Abstract
We examined whether p38 MAPK plays role in calcium oxalate monohydrate (COM) crystal-induced tight junction disruption. Polarized MDCK cells were pretreated with or without 20 μM SB239063 (p38 MAPK inhibitor) for 2-h, and then incubated with 100 μg/ml COM crystals for up to 48-h. Western blotting showed increased level of phospho-p38, not total p38, in COM-treated cells, whereas SB239063 pretreatment successfully maintained phospho-p38 at its basal level. COM crystals also caused decreased levels of two tight junction proteins, zonula occludens-1 (ZO-1) and occludin. Immunofluorescence study revealed disruption of tight junction, redistribution, and dissociation of ZO-1 and occludin. Moreover, transepithelial resistance (TER) showed defective barrier function, whereas Western blotting for Na+/K+-ATPase-α1 revealed defective fence function of tight junction in COM-treated cells. All these expression and functional defects were successfully prevented by SB239063 pretreatment. These findings indicate that COM crystals cause tight junction disruption in distal renal tubular epithelial cells through p38 MAPK activation.
Kidney stone disease is a common health problem worldwide1,2 with calcium oxalate monohydrate (COM) as the major crystalline composition of kidney stone matrix3. Adhesion of COM crystals on renal tubular epithelial cell surface is a crucial mechanism for kidney stone formation4,5. This pathogenic process leads to several cellular responses, including overproduction of reactive oxygen species (ROS)6,7, cellular injury8, and finally tissue inflammation9. Furthermore, our previous study has demonstrated that COM crystals can cause the increased paracellular permeability, loss of cell polarity and disruption of tight junction10. However, the cellular signaling involved in COM crystal-induced tight junction disruption remained unknown.
Mitogen-activated protein kinase (MAPK) causes intracellular signaling cascades that cells use to respond to various stimuli. p38 MAPK is one of signaling proteins in MAPK pathway that can be activated by various extracellular stresses, e.g., UV radiation11, hydrogen peroxide12, and proinflammatory cytokines13. Interestingly, p38 MAPK also plays role in modulation of tight junction function by regulating expression of tight junction proteins in response to several stimuli. In renal tubular cells, p38 MAPK can be activated by tumor necrotic factor α (TNFα) and interferon γ (IFNγ), leading to down-regulation of occludin, zonula occludens-1 (ZO-1) and claudin-2, which contributes to the increase in paracellular permeability14. In contrast, inhibition of basal p38 MAPK can enhance the barrier function of tight junction in mammary epithelial cells15. On these bases, we hypothesized that COM crystal-induced tight junction disruption may be mediated by p38 MAPK pathway.
This present study is an extended work of our previous report10 with a specific aim to address whether p38 MAPK plays a significant role in COM- crystal-induced tight junction disruption in polarized Madin-Darby canine kidney (MDCK) cells derived from distal nephron16,17. Expression levels of total p38, active form of p38 (phospho-p38), and two main tight junction proteins (occludin and ZO-1) were examined by Western blot analysis, whereas the distribution and co-localization of occludin and ZO-1 were evaluated by immunofluorescence study. In addition, transepithelial resistance (TER) was also measured to examine the barrier function, whereas translocation of Na+/K+-ATPase-α1 (a marker for basolateral membrane) to apical membrane was investigated to examine the fence function of tight junction in response to COM crystals. Moreover, a specific inhibitor of p38 MAPK activation, SB239063, was employed to examine whether the inhibition of p38 MAPK pathway could prevent the aforementioned defects of tight junction induced by COM crystals.
Results
Effects of differential doses of COM crystals on cell death
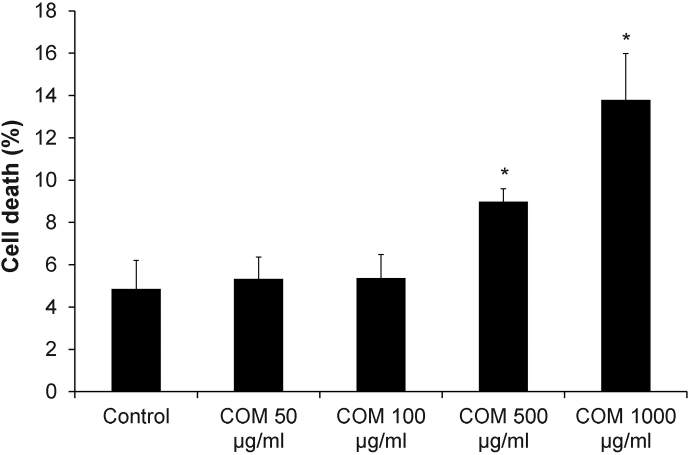
Annexin V/propidium iodide co-staining followed by flow cytometry was performed to examine the effects of differential doses of COM crystals on cell death. After cells were incubated with various doses of COM crystals for 48-h, the data revealed that low doses (50–100 μg/ml) of COM crystals had no effect on cell death, whereas higher doses (500–1000 μg/ml) caused significant increase of cell death in a dose-dependent manner (Figure 1).
Figure 1. Cell death assay.
Annexin V/propidium iodide co-staining followed by flow cytometry analysis was performed after MDCK cells were exposed to various doses of COM crystals for 48-h. Percentage of total cell death = [(number of both apoptotic and necrotic cells/number of all cells) × 100%]. N = 3 independent experiments for each bar; * = P<0.05 vs. control.
Effects of differential doses of COM crystals on the integrity of tight junction barrier
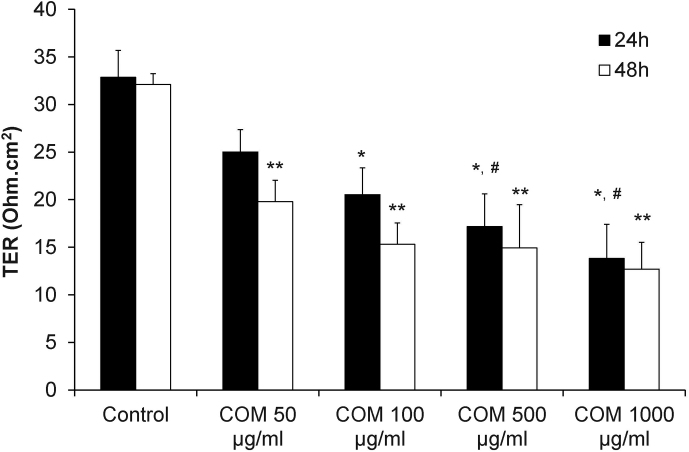
Transepithelial resistance (TER) was measured to examine the effects of differential doses of COM crystals on the integrity of tight junction barrier of polarized MDCK cells. The results showed significant decrease of TER in a dose-dependent manner at both 24-h and 48-h after exposure to COM crystals (Figure 2).
