Abstract
Objective
The objective of this study was to evaluate the development of functional auditory skills, language, and adaptive behavior in deaf children with cochlear implants (CI) who also have additional disabilities (AD).
Design
A two-group, pre-test versus post-test design was used.
Study sample
Comparisons were made between 23 children with CIs and ADs, and an age-matched comparison group of 23 children with CIs without ADs (No-AD). Assessments were obtained pre-CI and within 12 months post-CI.
Results
All but two deaf children with ADs improved in auditory skills using the IT-MAIS. Most deaf children in the AD group made progress in receptive but not expressive language using the Preschool Language Scale, but their language quotients were lower than the No-AD group. Five of eight children with ADs made progress in daily living skills and socialization skills; two made progress in motor skills. Children with ADs who did not make progress in language, did show progress in adaptive behavior.
Conclusions
Children with deafness and ADs made progress in functional auditory skills, receptive language, and adaptive behavior. Expanded assessment that includes adaptive functioning and multi-center collaboration is recommended to best determine benefits of implantation in areas of expected growth in this clinical population.
Keywords: Cochlear implant, pediatric
Children with deafness accompanied by additional disabilities (AD) constitute a significant proportion of the deaf pediatric population and pose a unique challenge to cochlear implant (CI) teams. A third or more of deaf children in the United States are believed to have an AD; however, what constitutes an AD varies. Commonly encountered ADs include cognitive or learning delays, motor impairment, autism spectrum disorders, visual deficits, global developmental delay, attention deficit/hyperactivity disorder, and an array of syndromic conditions (Daneshi & Hassanzadeh, 2007; Donaldson et al, 2004; Edwards et al, 2006; Holt & Kirk, 2005; Taitelbaum-Swead et al, 2006; Waltzman et al, 2000).
It is important to emphasize that ADs rarely affect only one domain alone (Conrad, 1979; Luria, 1973; Wallander et al, 1989). Functional challenges posed by ADs are often far-reaching and may result in inability to participate routinely in CI follow-up assessments and habilitation sessions, missed school days, increased caregiver stress levels, and fewer hours wearing the CI due to poor tolerance or frequently undergoing other medical procedures—all of which may impact a child’s subsequent CI performance. Moreover, the combination of a disability in addition to deafness is likely to result in a degree of functional impairment that is greater than the sum of each individual impairment (Nikolopoulos et al, 2008).
As candidacy criteria for cochlear implantation broaden, predicting outcomes in deaf children with ADs becomes increasingly important. Measures of speech perception and spoken language traditionally used to assess CI benefit may be ill-suited for this subgroup because their cognitive and intellectual development may be delayed relative to children without ADs (Edwards, 2007; Edwards et al, 2006). Additionally, the expectations of families and clinicians caring for children with deafness and ADs may differ significantly from traditional goals centered on domains of speech and language development (Archbold et al, 2002; Berrettini et al, 2008; Johnson et al, 2008; Wiley et al, 2008). While a great deal of evidence on speech perception, communication, language development, and speech intelligibility outcomes of cochlear implantation has been obtained for traditional pediatric CI recipients, there is a pressing need to obtain new knowledge about outcomes and benefits of cochlear implantation for deaf children with ADs in domains likely to be impacted by access to sound such as adaptive behavior.
Previous research suggests that deaf children who present with ADs make progress in a number of domains following implantation, but to a lesser degree and at a slower rate than children without ADs (Donaldson et al, 2004; Edwards, 2007; Waltzman et al, 2000). Nikolopoulos et al (2008) reported that after five years of implant use, children with ADs developed intelligible speech, but that only 16% of these children produced speech that was intelligible to all people or people with very little experience with deaf speech, compared to 61% of children without ADs. Wiley et al (2008) reported that children with ADs made measureable progress in detection and discrimination of sounds, but that their rate of progress was half that of children with developmental skills in the average range. Furthermore, they did not make gains in higher-level, more complex spoken language skills such as identification and comprehension. Holt and Kirk (2005) found that children with CIs and cognitive delays made progress in speech and language skills over time, but they struggled with higher-level, more complex language skills.
Although these earlier studies provide valuable information about speech and language development following cochlear implantation in samples of children with ADs, several critical issues remain to be addressed. First, studies to date have focused almost exclusively on speech and language outcomes. However, as a result of the broad functional impact of comorbid conditions, a significant concern in this population involves development of adaptive behaviors—areas extending beyond speech and language skills. Second, as a result of small sample sizes and significant heterogeneity across comorbid conditions, the generalizability of data from existing studies is limited and in need of replication (Eisenberg et al, 2007; Nikolopoulos et al, 2008). Finally, because children with CIs and ADs are frequently excluded from large CI outcome studies, comparison of adaptive behavior, speech, and language outcomes in this population with outcomes from the broad CI population is needed in order to inform expectations for outcomes and planning for intervention.
As a dedicated pediatric hospital with resources available to offer implantation to children with ADs who may pose a higher risk of complications, our center has a history of providing CIs to children with ADs. Therefore, the research questions in the present study are not a matter of informing decision-making regarding candidacy, but a matter of documenting the benefits provided by an implant to this clinical population, which is largely unknown in domains outside those directly related to auditory function. Not all implant centers share the same philosophy or have access to similar resources, which creates variability across centers that may affect reported outcomes with this clinical population and the findings reported in the literature.
