Abstract
Although, single tablet regimen (STR) efavirenz, emtricibine, and tenofovir disoproxil fumarate (EFV/FTC/TDF) may be appealing in HIV infected persons who are at high risk for non-adherence, the degree to which this simplified formulation affects adherence is not known. The virologic effectiveness of this STR in a potentially non-adherent population remains a concern, given the rapid selection of drug-resistance seen with these drugs. We performed a prospective observational study assessing adherence and virologic response to EFV/FTC/TDF STR among a cohort of homeless and marginally housed individuals. We compared adherence and viral suppression to historical controls followed in the same cohort. Adherence was higher in EFV/FTC/TDF STR regimen compared to non-one-pill once daily therapy (p=0.0060) after controlling for multiple confounders. Viral suppression (HIV RNA <50 c/ml) was greater in EFV/FTC/TDF STR than non-one pill daily regimens (69.2% vs 46.5%; p=0.02), but there was no difference in viral suppression after controlling for adherence. Once daily EFV/TNF/FTC STR appears to be a reasonable option for individuals with multiple barriers to adherence. Randomized clinical trials addressing various therapeutic strategies for this patient population are needed.
Introduction
Early highly active antiretroviral therapy (HAART) regimens contained in excess of 20 pills per day divided in three doses. Adherence to these early regimens was often between 60-70% [1-3]. Because adherence was difficult, some argued that treatment should be withheld from challenging patients, including the homeless, mentally ill, or drug users [4]. Given that the US domestic HIV epidemic is increasingly concentrated in marginalized communities who have many of these risk factors[5], efforts are needed to define optimal management for marginalized populations with multiple adherence challenges.
Modern HAART regimens are now more potent and relatively simple to administer. It is now possible to prescribe three drugs (efavirenz, emtricitabine, and tenofovir disoproxil fumarate, or EFV/FTC/TDF) in the form of a single tablet regimen taken once daily. While several studies have shown that decreasing the pill burden and dosing frequency is associated with increased adherence [6-12], there are no data comparing adherence between EFV/FTC/TDF STR and other regimens.
In order to define adherence patterns and treatment outcomes associated with STRs, we compared adherence and viral suppression to EFV/FTC/TDF STR in a population of homeless and marginally housed people in the Tenderloin, South of Market and Mission Districts of San Francisco. We compared our results to that observed earlier in this same cohort with other commonly used and still relevant regimens. Adherence was measured prospectively with unannounced pill counts at the home or usual place of residence which are highly correlated with viral suppression, electronic medication monitored adherence, evolution of drug resistance and disease progression [3, 13, 14].
Methods
Study Design and Participant Recruitment
Participants were identified from The Research on Access to Care in the Homeless (REACH) cohort, a systematic sample of HIV-positive adults recruited from San Francisco homeless shelters, free meal programs, and low-income single-room-occupancy hotels. The REACH cohort enrolled 658 HIV-positive participants between July 1996 and November 2008. The objectives, rationale, and sampling methods have been previously described[15]. The study sample taking EFV/FTC/TDF STR was enriched by recruitment from HIV clinical care centers serving the same population of the REACH Cohort. Both existing participants from the REACH Cohort and participants from clinics caring for the REACH cohort started ART within 6 months of the first unannounced pill count adherence measurement.
After informed consent, individuals received structured interviewer administered interviews on demographics, housing, drug and alcohol use, self reported adherence and the Beck Depression Inventory-II (BDI) [16].
Written consent was obtained from all participants for adherence monitoring, monthly phlebotomy and assessment of viral load and CD4 cell count. The University of California, San Francisco Committee on Human Subjects Research approved all study procedures.
Periodic Adherence Assessments
As previously described, participants received unannounced pill counts every 3 to 6 weeks over a period of 6 months[3]. All antiretroviral medications were counted. Unannounced pill counts do not interfere with the use of pillbox organizers (“medisets”) and participants are unlikely to empty bottles prior to assessment (“pill dump”) because the visits are unscheduled. The calculated number of pills taken was divided by the total number of prescribed tablets during the same period to determine percent of doses taken. Mean adherence was calculated as the average of the monthly pill count determination over 6 months.
Biologic Measurements
Plasma HIV RNA levels were determined after 6 months of adherence monitoring using the HIV-1 Amplicor Monitor Version 1.5 ultrasensitive assay (Roche Molecular Systems, Alameda, California, USA). Plasma was processed and stored at −20 °C within 6 hours of collection.