Figure 2. Measurement of transepithelial resistance (TER) revealed dose-dependent COM-induced decrease of TER.
TER was measured after the polarized MDCK cells were exposed to various doses of COM crystals for 24-h and 48-h. N = 3 independent experiments for each bar; * = P<0.01 vs. control at 24-h; ** = P<0.01 vs. control at 48-h; # = P<0.05 vs. 50 μg/ml COM-treated group at 24-h.
Based on the data shown in Figures 1 and 2, the dosage at 100 μg/ml of COM crystals was used for all subsequent experiments (as we aimed to address the effects of COM crystals on tight junction when the cells were not too toxic).
p38 MAPK in polarized MDCK cells was activated by COM crystals
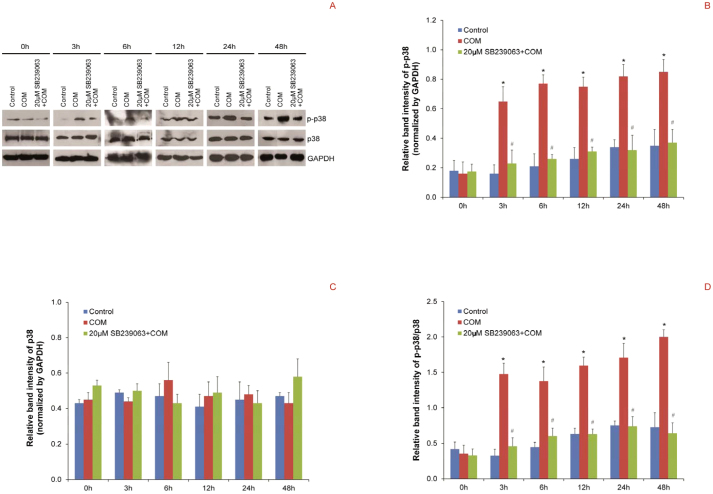
Western blot analysis for phospho-p38, the active form of p38, was performed to examine the effects of COM crystals on p38 MAPK pathway in polarized MDCK cells. The data revealed that exposure of polarized MDCK cells to COM crystals had caused significantly increased level of phospho-p38 since 3-h until 48-h after COM crystal exposure, whereas the level of total p38 remained unchanged at all time-points (Figure 3). Moreover, pretreatment of the cells with a specific inhibitor of p38 MAPK activation, SB239063, successfully maintained phospho-p38 at its basal level (Figure 3) at all time-points. These results indicate that p38 MAPK was activated in polarized MDCK cells by COM crystals, and SB239063 could prevent COM crystal-induced p38 MAPK activation. If p38 MAPK pathway is important for regulating COM crystal-induced tight junction disruption, SB239063 should be able to prevent expression and functional defects in tight junction protein complex induced by COM crystals.
Figure 3. Western blot analysis revealed that p38 MAPK in polarized MDCK cells was activated by COM crystals.
(A) Proteins derived from whole cell lysates of controlled and COM-treated (100 μg/ml) cells at various time-points, without or with 2-h pretreatment by 20 μM SB239063 (an inhibitor of p38 MAPK activation), were equally loaded into each of SDS-PAGE lane (30 μg/lane). The resolved proteins were subjected to Western blot analysis using rabbit polyclonal anti-phospho-p38 or mouse monoclonal anti-p38 antibody as the primary antibody. GAPDH served as the loading control. (B) and (C) Band intensities of phospho-p38 and total p38, respectively, were measured by a densitometer and normalized with that of GAPDH. (D) The ratio of band intensities of phospho-p38 and total p38 (p-p38/p38) was also determined. N = 3 independent experiments for each bar; * = P<0.05 vs. control at corresponding time-point; # = P<0.05 vs. COM-treated group at corresponding time-point.
Inhibition of p38 MAPK activation successfully prevented the decreased expression levels, redistribution and dissociation of tight junction proteins in polarized MDCK cells induced by COM crystals
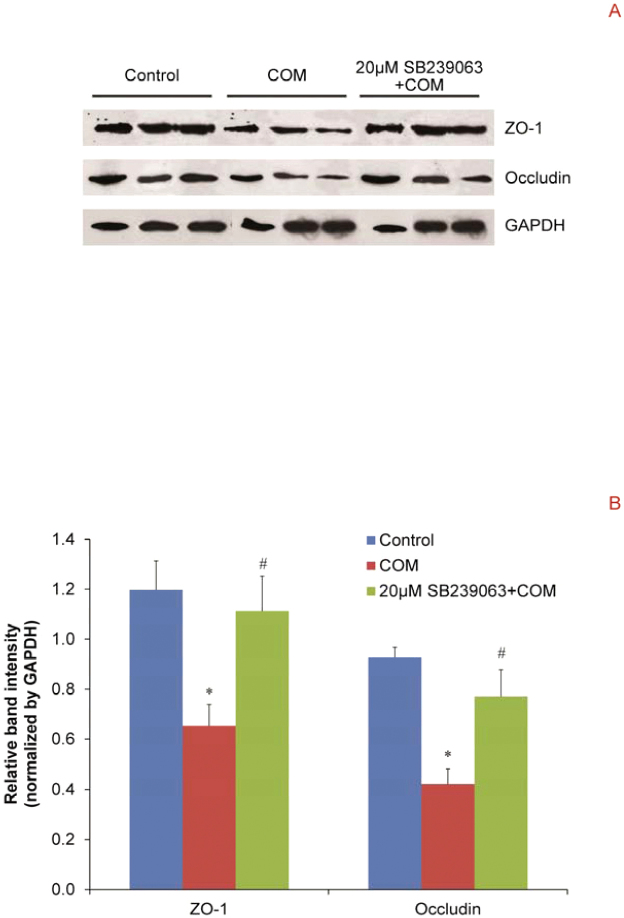
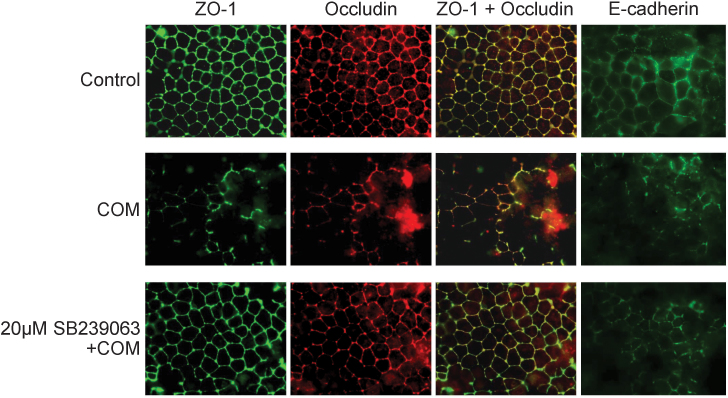
We evaluated whether p38 MAPK pathway plays an important role in regulation of COM crystal-induced decreased levels, redistribution and dissociation of two main tight junction proteins, ZO-1 and occludin, in polarized MDCK cells. Western blot analysis revealed that COM crystals significantly reduced expression levels of ZO-1 and occludin, whereas pretreatment with SB239063 successfully maintained the levels of these two tight junction proteins at their basal levels (Figure 4). Immunofluorescence study confirmed that COM crystals caused disruption of tight junction, decreased levels, redistribution and dissociation of ZO-1 and occludin in polarized MDCK cells (Figure 5). However, pretreatment with SB239063 could preserve tight junction structure, honey comb appearance of expression pattern of ZO-1 and occludin, as well as their co-localization (Figure 5).