The first objective of this study was to compare improvement in functional auditory skills during the first year of CI use in a group of children with ADs and in an age-matched comparison group of children without ADs. The second objective was to gather preliminary comparison data on improvement in language and adaptive behavior in a subset of these children. Similar to other studies that have successfully investigated outcomes in groups of children with deafness without regard to disability type (Berrettini et al, 2008; Waltzman et al, 2000; Wiley et al, 2005, 2008), our sample consisted of a largely heterogeneous cohort of children with a variety of functionally and etiologically distinct ADs. The presence of any ADs, regardless of type, could place a strain on the child, family, and habilitation team that might affect long-term CI performance; such general effects of other chronic conditions on adaptation and outcome have been shown to be important for both broad and specific adjustments and quality of life (Wallander et al, 1989). In addition, recent research has shown that pre-implant developmental level may be a better predictor of post-implant outcome than type of disability (Meinzen-Derr et al, 2009; Wiley et al, 2008). Therefore, we also collected a measure of pre-implant cognitive functioning and chose to investigate outcomes in this group as a whole without categorizing children into smaller sub-groups according to type of AD.
Materials and Methods
Participants
As part of a large ongoing long-term outcome study of deaf children who receive cochlear implants, children with at least one disability in addition to deafness were identified for inclusion in this investigation. We use the term “additional disability” as a blanket term that conceptualizes all of the children in our group; however we do include children with severe prematurity and children with autism, which may be better defined as developmental impairments. We chose to include these children because the sequelae of both are associated with atypical outcomes after implantation (Edwards, 2007; Holden-Pitt & Diaz, 1998; Van Naarden et al, 1999). To be enrolled, children had to be implanted prior to age five, have both pre- and post-implant scores on at least one outcome measure, and have been implanted after the year 2000. All eligible children with congenital deafness and an AD were chronologically age-matched (within 12 months) to a child with a CI but without an additional disability (No-AD).
A total of 32 children with ADs and a CI were identified for participation in the study. Eight children were excluded due to absence of a pre- and/or post-implant assessment, and one child was excluded because an age-matched comparison could not be found, yielding 23 children with CIs and ADs. Table 1 provides a summary of the individual demographic characteristics of the children in the AD and No-AD groups. The etiologies of hearing loss for the entire sample of 46 children with and without AD were unknown for 33 children, syndromal for five children, related to Mondini malformation, cytomegalovirus (CMV), neonatal meningitis, and auditory neuropathy for two children, respectively. All used currently available, state-of-the-art CI systems: 37 Nucleus, four MedEl, five Advanced Bionics devices. Table 2 provides a summary of hearing and device characteristics for each group.
Table 1.
Individual characteristics of children with additional disabilities (AD) and comparison group with no additional disabilities (No-AD).
| ID | Participants with additional disabilities
|
Comparison group
|
|||
|---|---|---|---|---|---|
| Age at implant (months) | Additional disability | Cognitive functiona | Age at implant (months) | Cognitive functiona | |
| 1 | 36.57 | ASD | — | 34.2 | — |
| 2 | 8.94 | Mitochondrial myopathy (motor) | — | 10.25 | 95 |
| 3 | 17.18 | Goldenhar syndrome | — | 14.49 | 110 |
| 4 | 17.28 | ASD, prematurity, dev delay | — | 13.54 | 100 |
| 5 | 10.74 | CP, vision | 118 | 13.83 | 97 |
| 6 | 47.77 | Dev delay | 82 | 27.34 | — |
| 7 | 20.07 | Prematurity, seizure disorder | — | 22.11 | — |
| 8 | 24.94 | CP, dev delay, prematurity | 54 | 22.64 | 94 |
| 9 | 16.49 | BOR syndrome (prematurity, motor, dev delay, vision) | 83 | 17.28 | 111 |
| 10 | 29.21 | Blindness, hypoparathyroidism (motor), dev delay | 50 | 27.93 | 85 |
| 11 | 16.72 | CP, dev delay, vision, prematurity | 73 | 17.28 | 104 |
| 12 | 18.37 | VATER syndrome (motor) | 94 | 15.97 | 75 |
| 13 | 34.6 | CHARGE (dev delay, vision, oral-motor, motor) | 66 | 39.16 | 73 |
| 14 | 33.77 | Leigh’s disease (motor, vision, oral-motor) | 55 | 31.74 | 95 |
| 15 | 13.31 | Prematurity | 100 | 13.6 | 85 |
| 16 | 32.23 | Dev delay, motor delay | 55 | 28.55 | 100 |
| 17 | 33.45 | CP, prematurity, dev delay | 55 | 32.53 | — |
| 18 | 21.88 | Motor delay, dev delay | 70 | 23.59 | 125 |
| 19 | 31.51 | Robinow syndrome (motor, oral-motor) | 65 | 24.15 | 90 |
| 20 | 31.28 | CP | 85 | 22.74 | 75 |
| 21 | 14.59 | CHARGE (oral-motor) | 85 | 12.75 | 90 |
| 22 | 24.94 | CP, prematurity, dev delay | 60 | 21.52 | — |
| 23 | 24.57 | Dev delay | 85 | 28.91 | 88 |
Note: ASD = autism spectrum disorder. CHARGE = coloboma, heart anomalies, choanal atresia, renal anomalies, genital anomalies, ear anomalies. Dev = developmental. CP = cerebral palsy. ADD = attention deficit disorder. BOR = branciootorenal syndrome. VATER = vertebrae, anus, trachea, esophagus, renal.