Analysis
Regimens were classified as (1) EFV/FTC/TDF STR, (2) ritonavir-boosted protease inhibitor plus two nucleoside reverse transcriptase inhibitors (r-PI) or (3) non-nucleoside reverse transcriptase inhibitor plus two nucleoside reverse transcriptase inhibitors (NNRTI). The EFV/FTC/TDF STR group was compared to all STRs as well as r-PI and NNRTI subgroups. Mean adherence was calculated from all pill counts and was classified as ≥ or < 90%.. Virologic suppression was defined as a plasma HIV RNA level below 50 copies/ml.
Adherence over 6 months was compared by regimen type using generalized estimating equations controlling for multiple confounders, including age, gender, race, education (completed high school), injection drug use, homelessness, Beck Depression Inventory, calendar year of treatment initiation, prior antiretroviral exposure (antiretroviral naïve: yes/no and total duration of prior treatment), and CD4 nadir. We also compared the proportion of individuals with viral suppression by regimen with Fisher's exact test. We then compared viral suppression by regimen controlling for adherence to determine if any differences in viral suppression were related differences in regimen potency or adherence.
Results
A total of 118 participants were recruited. For the current analysis, forty-seven participants were on EFV/FTC/TDF STR, and historically 57 were on r-PI and 14 were on NNRTI regimens. Participants were largely non-white (61%), male (73%) and had a high prevalence of lifetime injection drug use (63%). Forty-one percent were depressed, defined by a BDI-II score of > 13. Participants were also largely nucleoside experienced (65%) and had a median of 27.6 months of prior antiretroviral therapy. Most of the ritonavir-boosted PI-based regimens included either lopinavir-ritonavir (46%) or atazanavir-ritonavir (54%). NNRTI-treated individuals were on nevirapine (57%) or efavirenz (43%). The dosing frequency in the r-PI group was 47% once daily, 51% twice daily and 2% three times daily. The dosing frequency in the NNRTI was 36% once daily and 64% twice daily. There were no significant sociodemographic, prior treatment, or adherence differences between EFV/FTC/TDF STR, non-one-pill daily, r-PI-treated, and NNRTI groups (Table 1). There were significant differences in calendar year of HAART initiation among the regimen types. The median year of antiretroviral initiation was 2008 for EFV/FTC/TDF STR, 2006 for r-PI, and 2002.5 for NNRTI (p<0.0001, Savage Exactrank sum).
Table 1.
Participant Characteristics by Treatment Group.
| EFV/FTC/TDF STR | r-PI | NNRTI | Non-one pill daily (r-PI and NNRTI) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender X2 2.15, p= 0.34 | n | ||||||||||
| male | 37 | 78.7 | 38 | 66.7 | 11 | 78.6 | 49 | 69.0 | 86 | 72.9 | |
| female | 10 | 21.3 | 19 | 33.3 | 3 | 21.4 | 22 | 31.0 | 32 | 27.1 | |
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
| Race X2 4.05 p=0.67 | white | 18 | 38.3 | 21 | 36.8 | 7 | 50.0 | 28 | 39.4 | 46 | 39.0 |
| black | 17 | 36.2 | 21 | 36.8 | 6 | 42.9 | 27 | 38.0 | 44 | 37.3 | |
| hispanic | 4 | 8.5 | 8 | 14.0 | 1 | 7.1 | 9 | 12.7 | 13 | 11.0 | |
| other | 8 | 17.0 | 7 | 12.3 | 0 | 0.0 | 7 | 9.9 | 15 | 12.7 | |
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
| Homeless ever X2 13.61 p=0.001 | no | 20 | 42.6 | 8 | 14.3 | 1 | 7.1 | 9 | 12.9 | 29 | 24.8 |
| yes | 27 | 57.5 | 48 | 85.7 | 13 | 92.9 | 61 | 87.1 | 88 | 75.2 | |
| missing | 0 | 1 | 0 | 1 | 1 | ||||||
| High School X2 1.43, p=0.49 | no | 13 | 27.7 | 20 | 35.7 | 3 | 21.4 | 23 | 32.9 | 36 | 30.8 |
| yes | 34 | 72.3 | 36 | 64.3 | 11 | 78.6 | 47 | 67.1 | 81 | 69.2 | |