Figure 4. Western blot analysis revealed that inhibition of p38 MAPK activation successfully prevented the decreased expression levels of tight junction proteins in polarized MDCK cells induced by COM crystals.
(A) Proteins derived from whole cell lysate of controlled and COM-treated (100 μg/ml) cells without or with 2-h pretreatment by 20 μM SB239063 were equally loaded into each of SDS-PAGE lane (30 μg/lane). The resolved proteins were then subjected to Western blot analysis for ZO-1 and occludin, whereas GAPDH served as the loading control. (B) Band intensity levels were measured by a densitometer and the relative band intensity levels normalized with GAPDH were compared by one-way ANOVA. N = 3 independent experiments for each bar; * = P<0.05 vs. control; # = P<0.05 vs. COM-treated group.
Figure 5. Immunofluorescence study revealed that inhibition of p38 MAPK activation successfully prevented the decreased expression levels, redistribution and dissociation of tight junction proteins, and partially prevented defects in adherens junction of polarized MDCK cells induced by COM crystals.
The polarized MDCK cells were pretreated without or with 20 μM SB239063 for 2-h and then incubated with 100 μg/ml COM crystals for 48-h. Subsequently, the cells were subjected to co-staining for ZO-1(in green, first column) and occludin (in red, second column). The third column shows the merged view of both ZO-1 and occludin, and their co-localization is illustrated in yellow. The cells were also examined for E-cadherin (in green; last column). The polarized MDCK cells grown in COM-free medium served as the controls. Original magnification was 200× for all panels.
Effects of COM crystals on adherens junction in polarized MDCK cells
Tight junction formation is also associated with adherens junction. In addition to the investigations on tight junction protein complex, we also evaluated the effects of COM crystals on adherens junction and the role of p38 MAPK pathway in this response. Immunofluorescence study revealed that COM crystals induced disruption of adherens junction and decreased level of epithelial cadherin (E-cadherin) in the polarized MDCK cells (Figure 5). And this defect could be partially prevented by pretreatment with SB239063 (Figure 5).
Inhibition of p38 MAPK activation partially preserved the barrier function of tight junction in polarized MDCK cells treated with COM crystals
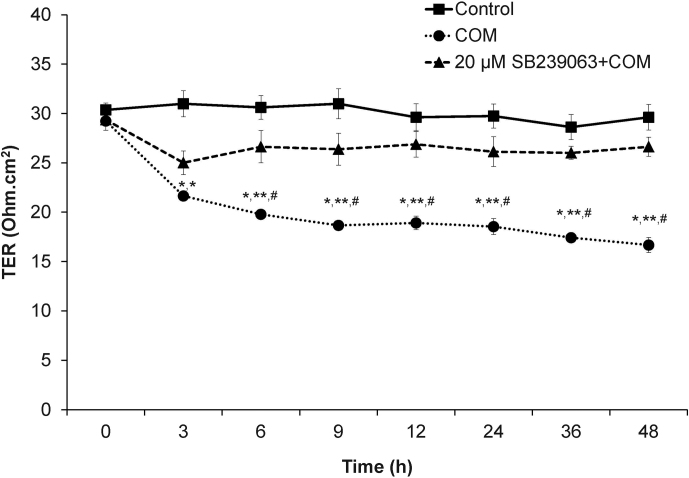
One of the main functions of tight junction is to serve as a paracellular “barrier” to control paracellular transports of water and solutes. We measured TER across polarized MDCK cell monolayer to examine the role of p38 MAPK in COM-induced increase of paracellular permeability. As expected, the results showed that COM crystals led to dramatic decrease in TER (increased permeability) across all time-points (Figure 6). Pretreatment with SB239063 partially prevented the defective barrier function of tight junction in polarized MDCK cells induced by COM crystals (Figure 6).
Figure 6. Measurement of transepithelial resistance (TER) revealed that inhibition of p38 MAPK activation partially preserved the barrier function of tight junction in polarized MDCK cells treated with COM crystals.
The polarized MDCK cells grown in TranswellsTM were pretreated without or with 20 μM SB239063 for 2-h before incubation with 100 μg/ml COM crystals for up to 48-h. TER was serially measured at 0, 3, 6, 9, 12, 24, 36 and 48-h after COM treatment. The polarized MDCK cells grown in COM-free medium served as the controls. N = 3 independent experiments for each data point; * = P<0.005 vs. TER of the controlled cells at the corresponding time-point; ** = P<0.0005 vs. the basal TER level; # = P<0.05 vs. TER of COM-treated cells pretreated with 20 μM SB239068 at the corresponding time-point.