Either the cognitive composite score from the Bayley Scales of Infant and Toddler Development, or the cognitive domain standard score from the Developmental Assessment of Young Children (DAYC) was used to assess cognitive functioning.
Table 2.
Group characteristics of children with additional disabilities (AD) and without additional disabilities (No-AD).
| Characteristic | AD group
|
No-AD group
|
|||
|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | p value | |
| Age at pre-CI testing (months) | 20 (9) | 6–40 | 20 (8) | 8–36 | 0.94 |
| Age at implantation (months) | 24 (10) | 9–48 | 22 (8) | 10–39 | 0.47 |
| Age at post-CI testing (months) | 29 (8) | 16–45 | 31 (10) | 16–54 | 0.53 |
| Unaided PTA at pre-CI (dB HL) | 112 (11) | 82–120 | 104 (13) | 73–118 | 0.05 |
| Cognitive ability (SS)a | 74 (19) | 50–118 | 94 (13) | 73–125 | 0.001 |
|
| |||||
| Percent | Percent | ||||
|
| |||||
| Device (unilateral) | 87 | 65 | — | ||
| Communication mode (Oral) | 39 | 70 | — | ||
| Gender (male) | 65 | 65 | — | ||
| Insertion (full) | 65 | 96 | — | ||
Note: M=mean. SD=standard deviation. PTA = pure-tone average in better ear. dB HL = decibels hearing loss. SS = standard score.
Either the cognitive composite score from the Bayley Scales of Infant and Toddler Development, or the cognitive domain standard score from the Developmental Assessment of Young Children (DAYC) was used to assess cognitive functioning.
Measures
Functional auditory skills
The Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS; Zimmerman-Phillips et al, 2001) assesses functional auditory skills through a structured clinician-parent interview. Scores range from 0 to 40 with higher scores indicating greater auditory skill development.
Receptive and expressive language
The Preschool Language Scale (PLS-4; Zimmerman et al, 2002) is a standardized assessment of receptive and expressive spoken language and communication for children from birth through 6 years, 11 months. The auditory comprehension subscale assesses how much language a child understands (receptive language) and although the name implies auditory comprehension, recently implanted children may have very limited comprehension that is truly auditory in nature. The expressive communication subscale assesses how well a child communicates with others (expressive language).
Because some children received a standard score of 50, which is the floor on the PLS, language quotients were calculated for all children by dividing the age-equivalent score by the child’s chronological age at the time of testing and multiplying by 100; language quotients closer to 100 suggest the child’s language level is age-appropriate. This method has been used in similar studies to assess language in clinical populations very similar to those in the present study (Meinzen-Derr et al, 2011). The PLS was administered in the child’s current communication mode and only responses using the child’s mode of communication were accepted. For children who use Total communication, administration of test items may have included signed exact English (SEE) in addition to spoken language. If a response on the expressive comprehension subtest required a word, then the child had to either sign or say the word if he/she used Total communication. If the child used Oral communication, he/she had to say the word, and a signed response was not accepted. In addition, if a response required a gesture, then all children, regardless of communication mode, were required to gesture. No modification of the PLS was needed for the child with a vision impairment because the level of testing did not require looking.
Adaptive behaviors
The Vineland Adaptive Behavior Scale second edition (Vineland-II; Sparrow et al, 2005) is a standardized assessment of adaptive behavior in everyday life. Adaptive behavior is the performance of daily activities required for personal and social sufficiency (Sparrow, 2010). Using a semi-structured interview, parents rate how often their child performs a specific behavior without help or reminders. Vineland items cluster into four domains: (1) communication, (2) daily living skills, (3) socialization, and (4) motor skills, each of which yields a standard score (M = 100, SD = 15) based on comparison with a typically-developing normative sample. We focused on daily living skills (e.g. self-care, caring for the home, and living in the community), socialization skills (e.g. social skills and relationships, playing and using leisure time, and adapting to people in the environment), and motor skills. The Vineland has been used to assess adaptive functioning in a wide range of clinical populations, including children with hearing loss (Bat-Chava et al, 2005; Horn et al, 2005; Kushalnagar et al, 2007; Nicholas & Geers, 2007).
Cognitive functioning
Cognitive functioning was assessed using the cognitive subtest of either the Bayley Scales of Infant and Toddler Development (Bayley, 1993, 2006), or the cognitive subtest of the Developmental Assessment of Young Children (DAYC; Voress & Maddox, 1998). The DAYC assesses five domains of development through observation, caregiver interview, or direct observation, and was used exclusively as a measure of developmental functioning in pre-CI evaluations until 2005, at which time, the Bayley was added to the standard pre-CI evaluation protocol. Therefore, depending on the year of enrollment and the time restrictions of the evaluation, a child may have received the DAYC, the Bayley, or both.
We used pre-CI cognitive scores from the Bayley for 19 children, the DAYC for 17 children, and the remaining 10 children did not receive pre-CI cognitive testing. There were no significant differences in cognitive standard scores obtained for children who received the Bayley compared to those who received the DAYC, t (34) = 0.05, p = 0.96. Although neither the Bayley-III nor the DAYC technical manuals publish correlations between the two measures, the Bayley was used in the development of the DAYC and there is overlap between DAYC and Bayley cognitive items.
