESTRUCTION
To advance our understanding of inflammatory bone destruction during periodontal disease suitable model and clinical systems are required. Currently, in vivo models using large numbers of rodents and primates, and genetic knock-out mice are utilised to investigate inflammatory disease and bone destruction. Such processes are modelled and investigated using in vivo rodent and primate models of experimental periodontitis using a ligature model to induce periodontitis and injections of various cytokines delivered to examine their influence of osteoclastogenesis and bone resorption [17]. This particular method of inducing periodontitis has also been used to investigate how in vivo administration of IL-1α accelerates ligature bone resorption in rats [18], whilst other studies have used established murine models of bone resorption induced by high dose LPS administration [19]. Infecting/immunizing rodents and primates with potential periodontal pathogens has been a tool used to investigate disease progression, inflammatory cell activity and tissue destruction. The use of A. actinomycetemcomitans (feeding model) and P. gingivalis (in an oral gavage model) to induce pro-inflammatory cytokine production and bone resorption in rats, mice and non-human primates has also been reported [20-23] along with experimental periodontitis in a humanized diabetic SCID mouse gavage model [22]. Even though such animal models have limitations, they are still considered as being superior to in vitro or clinical studies for addressing mechanistic questions and all are currently used in preference to other models. The current studies investigating the efficacy of the pro-resolving lipid molecules in resolving periodontal inflammation use in vivo induced experimental models of periodontitis. Studies using the P. gingivalis induced experimental rabbit model have demonstrated that the resolvin RvE1 prevents inflammation and bone loss in these animals when applied topically [24, 25]. The same rabbit model has also been used to investigate the efficacy of lipoxins to resolve periodontal inflammation and associated bone loss [26]. Similar studies using LPS induced periodontitis in rats have also suggested that polyunsaturated fatty acid (PUFA) derivatives inhibited disease progression [27]. In addition to these studies, it has also been suggested that over-expression of the 15-LOX type I lipoxygenase pathway in transgenic rabbits reduces the inflammatory phenotype in periodontitis [28].
As described above, studies using such in vivo models are numerous and have been used over many years however the cost of running such experiments and the difficulty in obtaining clear data suggests that we should be looking at more novel, laboratory based systems in our investigations prior to trialling novel treatments in a living system. Although there are many in vitro studies of the effects of cytokines and pro-resolving molecules on osteoclast differentiation [29, 30], results from such studies can be far removed from the in vivo situation as cell/cell interaction between differing cell types may influence behaviour. In vitro assays for rodent primary alveolar bone cells and periodontal ligament cells [31] allows us to investigate the behaviour of specific cells, reducing the number of animals being used. However these cells are difficult to extract and culture due to the very small area of tissue being dissected.
EX VIVO MODEL SYSTEMS AND PERIODONTAL DISEASE
As discussed earlier, ex vivo culture models have been developed to study a wide variety of developmental, physiological and pathological conditions and the development and use of such models significantly reduces the number of animals required for in vivo experimentation. The organ culture of cartilage has facilitated the understanding of cartilage repair and determination of defects [1] and has potential for use in toxicological research and drug activity [32], eliminating the need for expensive and unnecessarily numerous in vivo studies which have previously been used to study the biological effects of such tissues.
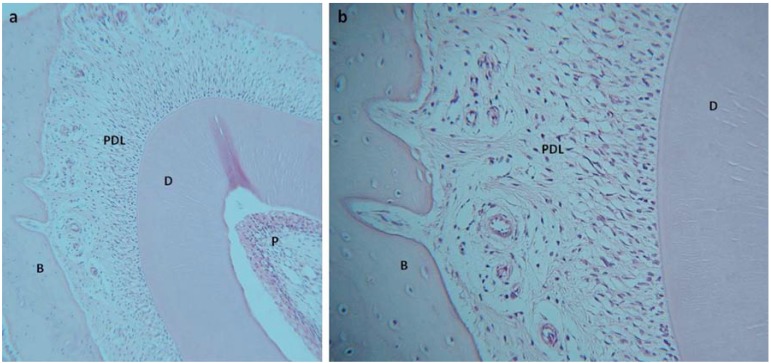
Further development of a mandibular slice culture system [33] to investigate periodontal inflammation and bone destruction has facilitated the development of an ex-vivo culture system which supports the differentiation, proliferation and activity of fibroblasts, osteoblasts, osteoclasts and immune system cells within cultured slices of mouse mandible [34] (Fig. 2). The system, which cultures 2mm thick slices of murine mandible in Trowel type cultures at the liquid – gas interface allows the culture of cells and tissue in situ providing a natural tissue environment to allow cells to be successfully cultured in 3D for up to 21 days. Although some reduction in cell numbers has been reported, viability of the cells and tissues within the mandible slice remain during all culture periods and no significant alterations in the numbers of dividing cells suggests viability is not significantly compromised by extended culture. Expression of bone matrix proteins, including bone sialoprotein and osteopontin, were maintained during culture with no significant alterations within the PDL or surrounding alveolar bone or in osteoblast cell numbers. Importantly, the system appeared to support the culture of osteoclasts present within the tissue slice. Although the number of tartrate resistant form of acid phosphatase (TRAP) positive osteoclasts were small, numbers did not significantly decrease with extended culture.