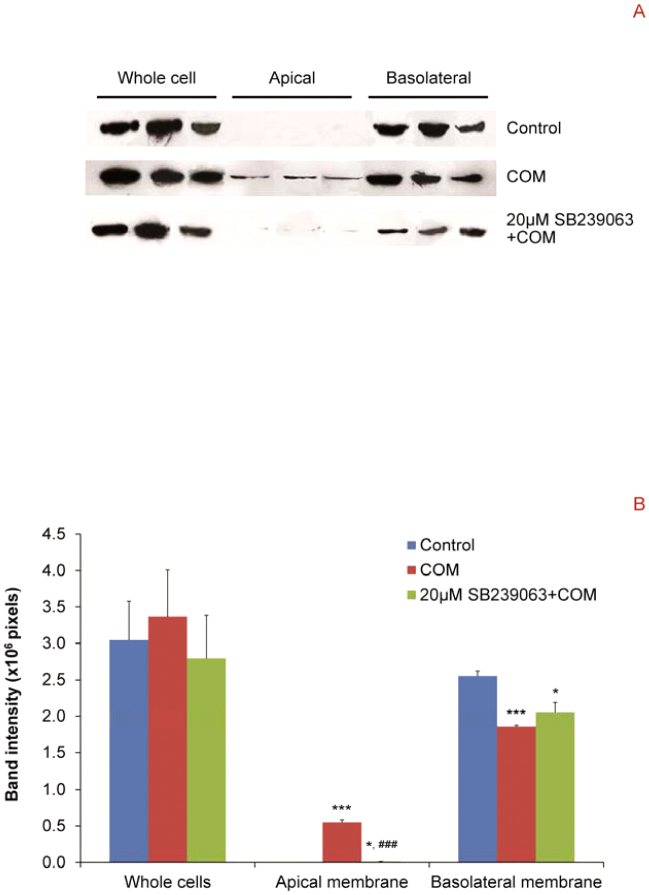
Inhibition of p38 MAPK activation successfully prevented defective fence function of tight junction in polarized MDCK cells induced by COM crystals
Another main function of tight junction is to serve as a “fence” to divide epithelial membranes into apical and basolateral membranes. We performed Western blot analysis to examine whether p38 is involved in regulation of defective fence function of tight junction in polarized MDCK cells induced by COM crystals. The data clearly demonstrated that COM crystals induced the translocation of Na+/K+-ATPase-α1 (a marker for basolateral membrane) from basolateral to apical membranes (Figure 7). However, the COM crystal-induced loss of fence function was attenuated by SB239063 pretreatment (Figure 7). Note that the attenuation of defective fence function by SB239063 was not entirely complete as there was a (very) minimal level of Na+/K+-ATPase-α1 remained detectable at apical membrane and its expression level at basolateral membrane was still less than the control (Figure 7).
Figure 7. Western blot analysis revealed that inhibition of p38 MAPK activation successfully prevented defective fence function of tight junction in polarized MDCK cells induced by COM crystals.
(A) Proteins derived whole cell lysate, apical and basolateral membranes of controlled and COM-treated (100 μg/ml) cells without or with 20 μM SB239063 pretreatment were subjected to Western blot analysis for Na+/K+ATPase-α1, which is a marker of basolateral membrane. Note that equal number of the cells (3.0 × 105 cells) was used for sample preparation of each sample. (B) Band intensity levels were measured by a densitometer and compared by one-way ANOVA. N = 3 independent experiments for each bar; * = P<0.05 vs. control; *** = P<0.0001 vs. control; ### = P<0.001 vs. COM-treated group.
Inhibition of p38 MAPK activation partially prevented overproduction of intracellular hydrogen peroxide in polarized MDCK cells induced by COM crystals
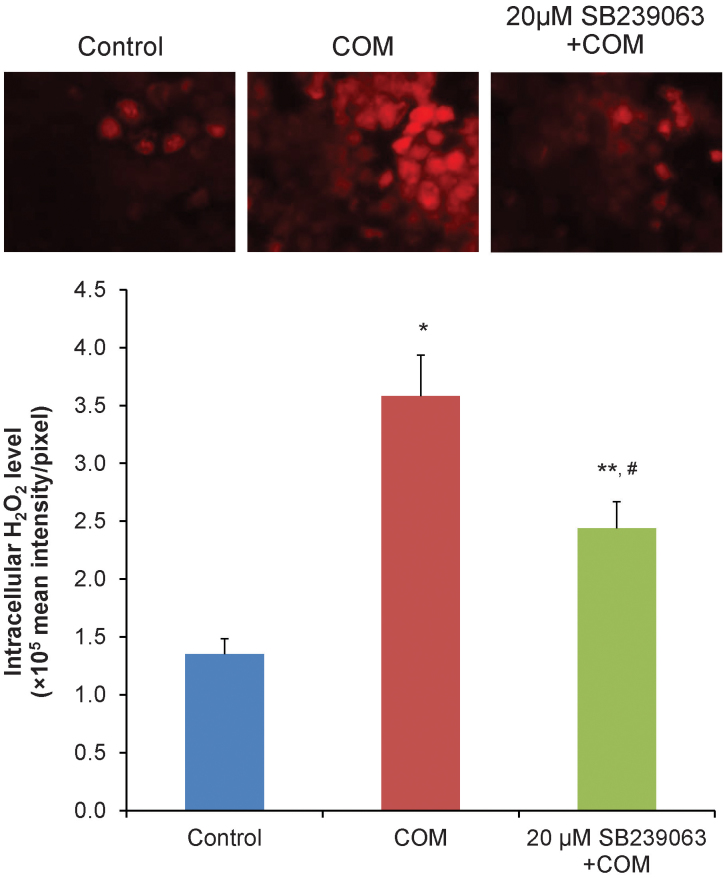
The ROS overproduction has been recognized as the p38 activator. We measured the intracellular hydrogen peroxide in polarized MDCK cells to examine the effects of COM crystals on ROS overproduction and the involvement of p38 MAPK. As expected, COM crystals caused the overproduction of the intracellular hydrogen peroxide (Figure 8). Interestingly, pretreatment with SB239063 partially prevented the overproduction of intracellular hydrogen peroxide induced by COM crystals (Figure 8).