Statistical analyses
Because participation in the long-term outcomes study is voluntary, not all children returned for their evaluation at each interval. If a child had data for one interval only, then only that interval was used in all analyses. If a child had both 6- and 12-month post-CI data, the 12-month interval was used. There were no differences in pre- to post-IT-MAIS gains in children with post-CI data at 6 months and those with post-CI data at 12 months, which supports the validity of using data from either of the two post-CI intervals. The same interval (either 6- or 12-months post-CI) was used for both children in the matched pair. A two-way repeated-measures ANOVA was used to compare pre- to post-CI scores in functional auditory skills (IT-MAIS) between the AD and No-AD groups. Language and adaptive behaviors were analysed descriptively due to the small sample size (n = 14 for language, n = 16 for adaptive behavior).
Results
Functional auditory skills
Pre- and post-CI IT-MAIS scores were available for all but two of the 46 children in the sample. The AD and No-AD groups scored similarly pre-CI with means of 2.5 and 4.7 respectively, however, differences between the groups increased at the post-CI interval with means of 12.9 and 23.9 for the AD and No-AD groups, respectively. With two exceptions (IDs 10 and 12), all children with ADs in our sample made progress in auditory skills within one year of implant use. Younger chronological age (r = −.52, p = .012) and earlier age at implantation (r = −.58, p = .005) were significantly correlated with larger gain in IT-MAIS scores for the No-AD group. There were no significant correlations between child characteristics and IT-MAIS gains for children with ADs. Two children in the AD group did not show an improvement in auditory skills from pre- to post-CI, with IT-MAIS scores of zero and one. One of these children (ID 10) experienced significant impairments including blindness and a cognitive score of 50. The second child (ID 12) was diagnosed with VATER syndrome, had a partial insertion of the electrode array, and achieved a cognitive score within normal limits.
To determine group differences in functional auditory skills over time, a two-way repeated measures ANOVA was conducted with group as the between- and pre- to post-CI IT-MAIS scores as the within-subjects factors. Two children in the No-AD group scored unusually high on the IT-MAIS pre-CI, with each child scoring 26 out of 40. These scores were identified as outliers and were not included in the analysis. Results revealed a significant main effect of CI use with IT-MAIS scores improving after one year of implant use for both groups, F (1, 40) = 91.91, p<.0001, partial η2 = 0.70, and a significant main effect of group with the No-AD group scoring higher on the IT-MAIS than the AD group, F (1, 40) = 7.41, p<.01, partial η2 = 0.16. In addition, a significant interaction was observed with the No-AD group making more progress within one year of CI use than the AD group, F (1, 40) = 9.37, p=.004, partial η2 = 0.19. A follow-up repeated measures ANOVA was carried out to identify the impact of cognitive ability on improvement in auditory skills between the two groups. After controlling for cognitive ability, both main effects (CI use and presence of ADs), as well as the interaction between the two main effects, were no longer significant. Children in the No-AD group scored significantly higher in cognitive ability than children in the AD group, F (1, 30) = 4.71, p=.04, partial η2 = 0.14.
Language skills
Nine of the 23 children in the AD group had pre-CI data on the PLS, however only seven of these children had post-CI data as well. The pre-CI means and standard deviations using language quotients for the entire sample of nine children with ADs were M=35, SD=13 for receptive language, and M=55, SD=16 for expressive language. These means are very similar to the means of the seven children who had both pre- and post-CI data (see Table 3). Neither the AD nor the No-AD group displayed language quotients that were age-appropriate after one year of CI use (i.e. scores around 100). However, the mean receptive language quotients increased from pre- to post-CI for both groups, although language quotients were lower overall for the AD group pre- and post-CI than for the No-AD group. Mean expressive language quotients decreased pre- to post-CI for both groups.
Table 3.
Pre- and post-CI means, standard deviations, and ranges for age equivalents and language quotients on the two subscales of the Preschool Language Scale.
| Auditory comprehension
|
Expressive communication
|
|||
|---|---|---|---|---|
| Pre-CI | Post-CI | Pre-CI | Post-CI | |
| AD Group
| ||||
| Age equivalent (months) | 8(4); 4–15 | 13(5);2–17 | 13(4); 9–20 | 14(4);11–22 |
| Language quotient | 33(13);16–51 | 42(20);5–69 | 56(17);39–81 | 46(16);28–69 |
|
| ||||
| No-AD Group
| ||||
| Age equivalent (months) | 10(3);4–13 | 18(6);10–29 | 15(3);10–19 | 20(5);15–29 |
| Language quotient | 50(10);41–71 | 57(13);43–82 | 75(24);57–124 | 69(19);51–101 |
Note: n=7 in each group. AD = additional disability. No-AD = no additional disability. Format for reading the cells is mean (standard deviation); range.