Fig. (2).
1.5mm slice of rat mandible cultured for 14 days. Low power microscopy (a) demonstrates maintenance of cell and tissue structure in the incisor tooth, periodontal ligament and alveolar bone. At higher power (b) preservation of ligament cells and osteocytes within the alveolar bone can be seen along with attachment of ligament to the bone through Sharpey’s fibres. D-dentin, P-pulp, PDL- periodontal ligament, B-bone.
It was also demonstrated that an inflammatory response can be invoked in the system, when stimulated appropriately with bacterial LPS or pro-inflammatory cytokines. Such stimulation leads to increases in osteoclast cell numbers within the slice and ultimately loss of tissue architecture and viability of ligament fibroblasts. Decreases in bone sialoprotein expression also appeared to be LPS dependent. Increases in monocytes and neutrophils within the system and the synthesis and secretion of inflammatory cytokines by resident cells in response to LPS and pro-inflammatory cytokine stimulation have also been noted and such cytokine responses have also shown to have been mediated by the addition of anti-inflammatory cytokines.
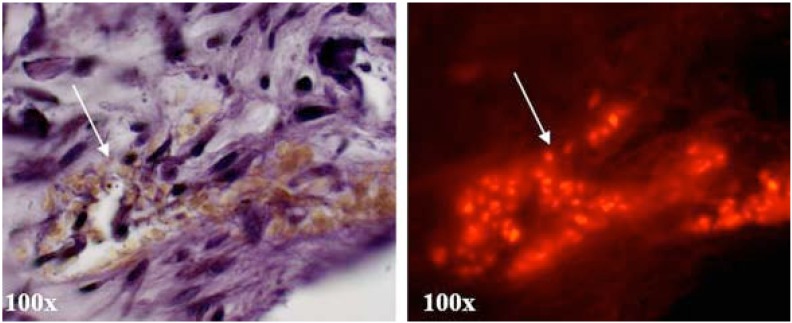
The system also supports insertion / transplantation of osteoclasts and cells of the innate immune system into the ligament tissue and allow for differentiation into osteoclasts (Fig. 3) which can be observed through long term culture by tracer fluorescence labelling. Pre-osteoclasts, isolated via non-adherence to tissue culture plastic following bone marrow aspirates and overnight stimulation with MSCF, were labelled with a cell tracker dye and 100 cells injected into the ligament of a murine mandible slice using a small guage needle. Cultures were maintained for 14 and 21 days in media supplemented with RANKL and MCSF. Following culture, TRAP positive multinuclear cells could be identified in areas within the mandible slice which correlated with red tracer fluorescence in the same sections examined with fluorescence microscopy (Fig. 3). The microinjection of pre-osteoclasts within this ex-vivo system may provide a viable model for generating a localised inflammatory response. Regeneration or engineering of tissues within compromised or inflamed areas is challenging, and being able to recreate such an environment not only benefits investigations into novel treatment modalities for inflammatory mediated tissue destruction in diseases such as periodontal disease and rheumatoid arthritis, but also development of novel methods to engineer tissues in situ.
Fig. (3).
Microinjection of monocytes labelled with the fluorescent cell tracker dye PKH-28 into the periodontal ligament of mandible slices cultured for 14 days demonstrated support of differentiation of these cells into TRAP positive osteoclasts as observed by a large multinuclear TRAP positive mass (arrow). This correlates with red fluorescence PKH-28 tracer in the same sections when examined using fluorescence microscopy (arrow).