Figure 8. Semiquantitative fluorescence assay revealed that COM crystals induced intracellular hydrogen peroxide production.
The polarized MDCK cells were pretreated without or with 20 μM SB239063 for 2-h and then incubated with 100 μg/ml COM crystals for 48-h. Subsequently, DHR123 was added into the culture medium and the fluorescence signal was detected by fluorescence microscope (upper panels). The mean florescence intensity was then quantitated (lower graph). N = 3 independent experiments of each bar; * = P<0.001 vs. control; ** = P<0.05 vs. control; # = P<0.05 vs. COM-treated group.
Discussion
Tight junction is the intercellular junction at apicolateral locale of epithelial cells18. This junction serves as a “paracellular barrier” to regulate and limit the passage of water, ions, macromolecules and pathogens through the paracellular route. Moreover, it also has a “fence” function to maintain the cell polarity by partitioning epithelial cell membranes into two parts – apical and basolateral membranes19. Tight junction protein complex composes of both transmembrane proteins (i.e., occludin20, claudin21 and junctional adhesion molecule22) and cytoplasmic proteins (i.e., ZO-123, ZO-224, ZO-325 and cingulin26). In addition, some proteins in this complex also bind to actin filament through distinct protein domains. Tight junction will function properly when it forms a stable protein complex. Dissociation of this protein complex or down-regulation of some proteins in this complex will lead to disruption and defects of the tight junction. Moreover, tight junction protein complex is also associated with signaling proteins that are involved in regulation of tight junction organization, integrity and functions27,28.
Our recent study has shown that COM crystals can induce tight junction disruption10. This may, at least in part, explain renal tubulointerstitial injury that frequently occurs in kidney stone disease. Moreover, COM crystal-induced tight junction disruption may be the linkage between intratubular and interstitial (Randall's plaque) theories of kidney stone pathogenesis10,29,30. Unfortunately, molecular mechanisms underlying COM crystal-induced tight junction disruption remained unknown. Several studies have suggested that tight junction can be disrupted by various stimuli through MAPK pathway14,31,32,33. A number of previous studies have indicated that p38 MAPK may be activated by oxalate34,35 and calcium36, both of which are the compositions of COM crystals. We therefore hypothesized that p38 MAPK might play significant role in COM crystal-induced tight junction disruption. To address our hypothesis, the inhibitor of p38 MAPK activation (SB239063) was employed to examine whether p38 MAPK activation was involved in expression and functional defects in tight junction of polarized MDCK cells induced by COM crystals. We first determined the appropriate dose and condition of COM crystal treatment that could be used to address effects of COM crystals on tight junction complex, as the cellular response to the crystal, not by severe cytotoxicity when the cells are too toxic to cope with the chemical stimulus. Based on the data shown in Figures 1 and 2, the dosage of 100 μg/ml COM crystals and 48-h incubation period were used for all subsequent experiments because the defect in tight junction barrier was clearly demonstrated whereas the total cell death remained unchanged. Using this condition, our previous study has reported that approximately 80% of MDCK cells were adhered by COM crystals and another 16% had the internalized COM crystals into the cells37.
We then examined whether p38 MAPK was activated in polarized MDCK cells upon COM crystal exposure. p38 is a member of MAPK pathway and can be activated by several stimuli, especially extracellular stresses11,12,13. The activated form of p38 MAPK is phosphorylated p38 MAPK38. Western blot analysis of time-course expression clearly showed that phospho-p38 level was increased in COM-treated polarized MDCK cells at all time-points, starting at 3-h after COM exposure, whereas total p38 remained unchanged (Figure 3). Moreover, SB239063 pretreatment successfully prevented the increased level of phospho-p38 in the COM-treated cells. These data indicated that p38 MAPK was activated in polarized MDCK cells by COM crystals. Furthermore, COM crystals could induce intracellular hydrogen peroxide overproduction, whereas pretreatment with SB239063 partially attenuate this effect (Figure 8). Our data were consistent with those reported in several previous studies demonstrating that the phosphorylated form of p38 MAPK was increased in a variety of cells, which were exposed to various stimuli, including oxidative stress12,33,39.
p38 MAPK is well recognized as a regulator of numerous genes, including transcription factors38,40. It plays significant roles in regulation of expression and phosphorylation of proteins. Several lines of evidence have also suggested that the activation of p38 MAPK by proinflammatory cytokines, pathogens and oxidative stress can lead to down-regulation of tight junction proteins, i.e., claudin 2, claudin 11, occludin and ZO-1, in diverse cells14,31,32,33. In addition, activation of p38 MAPK by alcohol can lead to phosphorylation of tight junction proteins and ultimately loss of the barrier function31. We thus hypothesized that COM crystal-induced p38 MAPK activation was associated with altered expression of tight junction proteins in polarized MDCK cells. Our hypothesis was addressed by Western blot analysis and immunofluorescence study of ZO-1 and occludin, which are commonly used to demonstrate the disruption of tight junction by various stimuli, e.g., oxidative stress, proinflammatory cytokines and pathogens41,42,43,44,45,46,47. The data clearly demonstrated that inhibition of p38 MAPK activation by SB239063 pretreatment successfully prevented the decreased expression levels (Figure 4), redistribution and dissociation of tight junction proteins in polarized MDCK cells induced by COM crystals (Figure 5). These data confirmed the hypothesis that COM crystal-induced p38 MAPK activation was associated with altered expression (i.e., decreased levels, redistribution and dissociation) of tight junction proteins in polarized renal tubular epithelial cells.
Adherens junction has been known as a crucial structure for tight junction formation48,49. Furthermore, there is evidence indicating that activation of p38 MAPK can lead to disruption of adherens junction50. We therefore hypothesized that COM crystals could also disrupt adherens junction using E-cadherin as the marker. The immunofluorescence study clearly confirmed our hypothesis. The data also revealed that inhibition of p38 MAPK activation by SB239063 pretreatment partially prevented the decreased expression level and disruption of adherens junction induced by COM crystals (Figure 5).