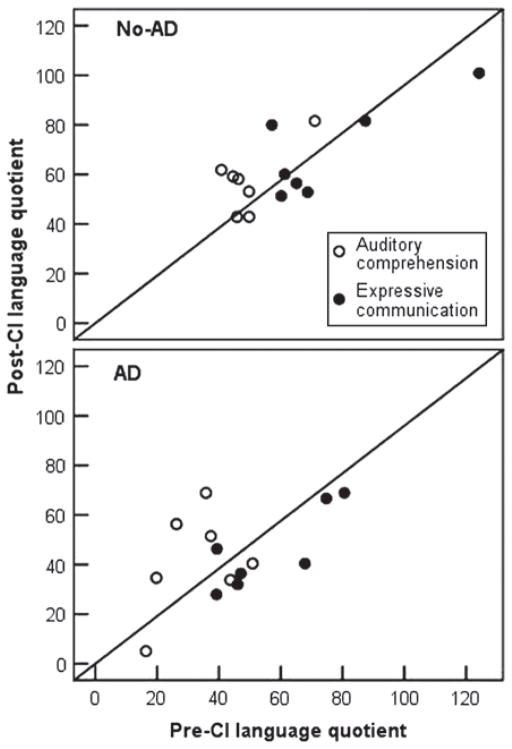
Figure 1 shows individual pre- and post-CI language quotients for both groups. The diagonal represents age appropriate gains in language quotients pre- to post-CI. Scores above the diagonal indicate the child is catching up to his or her chronological age faster than would be expected by development alone, and those below the diagonal indicate the child is making slower progress than would be expected based on development alone. Four of seven children with ADs displayed an increase in their receptive language quotient, and one child displayed an increase in their expressive language quotient within one year of CI use. Five of seven children in the No-AD group displayed an increase in receptive language quotient, and one child displayed an increase in expressive language quotient. We note here, however, that most language quotients for both groups that fell below the diagonal were not far from the diagonal for either group of children, indicating that although language quotients did not increase faster than would be expected by development alone in these children within one year of implant use, their language development did not slow by a great deal either.
Figure 1.
Scatter plots of language quotients pre- and post-CI for children with additional disabilities (AD), and without additional disabilities (No-AD).
Of the three children with ADs whose language quotients did not increase in either receptive or expressive language, two had cognitive ability scores at least two standard deviations below the mean and had diagnoses of Leigh’s disease (ID 14) and cytomegalovirus (CMV) (ID 16). Both children had full insertions of the electrode array and were implanted under age three. Alternately, the single child in the AD group who showed an increase in both receptive and expressive language quotients (ID 22) also had a cognitive score more than two standard deviations below the mean. This child was diagnosed with auditory neuropathy and also had a complex birth history of severe prematurity and cerebral palsy.
Adaptive behaviors
Twelve of the 23 children in the AD group had pre-CI data on the Vineland, however only eight of these children had pre- and post-CI data. The pre-CI means and standard deviations on the Vineland for the entire sample of 12 children with ADs were M=66, SD=20 for daily living skills; M=74, SD=12 for socialization; and M=67, SD=18 for motor. These means are very similar to the means of the eight children with both pre- and post-CI data (see Table 4). As a group, prior to CI, children with ADs scored within two standard deviations of the mean in socialization and motor skills and more than two standard deviations below the mean in daily living skills. Within one year of implant use, daily living skills increased to within two standard deviations of the mean, socialization increased modestly, and motor skills remained unchanged for children with ADs. As a group, deaf children without ADs scored within normal limits in all three domains of adaptive behavior both pre- and post-CI. Within one year of implant use, daily living skills and motor skills increased by four points, while socialization remained unchanged for children without ADs.
Table 4.
Pre- and post-CI means, standard deviations, and ranges for the Vineland Adaptive Behavior Scale.
| Group | Daily living skills
|
Socialization
|
Motor skills
|
|||
|---|---|---|---|---|---|---|
| Pre-CI | Post-CI | Pre-CI | Post-CI | Pre-CI | Post-CI | |
| AD | 65(15) | 74(16) | 74(9) | 77(12) | 70(17) | 69(21) |
| 42–88 | 51–95 | 63–90 | 59–96 | 50–97 | 40–94 | |
| No-AD | 93(13) | 97(9) | 91(10) | 90(7) | 94(8) | 98(11) |
| 75–119 | 79–107 | 76–102 | 79–102 | 83–102 | 82–114 | |
Note: n = 8 in each group. AD = additional disability. No-AD = no additional disability. Format for reading the cells is mean (standard deviation) with range immediately below.
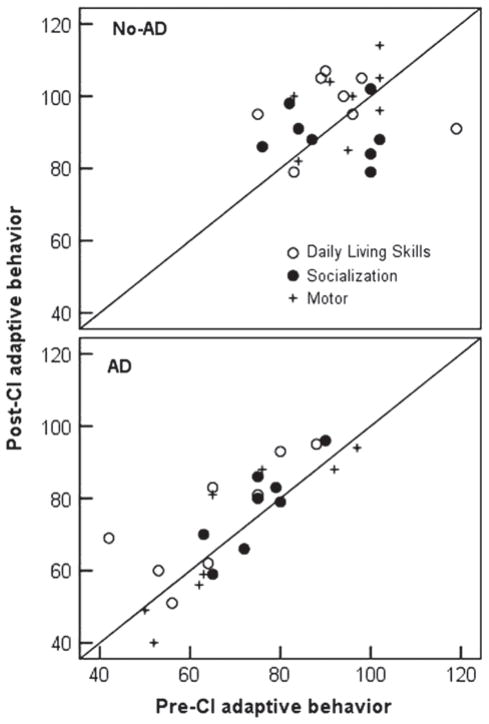
Figure 2 shows individual pre- and post-CI standard scores in daily living skills, socialization, and motor skills for both groups. The diagonal represents age-appropriate gains in adaptive behavior skills pre- to post-CI. Scores above the diagonal indicate the child is making gains greater than expected for his or her chronological age, and those below the diagonal indicate the child is making slower gains than would be expected for his or her age. Six out of eight children with ADs made greater than expected gains for their age in daily living skills, five of eight made greater than expected gains in socialization, and two made greater than expected gains for their age in motor skills within one year of implant use. Five of the six children who did not make progress in motor skills were diagnosed with a motor-related disability.