| missing | 0 | 1 | 0 | 1 | 1 | ||||||
| IDU_ever X2 4.08 p=0.13 | no | 22 | 46.8 | 16 | 28.1 | 6 | 42.9 | 22 | 31.0 | 44 | 37.3 |
| yes | 25 | 53.2 | 41 | 71.9 | 8 | 57.1 | 49 | 69.0 | 74 | 62.7 | |
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
| Prior ARV treatment | no | 22 | 46.8 | 46 | 80.7 | 8 | 57.1 | 54 | 76.1 | 76 | 64.4 |
| yes | 25 | 53.2 | 11 | 19.3 | 6 | 42.9 | 17 | 23.9 | 42 | 35.6 | |
| missing | 0 | 0 | 0 | ||||||||
| age F=2.21,p=0.11 | Mean Std dev | 47.2 | 8.2 | 44.3 | 7.3 | 43.6 | 8.6 | 44.2 | 7.5 | 45.4 | 7.9 |
| 7.3 | |||||||||||
| Median iqr | 47.0 | 8.0 | 45.0 | 9.0 | 43.5 | 10.0 | 45.0 | 9.0 | 46.0 | 9.0 | |
| 9.0 | |||||||||||
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
| income F=1.16,p=0.32 | Mean Std dev | 821.6 | 394.9 | 730.8 | 471.7 | 638.5 | 371.1 | 712.6 | 452.7 | 756.0 | 432.2 |
| Median iqr | 853.0 | 623.0 | 747.0 | 463.0 | 677.5 | 411.0 | 747.0 | 496.0 | 762.0 | 503.0 | |
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
| nadir F=5.97,p=0.003 | Mean Std dev | 298 | 204 | 176 | 165 | 211 | 153 | 183 | 162 | 229 | 188 |
| Median iqr | 270 | 283 | 154 | 140 | 185 | 207 | 158 | 182 | 180 | 210 | |
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
| Log HIV RNA F=4.47,p=0.01 | Mean Std dev | 1.9 | 1.6 | 2.8 | 1.7 | 2.8 | 1.8 | 2.9 | 1.7 | 2.5 | 1.7 |
| Median iqr | 1.7 | 2.7 | 2.9 | 2.6 | 2.8 | 3.3 | 3.0 | 2.9 | 2.6 | 2.7 | |
| missing | 1 | 0 | 0 | 0 | 1 | ||||||
| BDI-II F=1.28,p=0.28 | Mean Std dev | 12.5 | 10.4 | 11.9 | 10.9 | 17.0 | 11.5 | 12.9 | 11.1 | 12.2 | 10.6 |
| Median iqr | 12.0 | 18.0 | 9.0 | 14.0 | 16.0 | 15.0 | 10.0 | 14.0 | 9.5 | 15.0 | |
| missing | 2 | 0 | 0 | 0 | 2 | ||||||
| Calendar year F=85.59,p=<0.0001 | Mean Std dev | 2007.5 | 0.5 | 2005.0 | 1.6 | 2003.2 | 1.4 | 2004.7 | 1.7 | 2005.8 | 2.0 |
| Median iqr | 2008 | 1.0 | 2006.0 | 1.0 | 2002.5 | 3.0 | 2005.0 | 3.0 | 2006.0 | 2.0 | |
| missing | 0 | ||||||||||
| Months on HAART F=3.12,p<0.048 | Mean Std dev | 24.9 | 34.3 | 33.6 | 29.5 | 12.4 | 12.8 | 29.5 | 28.3 | 27.6 | 30.8 |
| Median iqr | 6.0 | 37.0 | 28.0 | 49.0 | 8.5 | 14.0 | 20.0 | 48.0 | 16.0 | 42.0 | |
| missing | 0 | 0 | 0 | 0 | 0 | ||||||
Legend: Chi square for categorical variables and ANOVA for continuous variables were used to test for a non-random distribution for the EFV/FTC/TDF STR, NNRTI and r-PI groups
The mean adherence to EFV/FTC/TDF STR was 86% (SD±18%). This was higher than the mean adherence to all non-one-pill daily regimens (73%, SD±0.23%, P=0.001), to all r-PI regimens (75%, SD±21%, P=0.006), and all NNRTI regimens (68%, SD±26%, P=0.02). The proportion achieving 90% adherence was higher (58%) in the EFV/FTC/TDF STR than in the combined non-one-pill (35% p=0.02) group, the r-PI (37%, p=0.035) group, but did not reach statistical significance in the NNRTI (29%, p=0.072) group in univariable analyses. Adherence was greater in the EFV/FTC/TDF STR group than the non-one-pill daily subgroup in a generalized estimating equation (GEE) analysis controlling for gender, race, high school education, income, homelessness, injection drug use, nadir CD4, BDI, prior antiretroviral use, and calendar year (Table 2, p=0.0060). Adherence was also greater in the EFV/FTC/TDF STR group than the r-PI subgroup GEE analysis controlling for the same confounders (p=0.004). Higher CD4 nadir was also associated with better adherence, which may suggest that individuals presenting early for care may be better able to adhere than those delaying presentation with advanced disease. There were insufficient individuals (n=14) for a similar multivariable NNRTI subgroup analysis.