In addition to expression study, we also performed functional analyses of the role for p38 MAPK in tight junction functions. As a “barrier”, tight junction regulates the paracellular permeability by regulating the paracellular passage of water, ions, macromolecules and other substances. The impairment of barrier function is associated with leakage of these substances from the intratubular lumen to the interstitial compartment, or vice versa, which may eventually lead to cellular injury and tissue inflammation. TER was measured to reflect tight junction integrity and epithelial permeability to ions51. The data revealed that TER was dramatically decreased in COM-treated polarized MDCK cells, whereas the inhibition of p38 MAPK activation by SB239063 pretreatment could partially preserve the barrier function of tight junction (Figure 6). Our data were consistent with those of other previous studies, which have demonstrated that effects of some stimuli on epithelial permeability are less when p38 MAPK activation is inhibited39,52, and that the inhibition of basal p38 MAPK activity can enhance the epithelial barrier function15. As a “fence”, tight junction plays critical roles in maintaining epithelial cell polarity and preventing translocation of apical membrane proteins and lipids to basolateral membranes, or vice versa. Western blot analysis of basolateral membrane protein, Na+/K+-ATPase-α1, revealed that COM crystal-induced translocation of this protein from basolateral to apical membranes of polarized MDCK cells could be prevented (although partially) by inhibition of p38 MAPK activation with SB239063 pretreatment. These data confirmed that the defective barrier and fence functions of tight junction induced by COM crystals were regulated by p38 MAPK pathway.
It should be noted that COM crystal-induced defects in barrier and fence functions of tight junction, as well as overproduction of intracellular hydrogen peroxide, were not completely protected by SB239063 pretreatment (Figures 6–8), probably due to two main possibilities. First, the condition for inhibition of p38 MAPK activation might not yet be optimal. The most appropriate dosage and incubation period of pretreatment with SB239063 should be further defined to achieve the optimal yield of such prevention. Second, p38 MAPK might not be the only pathway involved in COM crystal-induced tight junction disruption. There might be some other signaling pathways that also played roles (although not as the major players) in this process.
In conclusion, we have demonstrated for the first time that COM crystals cause disruption and defective barrier and fence functions of tight junction of distal renal tubular epithelial cells through p38 MAPK activation. Our findings add significant information on the pathophysiology of kidney stone disease.
Methods
Cell polarization and treatment with COM crystals with or without pretreatment by a p38 MAPK activation inhibitor
Madin-Darby canine kidney (MDCK) cells were cultivated and polarization was performed as described in Supplementary Methods. COM crystals were prepared as described previously53,54. The polarized MDCK cells were subjected to pretreatment with or without 20 μM SB239063 (Tocris Bioscience; Bristol, UK), which is a specific inhibitor of p38 MAPK activation, for 2-h. Thereafter, the cells were incubated with COM crystals (by adding COM crystals into the culture medium with a ratio of 100 μg crystals/ml culture medium) for 0, 3, 6, 12, 24 and 48-h. The controlled cells were maintained in COM-free medium that was initially exposed to 100 μg/ml COM crystals (for 30 min), which were then removed from the medium to obtain identical concentrations of free calcium and oxalate ions in the medium comparing to those of COM crystal-containing medium (as a minimal, but non-negligible, amount of solid COM crystals could be solubilized in the culture medium). The controlled and COM-treated cells were then subjected to the following expression and functional analyses, all of which were done in triplicate.
Isolation of apical and basolateral membranes, and protein extraction
Apical membranes were isolated by a recently established peeling method55 based on hydrous affinity and ionic interaction between a surface (paper, membrane, or a solid surface) and apical membranes of the cells (additional details for this isolation technique can be found in Supplementary Methods). Proteins were extracted from whole cells, apical and basolateral membranes for subsequent Western blot analysis using 1× Laemmli's buffer. Protein concentrations in individual samples were measured by the Bradford method using Bio-Rad Protein Assay (Bio-Rad Laboratories; Hercules, CA).
Western blot analyses
Proteins derived from whole cell lysate with an equal amount of 30 μg/lane were resolved by 10% SDS-PAGE for Western blot analyses of p38 MAPK, phospho-p38, ZO-1, and occludin. For Western blot analysis of Na+/K+-ATPase-α1, an equal number of priming cells (3×105 cells/sample/lane) was used for isolation of apical and basolateral membranes, and all the recovered proteins derived from each sample were resolved in each lane of 10% SDS-PAGE gel. The resolved proteins were then transferred onto nitrocellulose membranes using a semi-dry transfer apparatus (Bio-Rad; Milano, Italy). Non-specific bindings were blocked with 5% skim milk in PBS for 1-h at room temperature. The membranes were then incubated with mouse monoclonal anti-p38 (Santa Cruz Biotechnology; Santa Cruz, CA), rabbit polyclonal anti-phospho-p38 (Santa Cruz Biotechnology), mouse monoclonal anti-ZO-1 (Zymed; San Francisco, CA), rabbit polyclonal anti-occludin (Santa Cruz Biotechnology), mouse monoclonal anti-Na+/K+-ATPase-α1 (Santa Cruz Biotechnology), or mouse monoclonal anti-GAPDH (glyceroldehyde-3-phosphate dehydrogenase) (Santa Cruz Biotechnology) antibody at 4°C overnight. All these primary antibodies were diluted 1:1000 with 1% skim milk/PBS. After three washes with PBS, the membranes were incubated with corresponding secondary antibody conjugated with horseradish peroxidase (1:2000 in 1% skim milk/PBS) at room temperature for 1-h. Immunoreactive protein bands were then visualized by SuperSignal West Pico chemiluminascence substrate (Pierce Biotechnology; Rockford, IL) and autoradiogram. Band intensity levels were measured by Image Master densitometer (GE Healthcare; Uppsala, Sweden).