Figure 2.
Scatter plots of adaptive behavior pre- and post-CI for children with additional disabilities (AD), and without additional disabilities (No-AD).
Five out of eight children in the No-AD group made greater than expected gains for their age in daily living skills and socialization, and two of eight children made greater than expected gains in motor skills within one year of implant use. Again, most of the scores for both groups that fell below the diagonal were not far from the diagonal, indicating that although these children did not maintain age-appropriate gains in adaptive behavior within one year of implant use, they did not slow by much.
The child diagnosed with Leigh’s disease was one of the two children with ADs that did not make progress in any of the three domains of adaptive functioning. The second child that did not make progress was diagnosed with Robinow syndrome (ID 19) and experienced a partial insertion of the electrode array. Aided thresholds for this child were greater than 95 dB HL suggesting that the child was receiving little auditory input from the CI. This child dropped two standard score points in daily living skills, and six points in both socialization and motor skills. However, the child went from scoring 1 to 7 points on the IT-MAIS and went from a language quotient of 19 to 24 on the auditory comprehension subscale of the PLS within one year of implant use, suggesting some limited benefit from implantation.
We were curious if children with ADs who did not make language gains, were in fact able to make gains in adaptive behavior. We found that two of the three children (ID 16 and ID 20) who made less than expected gain for their age in language within one year of implant use as indicated by a decline in their language quotient after implantation (i.e. scores below the diagonal in Figure 1), nonetheless did made gains in adaptive behavior. The child diagnosed with CMV (ID 16) improved seven standard score points in both daily living skills and socialization within one year of implant use; this child’s motor skills score fell only one point. Although the second child (ID 20) did not make gains in motor skills or socialization, this child did gain seven standard score points in daily living skills within one year of implantation.
Discussion
The first objective of this study was to compare improvement in functional auditory skills within one year of implant use in a group of deaf children with ADs and in an age-matched comparison group of children without AD. We found that as a group, children with ADs made progress in functional auditory skills within one year of implant use. Individually, however, there were two children who did not make progress. Interestingly, none of the traditional audiological or demographic correlates of benefit (degree of loss, age, age at implantation) were associated with progress in functional auditory skills in the children with ADs. However, in the No-AD group, younger age at implantation and younger chronological age were found to be significantly correlated with greater gains in functional auditory skills within one year of implant use. This finding suggests that established early predictors of functional auditory benefit may not be as critical or meaningful in predicting benefit in deaf children with ADs.
As a group, children with and without ADs made progress in auditory skills within one year of implant use, but children without ADs made more progress. However, after controlling for cognitive ability, the differences between the groups in auditory skills were no longer significant. It is likely that the effects of time and group were eliminated because children without ADs had significantly higher cognitive abilities than children with ADs. This interpretation is supported by several studies that have shown that cognitive functioning is a reliable predictor of benefit in children with ADs (Edwards et al, 2006; Meinzen-Derr et al, 2009; Wiley et al, 2008).
A second objective of this study was to gather preliminary comparison data on the development of language and adaptive behavior in a subset of children with CIs and ADs, and a comparison group of children with CIs who do not have ADs. Although children in both groups scored well below age-appropriate language levels both pre- and within one year of implant use, language quotients indicated that four of seven children in the AD group and five of seven children in the No-AD group were catching up faster than development would predict in receptive language within one year of CI use. However, children in the No-AD group showed closer to age-appropriate receptive language prior to and within one year of implant use than children in the AD group. With regard to expressive language, all children in the AD and No-AD groups, with the exception of one child in each group, made slower than expected gains over one year of implant use. It is possible that although receptive language levels increased for most children within one year of implant use, levels were not yet sufficient to impact the development of expressive communication as measured by the PLS. Supporting this assumption, Meinzen-Derr and colleagues (2009) reported an increase in both receptive and expressive language quotients on the PLS in a majority of children with ADs after a mean of two years of implant use. Continued post-CI language testing will allow us to better understand the rate of development in receptive and expressive language in children with ADs.
As is typical in the population of deaf children who receive CIs, considerable individual differences were observed in performance in both the AD and No-AD group; two children had significantly below average cognitive functioning, however, the third child was within normal limits. Similarly, the only child to make progress in both language areas had significantly below average cognitive functioning and a complicated birth history. Although, these findings are from a small sample of children, they highlight the difficulty of predicting if a specific child with ADs will make progress in language after cochlear implantation.
Finally, in order to investigate gains in areas outside speech and language functioning that may be affected positively from increased auditory access provided by a CI, we measured progress in adaptive behavior in both groups of children. Similar to the performance of children with ADs such as autism, mental retardation, and visual impairment but without deafness, the majority of deaf children in our sample with ADs scored well below the mean in adaptive behavior. However, after implantation, six of eight children made progress in daily living skills and five of eight made progress in socialization. In the group without ADs, five of eight children made progress in two out of three adaptive behavior domains. Only two children in the AD group made progress in motor skills, one of whom did not have a motor component to the diagnosis. Five of the six children who did not make progress in motor skills displayed a motor component to their diagnosis which may explain the poor progress in this domain. Notably, the two children who did not make any progress in language skills did make progress in daily living skills and/or socialization. Daily living skills (i.e. letting someone know when he/she needs diaper changing, being careful around hot objects, being aware of appropriate behavior) and socialization behaviors (i.e. smiling around familiar people, attempting to make social contact, showing affection towards caregivers) are likely to make meaningful differences to families of children with complex needs. These findings, although preliminary and based on a small sample, are encouraging and provide the first evidence of benefits in adaptive behavior after cochlear implantation in children with extremely complex developmental needs. Our findings support the need for assessment in areas outside traditional speech and language domains, which are areas of expected benefit for children without ADs, but may not be expected in the growing population of deaf children with complex needs.
As with most CI studies, the data reported here may be subject to selection bias. Families that are not highly motivated by perceived progress and benefits to their child may be less likely to participate in research studies, leaving out an important segment of the population of children with ADs. While the extent to which this occurs in this study is unknown, it should be kept in mind because each of the outcome measures in this study has a parent-report component. In addition, many of the children in our sample were younger than three years of age at the time of implantation, and it is possible that preoperatively identified diagnoses may become more well-defined and that the full impact of a child’s special needs on developmental outcomes will become more complex over time. Long term longitudinal follow-up is necessary to understand more fully the impact of auditory access to sound on various outcomes after implantation.
Another consideration is the heterogeneous nature of this sample of children that includes children with a wide range of motor, visual, developmental, and neurocognitive conditions. Combined with the small sample size, a comparison of progress between specific handicapping conditions was not possible. However, even within a specific diagnosis category such as cerebral palsy, individual differences in the severity of the diagnosis and its impact on day-to-day functioning, family resources, and parenting stress may be better predictors of the benefit a child receives from a CI than the specific diagnosis itself. As a result, homogenizing the sample by type of diagnosis may not be the answer; rather, measuring the functional impact of a disability—regardless of the type—may be a more ecologically valid method for evaluating progress across children with a variety of handicapping conditions.
Given the wide-ranging consequences of deafness on child development, we know very little about the real-world impact of cochlear implantation on this special population of children with ADs. Our current lack of knowledge places children and their families at greater risk for uncertainty, distress, and negative outcomes. Moreover, families of children with ADs may have fundamentally different expectations regarding the effectiveness of cochlear implantation than families of deaf children without an AD. For this reason, broadening post-CI outcome measures to include quality of life, parental stress, behavior regulation, socio-emotional development, family integration, and adaptive functioning is imperative for children with ADs. Multi-center collaborations along with comprehensive, multidisciplinary, and multi-trait pre- and post-implant assessments will provide additional new knowledge of pre-implant predictors and expected benefits across several domains of functioning. This knowledge has direct clinical applications, with the ultimate goal of providing evidence-based expectations to parents of deaf children with ADs.
Acknowledgments
We are grateful to the families who participated in this research, and to clinical research associates Shirley Henning and Bethany Colson for their valued consultation. Excerpts of this paper were presented as a poster: Beer J., Harris M.S., Kronenberger W. & Pisoni D.B. (June, 2010). Auditory skills, language, and adaptive behavior in multiple involved children following cochlear implantation. Poster presented at 11th International Conference on Cochlear Implants and Other Auditory Implantable Technologies. Stockholm, Sweden.
Abbreviations
- AD
Additional disability
- CI
Cochlear implant
- IT-MAIS
Infant toddler meaningful auditory integration scale
- OC
Oral communication
- PLS
Preschool language scale
- TC
Total communication
Footnotes
Declaration of interest: This research was supported by NIH/NIDCD Training Grant T32DC00012 (Pisoni) and NIH/NIDCD R01DC000064.
References
- Archbold SM, Lutman ME, O’Neill C, Nikolopoulos TP. Parents and their deaf child: Their perceptions three years after cochlear implantation. Deafness and Education International. 2002;4(1):12–40. [Google Scholar]
- Bat-Chava Y, Martin D, Kosciw JG. Longitudinal improvements in communication and socialization of deaf children with cochlear implants and hearing aids: Evidence from parental reports. J Child Psychol Psychiatry. 2005;46(12):1287–1296. doi: 10.1111/j.1469-7610.2005.01426.x. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio, USA: The Psychological Corporation; 1993. [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, USA: Pearson Education, Inc; 2006. [Google Scholar]
- Berrettini S, Forli F, Genovese E, Santarelli R, Arslan E, et al. Cochlear implantation in deaf children with associated disabilities: Challenges and outcomes. Int J Audiol. 2008;47(4):199–208. doi: 10.1080/14992020701870197. [DOI] [PubMed] [Google Scholar]
- Conrad R. The Deaf School Child. London: Harper Row; 1979. [Google Scholar]
- Daneshi A, Hassanzadeh S. Cochlear implantation in prelingually deaf persons with additional disability. J Laryngol Otol. 2007;121:635–638. doi: 10.1017/S0022215107005051. [DOI] [PubMed] [Google Scholar]
- Donaldson AI, Heavner KS, Zwolan TA. Measuring progress in children with autism spectrum disorder who have cochlear implants. Arch Otolaryngol Head Neck Surg. 2004;130(5):666–671. doi: 10.1001/archotol.130.5.666. [DOI] [PubMed] [Google Scholar]
- Edwards L. Children with cochlear implants and complex needs: A review of outcome research and psychological practice. J Deaf Stud Deaf Educ. 2007;12:258–268. doi: 10.1093/deafed/enm007. [DOI] [PubMed] [Google Scholar]
- Edwards L, Frost R, Witham F. Developmental delay and outcomes in paediatric cochlear implantation: Implications for candidacy. Int J Pediatr Otorhinolaryngol. 2006;70:1593–1600. doi: 10.1016/j.ijporl.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Eisenberg LS, Widen JE, Yoshinaga-Itano C, Norton S, Thal D, et al. Current state of knowledge: Implications for developmental research: Key issues. Ear Hear. 2007;28(6):773–777. doi: 10.1097/AUD.0b013e318157f06c. [DOI] [PubMed] [Google Scholar]
- Holden-Pitt L, Diaz JA. Thirty years of the Annual Survey of Deaf and Hard-of-Hearing Children & Youth: A glance over the decades. Am Ann Deaf. 1998;143:72–76. [PubMed] [Google Scholar]
- Holt RF, Kirk KI. Speech and language development in cognitively delayed children with cochlear implants. Ear Hear. 2005;26:132–148. doi: 10.1097/00003446-200504000-00003. [DOI] [PubMed] [Google Scholar]
- Horn DL, Davis RAO, Pisoni DB, Miyamoto RT. Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear Hear. 2005;26:389–408. doi: 10.1097/00003446-200508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KC, DesJardin JL, Barker DH, Quittner AL, Winter ME. Assessing joint attention and symbolic play in children with cochlear implants and multiple disabilities: Two case studies. Otol Neurotol. 2008;29:246–250. doi: 10.1097/mao.0b013e318162f1f3. [DOI] [PubMed] [Google Scholar]
- Kushalnagar P, Krull K, Hannay J, Mehta P, Caudle S, et al. Intelligence, parental depression, and behavior adaptability in deaf children being considered for cochlear implantation. J Deaf Stud Deaf Educ. 2007;12(3):335–349. doi: 10.1093/deafed/enm006. [DOI] [PubMed] [Google Scholar]
- Luria AR. The Working Brain: An Introduction to Neuropsychology. Penguin; 1973. [Google Scholar]
- Meinzen-Derr J, Wiley S, Grether S, Choo D. Language performance in children with cochlear implants and additional disabilities. Laryngoscope. 2009;120:405–413. doi: 10.1002/lary.20728. [DOI] [PubMed] [Google Scholar]
- Meinzen-Derr J, Wiley S, Grether S, Choo DI. Children with cochlear implants and developmental disabilities: A language skills study with developmentally matched hearing peers. Res Dev Disabil. 2011;32(2):757–767. doi: 10.1016/j.ridd.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. J Speech Lang Hear Res. 2007;50(4):1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos TP, Archbold SM, Wever CC, Lloyd H. Speech production in deaf implanted chidlren with additional disabilities, and comparison with age-equivalent implanted children without such disorders. Int J Pediatr Otorhinolaryngol. 2008;72:1823–1828. doi: 10.1016/j.ijporl.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Sparrow SS. Adaptive Behavior and the Vineland Then and Now. House Ear Institute; Los Angeles, USA: 2010. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Vineland-II: AGS Publishing; 2005. [Google Scholar]
- Taitelbaum-Swead R, Brownstein Z, Muchnik C, Kishon-Rabin L, Kronenberg J, et al. Connexin-associated deafness and speech perception outsome of cochlear implantation. Arch Otolaryngol Head Neck Surg. 2006;132:495–500. doi: 10.1001/archotol.132.5.495. [DOI] [PubMed] [Google Scholar]
- Van Naarden K, Decoufle P, Caldwell K. Prevalence and characteristics of children with serious hearing impairment in metropolitan Atlanta, 1991–1993. Pediatrics. 1999;103:570–575. doi: 10.1542/peds.103.3.570. [DOI] [PubMed] [Google Scholar]
- Voress J, Maddox T. Developmental Assessment of Young Children. Austin, USA: Pro-Ed; 1998. [Google Scholar]
- Wallander JL, Varni JW, Babani L, Tweddle Banis H, DeHaan CB, et al. Disability parameters, chronic strain, and adaptation of physically handicapped children and their mothers. Journal Pediatr Psychol. 1989;14:23–42. doi: 10.1093/jpepsy/14.1.23. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Scalchunes V, Cohen NL. Performance of multiple handicapped children using cochlear implants. Am J Otol. 2000;21(3):329–335. doi: 10.1016/s0196-0709(00)80040-x. [DOI] [PubMed] [Google Scholar]
- Wiley S, Jahnke M, Meinzen-Derr J, Choo D. Perceived qualitation benefits of cochlear implants in children with multi-handicaps. Int J Pediatr Otorhinolaryngol. 2005;69:791–798. doi: 10.1016/j.ijporl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wiley S, Meinzen-Derr J, Choo D. Auditory skills development among children with develomental delays and cochlear implants. Ann Otol Rhinol Laryngol. 2008;117:711–718. doi: 10.1177/000348940811701001. [DOI] [PubMed] [Google Scholar]
- Zimmerman-Phillips S, Robbins AM, Osberger MJ. Infant-Toddler Meaningful Auditory Integration Scale 2001 [Google Scholar]
- Zimmerman IL, Steiner BS, Pond R. Preschool Language Scale. 4. PsychCorp; 2002. (PLS-4) [Google Scholar]