Table 2.
Generalized estimating equation predictors of adherence: EFV/FTC/TDF STR vs. non-one pill, once daily regimens (NNRTI and rPI combined).
| z | P | ||
|---|---|---|---|
| Treatment | EFV/FTC/TDF STR versus other | 2.75 | 0.006 |
| Male gender | 0.44 | 0.660 | |
| Race | White versus non-White | 1.25 | 0.210 |
| Education | High school versus no high school | 1.35 | 0.177 |
| Income | Per dollar | −1.66 | 0.097 |
| Homeless ever | 0.42 | 0.676 | |
| Injection drug use ever | 0.43 | 0.664 | |
| Nadir CD4 | Per cell/ml | 3.34 | 0.001 |
| BDI | Per point | 0.26 | 0.792 |
| Prior ARV treatment | 0.43 | 0.667 | |
| Calendar year | Per year | −0.02 | 0.985 |
| Prior cumulative months on HAART | Per month | 1.16 | 0.245 |
Viral suppression (defined as HIV RNA <50 copies/ml) was greater in the EFV/FTC/TDF STR (69%) group compared to either the non-one pill daily group (46%, p=0.02) or the r-PI (47%, p=0.034) group, but not in the NNRTI (43%, p=0.111) group. The high rates of viral suppression to NNRTI-based regimens at moderate adherence is consistent previous published results from this cohort[17]. The difference between EFV/FTV/TDF STR and r-PI group was not significant when controlling for adherence, suggesting that this difference was more closely related to differences in adherence than differences in regimen potency.
Many individuals with less than perfect adherence to an NNRTI based regimen exhibited viral suppression. Among the individuals on EFV/FTC/TDF STR, the virologic suppression rates at month 6 were 50%, 50%, 33%, 67% and 83% for the adherence categories 0-49% 50-74%, 75-79%, 80-89%, and 90-100% respectively. Viral suppression in the r-PI group was 20%, 18%, 50%, 56%, and 71% in the same adherence categories.
Discussion
In a difficult to treat population with a high prevalence of substance abuse, mental illnesses and limited access to housing, we found that adherence to EFV/FTC/TDF STR was higher than non-one-pill-daily regimens. Historically, adherence to most regimens has been between 60-80%. It is notable that this challenging patient population achieved 86% average adherence, despite many adherence barriers. These data are generally consistent with an emerging series of studies indicating simpler regimens are each associated with higher adherence[6-8, 10-12, 18].
It is also notable that virologic suppression rates were comparable to that seen in other clinic-based cohorts[19-21]. Although the non-randomized nature of our study makes it impossible to directly compare the relative effectiveness of various treatment options for patients at high risk of non-adherence, our study does provide support for the use of a regimen that many have argued should be avoided given the common perception that NNRTI-based regimens are “fragile” (where fragility is defined based on the propensity to develop resistance in the context of ongoing viral replication)..
Although these data support the use of the EFV/FTC/TDF STR in this patient population, several limitations to our study design should be considered. First, treatment regimens were not assigned randomly and unmeasured confounders may have affected our comparisons between various regimens. Many more patients receiving the non-one-pill-once-daily regimens were treatment naïve prior to HAART than patients receiving the other regimens included in our analysis. However, the differences in viral suppression between regimens were closely related to differences in adherence, but not calendar time or prior treatment history in multivariable analyses. Adherence was measured for a relatively short period and can change over time. Finally, drug resistance accumulation among those exhibiting incomplete viral suppression was not measured in this study, and would be predicted to be more common in those receiving EFV/FTC/TDF STR than those receiving boosted PI based regimens.
In summary we found that a one-pill per day STR was associated with good adherence and viral suppression in a challenging population. While current treatment guidelines[22] acknowledge the role of adherence and regimen convenience as factors for choosing a regimen, there is not an explicit recommendation for EFV/FTC/TDF STR for patients with multiple adherence barriers. Our findings suggest that EFV/FTC/TDF STR performs well in such a population. While a randomized clinical trial that targeted patients with significant adherence barriers is needed to confirm our results, such a study would be difficult to perform and to our knowledge no such study is currently being considered. In the absence of more definitive data, our study supports the use of EFV/FTC/TDF STR in this patient population. Simplification of therapy represents an important step forward in supporting adherence and treatment success.