Immunofluorescence study
MDCK cells (approximately 5×105 cells) were cultivated on cover slips (cleaved mica disk diameter: 9.5 mm, V-1 grade; SPI Supplier; Toronto, Canada). After the cells were pretreated with or without 20 μM SB239063 for 2-h followed by incubation in COM crystal-containing or COM-free medium for 48-h, they were rinsed with ice-cold membrane-preserving buffer. The cells were then fixed with 3.7% formaldehyde for 15-min and permeabilized with 0.1% Triton X-100 for 15-min. For co-staining of ZO1 and occludin, after another washing step with ice-cold membrane-preserving buffer, the cells were incubated with mouse monoclonal anti-ZO1 and rabbit polyclonal anti-occludin (both were purchased from Santa Cruz Biotechnology and diluted 1:50 in 1% skim milk/PBS) at 37°C for 1-h. Thereafter, the cells were incubated with AlexaFluor-488-conjugated goat anti-mouse IgG (1:5000 in 1% skim milk/PBS; Invitrogen/Molecular Probes; Burlington, Canada), Cy3-conjugated donkey anti-rabbit IgG (1:5000 in 1% skim milk/PBS; Jackson ImmunoResearch Laboratories; West Grove, PA) and Hoechst dye (1:1000 in PBS for nuclear stain; Invitrogen/Molecular Probes) at 37°C for 1-h. For single staining of E-cadherin after cells were fixed and permeabilized, the cells were incubated with rat monoclonal anti-E-cadherin (1:50 in 1% skim milk/PBS; Santa Cruz Biotechnology) at 37°C for 1-h. The cells were then incubated with AlexaFluor-488-conjugated goat anti-rat IgG (1:5000 in 1% skim milk/PBS; Invitrogen/Molecular Probes) and Hoechst dye (1:1000 in PBS for nuclear stain; Invitrogen/Molecular Probes) at 37°C for 1-h. The cover slips were then mounted with 50% glyceral/PBS and examined by using a laser-scanning confocal microscope equipped with LSM5 Image Browser (LSM 510 META, Carl Zeiss; Oberkochen, Germany).
Measurement of transepithelial resistance (TER)
The resistance across the polarized MDCK cell monolayer was serially measured using Millicell-ERS resistance system (Millipore; Bedford, MA) after the cells were pretreated with or without 20 μM SB239063 followed by incubation in COM crystal-containing or COM-free medium for 0, 3, 6, 9, 12, 24, 36 and 48-h. TER was measured at 3 different sites in each transwell and the background obtained from the blank control was subtracted. The net resistance was multiplied by the membrane area to give the resistance in Ohm·cm2.
Semiquantitative analysis of intracellular hydrogen peroxide by fluorescence microscopy
MDCK cells (approximately 5×105 cells) were cultivated on cover slips with or without 2-h pretreatment by 20 μM SB239063 followed by incubation in COM crystal-containing or COM-free medium for 48-h. Thereafter, the cells were rinsed with PBS and then incubated with 10 μM dihydrorhodamine 123 (Sigma; St Louis, MO) at 37°C for 15 min. After the excess dye was removed, the cover slips were mounted with 50% glycerol/PBS and the fluorescence signal was detected using a fluorescence microscope. The quantitative data (fluorescence intensity) was obtained from 15 high power fields (HPF) of each group using ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical analysis
All the experiments were done in triplicate and quantitative data are presented as mean ± SEM. Comparisons of the data among different groups were performed by one-way ANOVA using SPSS software version 13.0 (SPSS; Chicago, IL). P values less than 0.05 were considered statistically significant.
Author Contributions
PP and VT designed research; PP performed experiments; PP and VT analyzed data; PP and VT wrote the manuscript.
Supplementary Material
Supplementary Methods
Acknowledgments
This study was supported by Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative, and The Thailand Research Fund (RTA5380005) to VT. PP is supported by the Royal Golden Jubilee PhD Program of The Thailand Research Fund, whereas VT is also supported by “Chalermphrakiat” Grant and Faculty of Medicine Siriraj Hospital.
References
- Amato M., Lusini M. L. & Nelli F. Epidemiology of nephrolithiasis today. Urol. Int. 72 Suppl 1, 1–5 (2004). [DOI] [PubMed] [Google Scholar]
- Coe F. L., Evan A. & Worcester E. Kidney stone disease. J Clin. Invest 115, 2598–2608 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert G. Stone analysis. Urol. Res. 34, 146–150 (2006). [DOI] [PubMed] [Google Scholar]
- Evan A. P., Coe F. L., Lingeman J. E. & Worcester E. Insights on the pathology of kidney stone formation. Urol. Res. 33, 383–389 (2005). [DOI] [PubMed] [Google Scholar]
- Lieske J. C., Deganello S. & Toback F. G. Cell-crystal interactions and kidney stone formation. Nephron 81 Suppl 1, 8–17 (1999). [DOI] [PubMed] [Google Scholar]
- Davalos M., Konno S., Eshghi M. & Choudhury M. Oxidative renal cell injury induced by calcium oxalate crystal and renoprotection with antioxidants: a possible role of oxidative stress in nephrolithiasis. J Endourol. 24, 339–345 (2010). [DOI] [PubMed] [Google Scholar]
- Thamilselvan S., Khan S. R. & Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol. Res. 31, 3–9 (2003). [DOI] [PubMed] [Google Scholar]
- Hackett R. L., Shevock P. N. & Khan S. R. Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol. Res. 22, 197–203 (1994). [DOI] [PubMed] [Google Scholar]
- Khan S. R. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin. Exp. Nephrol. 8, 75–88 (2004). [DOI] [PubMed] [Google Scholar]
- Peerapen P. & Thongboonkerd V. Effects of calcium oxalate monohydrate crystals on expression and function of tight junction of renal tubular epithelial cells. Lab Invest 91, 97–105 (2011). [DOI] [PubMed] [Google Scholar]
- Staples C. J., Owens D. M., Maier J. V., Cato A. C. & Keyse S. M. Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J Biol. Chem. 285, 25928–25940 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. C., Klausen C. & Leung P. C. Hydrogen peroxide mediates EGF-induced down-regulation of E-cadherin expression via p38 MAPK and snail in human ovarian cancer cells. Mol. Endocrinol. 24, 1569–1580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Cytokine-induced epithelial permeability changes are regulated by the activation of the p38 mitogen-activated protein kinase pathway in cultured Caco-2 cells. Shock 29, 531–537 (2008). [DOI] [PubMed] [Google Scholar]
- Patrick D. M., Leone A. K., Shellenberger J. J., Dudowicz K. A. & King J. M. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC. Physiol 6, 2 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozzino F., Pugnale P., Feraille E. & Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am. J Physiol Cell Physiol 297, C775–C787 (2009). [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Chuman L. M., Shaffer L. & Saier M. H. Jr. Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J Cell Biol. 81, 635–648 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H. Jr. Growth and differentiated properties of a kidney epithelial cell line (MDCK). Am. J Physiol 240, C106–C109 (1981). [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Betanzos A., Nava P. & Jaramillo B. E. Tight junction proteins. Prog. Biophys. Mol. Biol. 81, 1–44 (2003). [DOI] [PubMed] [Google Scholar]