CONCLUSION
The development of such novel, reproducible and functionally viable ex vivo culture systems allows us to recapitulate and measure biological responses of dentin/pulp regeneration and periodontal disease. They allow us to monitor cell and molecular responses and understand how the tissues respond to injury. This gives us the ability to understand the complex, but exquisite natural regenerative processes these tissues have and allow us to intervene and manipulate these responses and provide biological evidence on which we can base the development of new clinical treatments. In turn this provides us with a very powerful tool to advance the development of novel tissue regeneration strategies in dental tissue repair and the creation and assessment of novel bioactive treatment modalities.
ACKNOWLEDGEMENTS
This work was supported in part by a Chief Medical Officers Research Grant from the Welsh Assembly Government, NC3Rs research grant 77844 and an Oral and Dental Research Trust Award.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1. Graichen H, Al-Shamari D, Hinterwimmer S, von Eisenhart-Rothe R, Vogl T, Eckstein F. Accuracy of quantitative magnetic resonance imaging in the detection of ex vivo focal cartilage defects. Am Rheum Dis. 2005;64:1120–5. doi: 10.1136/ard.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sloan AJ, Shelton RM, Hann AC, Moxham BJ, Smith AJ. An in vitro approach for the study of dentinogenesis by organ culture of the dentine-pulp complex from rat incisor teeth. Archs Oral Biol . 1998; 43:421. doi: 10.1016/s0003-9969(98)00029-6. [DOI] [PubMed] [Google Scholar]
- 3. Sloan AJ, Smith AJ. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-ß isoforms 1-3 in vitro. Archs Oral Biol. 1999;44:149. doi: 10.1016/s0003-9969(98)00106-x. [DOI] [PubMed] [Google Scholar]
- 4. Magloire H, Joffre A, Bleicher F. An in vitro model of human dental pulp repair. J Dent Res. 1996;75:1971–8. doi: 10.1177/00220345960750120901. [DOI] [PubMed] [Google Scholar]
- 5. Bègue-Kirn C, Smith AJ, Ruch JV, et al. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992; 36:491–503. [PubMed] [Google Scholar]
- 6. Bègue-Kirn C, Smith AJ, Loriot M, Kupferle C, Ruch JV, Lesot H. Comparative analysis of TGF bs, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int J Dev Biol . 1994; 38:405–20. [PubMed] [Google Scholar]
- 7. Nakashima M. Establishment of primary cultures of pulp cells from bovine permanent incisors. Archs Oral Biol. 1991; 36:655–63. doi: 10.1016/0003-9969(91)90018-p. [DOI] [PubMed] [Google Scholar]
- 8. Munksgaard EC, Richardson WS III, Butler WT. Biosynthesis of phosphoprotein by rat incisor odontoblast in in vitro culture. Archs Oral Biol. 1978; 23:583–7. doi: 10.1016/0003-9969(78)90275-3. [DOI] [PubMed] [Google Scholar]
- 9. Heywood BR, Appleton J. The ultrastructure of the rat incisor odontoblast in organ culture. Archs Oral Biol. 1984; 29:327–9. doi: 10.1016/0003-9969(84)90107-9. [DOI] [PubMed] [Google Scholar]
- 10. Sloan AJ, Rutherford RB, Smith AJ. In Vitro stimulation of the dentine-pulp complex by BMP-7. Archs Oral Biol. 2000; 45:173. doi: 10.1016/s0003-9969(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 11. Murray PE, Lumley PJ, Ross HF, Smith AJ. Tooth slice organ culture for cytotoxicity assessment of dental materials. Biomaterials . 2000; 21:1711. doi: 10.1016/s0142-9612(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 12. Moseley R, Sloan AJ, Waddington RJ, Smith AJ, Hall RC, Embery G. The influence of fluoride on the cellular morphology and synthetic activity of the rat dentine-pulp complex in vitro. Archs Oral Biol. 2003; 48:39–46. doi: 10.1016/s0003-9969(02)00160-7. [DOI] [PubMed] [Google Scholar]
- 13. Moseley R, Waddington RJ, Sloan AJ, Smith AJ, Hall RC, Embery G. The influence of fluoride exposure on dentin mineralisation using an in vitro organ culture method. Calcif Tiss Int. 2003; 73:70–5. doi: 10.1007/s00223-003-0022-8. [DOI] [PubMed] [Google Scholar]
- 14. Waddington RJ, Moseley R, Smith AJ, Sloan AJ, Embery G. Fluroide-induced changes to proteoglycan structure synthesised within the dentine-pulp complex in vitro. Biochem Biophys Acta . 2004; 1689:142. doi: 10.1016/j.bbadis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 15. Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis Embryonic events as a template for dental tissue repair. Crit Rev Oral Biol Med. 2001; 12:425. doi: 10.1177/10454411010120050501. [DOI] [PubMed] [Google Scholar]
- 16. Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-b 1 on human dental pulp repair in vitro. Conn. Tiss Res. 2002; 43:381–6. doi: 10.1080/03008200290000574. [DOI] [PubMed] [Google Scholar]
- 17. Assuma R, Oates T, Cochran D, Amar S, Graves D. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–9. [PubMed] [Google Scholar]
- 18. Chiang C, Kyritsis G, Graves D, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun . 1999; 67:4231–6. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koide M, Suda S, Saitoh S, et al. In vivo administration of IL-1 beta accelerates silk ligature-induced alveolar bone resorption in rats. J Oral Path Med. 1995; 24:420–34. doi: 10.1111/j.1600-0714.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 20. Doxey DL, Cutler CW, Iacopino AM. Diabetes prevents periodontitis- induced increases in gingival platelet derived growth factor-B and interleukin 1-beta in a rat model. J Periodontol. 1998; 69:113–9. doi: 10.1902/jop.1998.69.2.113. [DOI] [PubMed] [Google Scholar]
- 21. Baker PJ, Garneau J, Howe L, Roopenian DC. T-cell contributions to alveolar bone loss in response to oral infection with Porphyromonas gingivalis. Acta Odontol Scand. 2001; 59:222–5. doi: 10.1080/00016350152509247. [DOI] [PubMed] [Google Scholar]
- 22. Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host–bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008; 35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol . 2000; 164:2102–9. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 24. Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006; 20:401–3. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 25. Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–9. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 26. Serhan CN. Clues for new therapeutics in osteoporosis and periodontal disease: new roles for lipoxygenases? Expert Opin Ther Targets. 2004;8:643–52. doi: 10.1517/14728222.8.6.643. [DOI] [PubMed] [Google Scholar]
- 27. Vardar S, Buduneli E, Baylas H, Berdeli AH, Buduneli N, Atilla G. Individual and combined effects of selective cyclooxygenase-2 inhibitor and omega-3 fatty acid on endotoxin-induced periodontitis in rats. J Periodontol. 2005; 76:99. doi: 10.1902/jop.2005.76.1.99. [DOI] [PubMed] [Google Scholar]
- 28. Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008; 79:601–8. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taubmann M, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect bone resorption. Crit Rev Oral Biol Med. 2001; 12:125–35. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 30. Herrera BS, Ohira T, Gao L, et al. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol. 2008; 155:1214–22. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roberts HC, Moseley R, Sloan AJ, Youde SJ, Stringer BMJ, Waddington RJ. Lipopolysaccharide alters decorin and biglycan synthesis in alveolar bone osteoblasts – consequences for bone repair during periodontal disease. Eur J Oral Sci. 2008; 116:207. doi: 10.1111/j.1600-0722.2008.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turner DJ, Hoyle SL, Snewin VA, Gares MP, Brown IN, Young DB. An ex vivo culture model for screening drug activity against in vivo phenotypes of Mycobacterium tuberculosum. Microbiology. 2002; 148:2929. doi: 10.1099/00221287-148-10-2929. [DOI] [PubMed] [Google Scholar]
- 33. Dhopatkar AA, Sloan AJ, Rock WP, Cooper PR, Smith AJ. A novel in vitro organ culture model to investigate the reaction of the dentine-pulp complex to orthodontic force. J Orthod. 2005; 32:122–32. doi: 10.1179/146531205225020979. [DOI] [PubMed] [Google Scholar]
- 34. Taylor SY, Smith EL, Waddington RJ, Wei XQ, Sloan AJ. Development of an ex vivo mandible model of local inflammation. J DentRes. 2008;87(Spec Iss C ):0302. [Google Scholar]