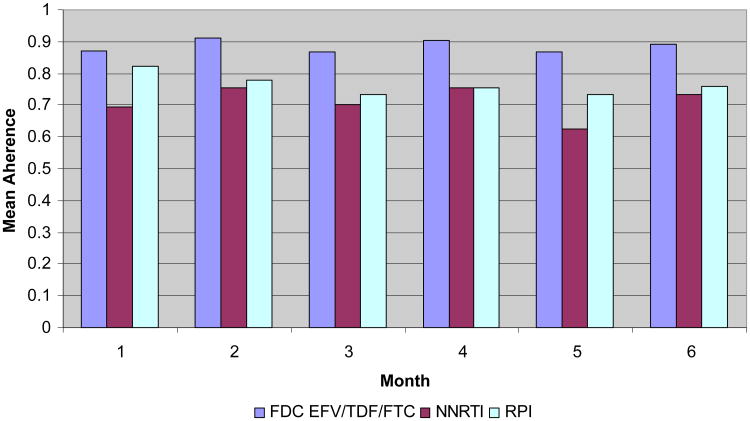
Figure 1. Mean Adherence by Regimen and Month.
Acknowledgments
This work was supported by National Institutes of Health (MH54907, AI069994); the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), Gilead Sciences and Bristol-Myers Squibb. Dr. Bangsberg received additional funding from MH87227. HIV RNA kits were donated by Roche. The funders had no role in the data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. Aids. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 4.Sontag D, Richardon L. Doctors withhold HIV pill regimen from some. Vol. 1997. New York Times; Sect A1. [PubMed] [Google Scholar]
- 5.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America -- Forgotten but Not Gone. N Engl J Med. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina JM, Podsadecki TJ, Johnson MA, Wilkin A, Domingo P, Myers R, et al. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 weeks. AIDS Res Hum Retroviruses. 2007;23:1505–1514. doi: 10.1089/aid.2007.0107. [DOI] [PubMed] [Google Scholar]
- 7.Portsmouth SD, Osorio J, McCormick K, Gazzard BG, Moyle GJ. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: a 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med. 2005;6:185–190. doi: 10.1111/j.1468-1293.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Clin Infect Dis. 2009. Better Adherence with Once-Daily Antiretroviral Regimens: A Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapper ML, Flexner C, Eron JJ, Molina JM. Simplifying antiretroviral therapy. AIDS Read. 2004;14:355-360–367-371. [PubMed] [Google Scholar]
- 10.Boyle BA, Jayaweera D, Witt MD, Grimm K, Maa JF, Seekins DW. Randomization to once-daily stavudine extended release/lamivudine/efavirenz versus a more frequent regimen improves adherence while maintaining viral suppression. HIV Clin Trials. 2008;9:164–176. doi: 10.1310/hct0903-164. [DOI] [PubMed] [Google Scholar]
- 11.Wright D, Rodriguez A, Godofsky E, Walmsley S, Labriola-Tompkins E, Donatacci L, et al. Efficacy and safety of 48 weeks of enfuvirtide 180 mg once-daily dosing versus 90 mg twice-daily dosing in HIV-infected patients. HIV Clin Trials. 2008;9:73–82. doi: 10.1310/hct0902-73. [DOI] [PubMed] [Google Scholar]
- 12.Airoldi M, Zaccarelli M, Bisi L, Bini T, Antinori A, Mussini C, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence. 4:115–125. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, Harrigan PR, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. Aids. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 14.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, Moss A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. Aids. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 15.Moss AR, Hahn JA, Perry S, Charlebois ED, Guzman D, Clark RA, Bangsberg DR. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 16.Beck A, Ward C, Mendelson M. An inventory for measuring depression. Archives of Psychiatry. 1961;4:561–567. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 18.Maggiolo F, Ripamonti D, Arici C, Gregis G, Quinzan G, Camacho GA, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3:371–378. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- 19.Jayaweera D, Dejesus E, Nguyen KL, Grimm K, Butcher D, Seekins DW. Virologic suppression, treatment adherence, and improved quality of life on a once-daily efavirenz-based regimen in treatment-Naive HIV-1-infected patients over 96 weeks. HIV Clin Trials. 2009;10:375–384. doi: 10.1310/hct1006-375. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Del Cacho E, Codina C, Tuset M, De Lazzari E, Mallolas J, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008;24:1263–1268. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- 21.Street E, Curtis H, Sabin CA, Monteiro EF, Johnson MA. British HIV Association (BHIVA) national cohort outcomes audit of patients commencing antiretrovirals from naive. HIV Med. 2009;10:337–342. doi: 10.1111/j.1468-1293.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 22.DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Washington, DC: Department of Health and Human Services; 2009. [Google Scholar]