- Matter K. & Balda M. S. Functional analysis of tight junctions. Methods 30, 228–234 (2003). [DOI] [PubMed] [Google Scholar]
- Furuse M. et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 123, 1777–1788 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Furuse M., Fujimoto K. & Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A 96, 511–516 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Padura I. et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 142, 117–127 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S. & Goodenough D. A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 103, 755–766 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis L. A. & Goodenough D. A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 124, 949–961 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J., Gu L., Wittchen E. S., Hibbard J. & Stevenson B. R. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 141, 199–208 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S., Sabanay H., Jakes R., Geiger B. & Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature 333, 272–276 (1988). [DOI] [PubMed] [Google Scholar]
- Matter K. & Balda M. S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 4, 225–236 (2003). [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Tapia R. & Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 1778, 729–756 (2008). [DOI] [PubMed] [Google Scholar]
- Evan A. P. et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin. Invest 111, 607–616 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushinsky D. A. Nephrolithiasis: site of the initial solid phase. J Clin. Invest 111, 602–605 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K., Jiang Y. & Gupta S. Effects of chronic alcohol drinking on receptor-binding, internalization, and degradation of human immunodeficiency virus 1 envelope protein gp120 in hepatocytes. Alcohol 41, 591–606 (2007). [DOI] [PubMed] [Google Scholar]
- Xia W., Mruk D. D., Lee W. M. & Cheng C. Y. Differential interactions between transforming growth factor-beta3/TbetaR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol. Chem. 281, 16799–16813 (2006). [DOI] [PubMed] [Google Scholar]
- Lui W. Y., Wong C. H., Mruk D. D. & Cheng C. Y. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology 144, 1139–1142 (2003). [DOI] [PubMed] [Google Scholar]
- Chaturvedi L. S. et al. Oxalate selectively activates p38 mitogen-activated protein kinase and c-Jun N-terminal kinase signal transduction pathways in renal epithelial cells. J Biol. Chem. 277, 13321–13330 (2002). [DOI] [PubMed] [Google Scholar]
- Han H. J., Lim M. J. & Lee Y. J. Oxalate inhibits renal proximal tubule cell proliferation via oxidative stress, p38 MAPK/JNK, and cPLA2 signaling pathways. Am. J Physiol Cell Physiol 287, C1058–C1066 (2004). [DOI] [PubMed] [Google Scholar]
- Semenova M. M. et al. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat. Neurosci. 10, 436–443 (2007). [DOI] [PubMed] [Google Scholar]
- Chaiyarit S. & Thongboonkerd V. Changes in mitochondrial proteome of renal tubular cells induced by calcium oxalate monohydrate crystal adhesion and internalization are related to mitochondrial dysfunction. J Proteome. Res. 11, 3269–3280 (2012). [DOI] [PubMed] [Google Scholar]
- Ono K. & Han J. The p38 signal transduction pathway: activation and function. Cell Signal 12, 1–13 (2000). [DOI] [PubMed] [Google Scholar]
- Oshima T., Miwa H. & Joh T. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am. J Physiol Cell Physiol 295, C800–C806 (2008). [DOI] [PubMed] [Google Scholar]
- Zarubin T. & Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 (2005). [DOI] [PubMed] [Google Scholar]
- Basuroy S., Seth A., Elias B., Naren A. P. & Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J 393, 69–77 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. K., Baker R. D., Baker S. S., Gupta A. & Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am. J Physiol 273, G812–G823 (1997). [DOI] [PubMed] [Google Scholar]
- Sheth P., Basuroy S., Li C., Naren A. P. & Rao R. K. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol. Chem. 278, 49239–49245 (2003). [DOI] [PubMed] [Google Scholar]
- Rodriguez P., Heyman M., Candalh C., Blaton M. A. & Bouchaud C. Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl.19A cell monolayers. Cytokine 7, 441–448 (1995). [DOI] [PubMed] [Google Scholar]
- Tazuke Y., Drongowski R. A., Teitelbaum D. H. & Coran A. G. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr. Surg. Int. 19, 321–325 (2003). [DOI] [PubMed] [Google Scholar]
- Al Sadi R. M. & Ma T. Y. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 178, 4641–4649 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. et al. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol. 218, 210–221 (2009). [DOI] [PubMed] [Google Scholar]
- Contreras R. G. et al. E-Cadherin and tight junctions between epithelial cells of different animal species. Pflugers Arch. 444, 467–475 (2002). [DOI] [PubMed] [Google Scholar]
- West M. R., Ferguson D. J., Hart V. J., Sanjar S. & Man Y. Maintenance of the epithelial barrier in a bronchial epithelial cell line is dependent on functional E-cadherin local to the tight junctions. Cell Commun. Adhes. 9, 29–44 (2002). [DOI] [PubMed] [Google Scholar]
- Lv Z. M. et al. The role of the p38 MAPK signaling pathway in high glucose-induced epithelial-mesenchymal transition of cultured human renal tubular epithelial cells. PLoS. One. 6, e22806 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak A. S. & Miller W. M. Measurement of trans-epithelial electrical resistance in perfusion: Potential application for in vitro ocular toxicity testing. Biotechnol. Bioeng. 50, 568–579 (1996). [DOI] [PubMed] [Google Scholar]
- Costantini T. W. et al. Role of p38 MAPK in burn-induced intestinal barrier breakdown. J Surg. Res. 156, 64–69 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongboonkerd V., Semangoen T. & Chutipongtanate S. Factors determining types and morphologies of calcium oxalate crystals: Molar concentrations, buffering, pH, stirring and temperature. Clin. Chim. Acta 367, 120–131 (2006). [DOI] [PubMed] [Google Scholar]
- Thongboonkerd V., Chutipongtanate S., Semangoen T. & Malasit P. Urinary trefoil factor 1 is a novel potent inhibitor of calcium oxalate crystal growth and aggregation. J Urol. 179, 1615–1619 (2008). [DOI] [PubMed] [Google Scholar]
- Fong-ngern K., Chiangjong W. & Thongboonkerd V. Peeling as a novel, simple, and effective method for isolation of apical membrane from intact polarized epithelial cells. Anal. Biochem 395, 25–32 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods