Abstract
Computerized physician order entry (CPOE) systems can create unintended consequences. These include medication errors and adverse drug events. We look at a less understood error; patient misidentification. First, two email surveys were used to establish potential risk factors for this error. Next, an automated detection trigger was designed and validated with inpatient medication orders at a large pediatric hospital. The incidence was 0.064% per medication ordered. Finally, a case-control study identified the following as significant risk factors on multivariate analysis: patient age, last name spelling, bed proximity, medical service, time/date of order, and ordering intensity. These results can be used to improve patient safety by increasing awareness of high risk situations and guiding future research.
Introduction
Computerized physician order entry (CPOE) systems are widely thought to increase efficiency and patient safety and reduce costs. The systems reduce errors by eliminating illegible orders, improving communication and order tracking, and reducing the time of retrieving information.1,2 A CPOE system with decision support of dose selection, simple drug-allergy and drug-drug interaction checking, and route and frequency indication can significantly reduce errors.1,2 In one study, the implementation of CPOE reduced dosing errors by 23% and allergy errors by 56%.1 CPOE systems can reduce medication prescription errors3 and adverse drug events.1
However, there are some unintended consequences that result from the implementation of CPOE systems. The systems could facilitate errors by creating a dependence on the suggestions or through alert fatigue, computer crashes, complicated computer interfaces, and decreasing direct communication.2,3 One type of potentially dangerous error is ordering on the wrong patient. Patient identification errors can lead to unnecessary treatment, serious harm, or loss of life.4 Unless the error is caught, a closed-loop medication system,5 including pharmacy integration and bar-coded administration, virtually guarantees that a medication ordered for the wrong patient will be administered to the wrong patient.
In laboratory medicine, several studies have reported on patient misidentification.6,7 Like order entry, this is another example of a highly automated process that can be derailed by a human error at the first step. Mislabeled specimens can result in unnecessary hospitalization and surgical procedures.6 In one study, 73% of the 253 adverse events in laboratory medicine, extracted from the database of the Veterans Affairs National Center for Patient Safety, were due to patient misidentification.6 Another study reported that 22% of this type of patient misidentification was attributed to CPOE.7 Approximately 1 in 18 patient identification errors resulted in adverse events.7
There are no data on the observed frequency of patient misidentification at the time of inpatient medication ordering, with or without CPOE. Voluntary reporting systems underrepresent the actual frequency.4 Voluntary reports of patient identification errors only account for 2–3% of the total errors and often do not include the near misses. Despite this, qualitative research on patient identification errors suggests this is a common problem. In one study, 23% of house staff reported this occurring a few times a week or more.8 The risk factors of this type of error are thought to include poor screen designs, similar names, hectic workstations, and interruptions.8
To efficiently detect medication errors without relying on voluntarily reporting, qualitative research, or tedious review of medical records, the “trigger” approach has recently been applied. The trigger approach identifies an event in the electronic medical record (EMR), which serves as a proxy for the actual error. For example, a trigger tool detected 10 times more confirmed serious adverse events than hospital reporting systems.9 Establishing triggers from modern EMRs can take advantage of audit trails, which include exact time and user stamps of all system interactions. Abrupt medication stop has previously been used as a trigger, primarily to identify medications that need to be discontinued after reaching the patient, due to an adverse drug effect.10–13 To our knowledge, this approach has never previously been extended to specifically identify CPOE patient misidentification errors.
Knowing the frequency at which patient identification errors occur will allow clinicians and hospital administrators to accurately weigh the trade-offs of potential improvements. Establishing risk factors that increase the likelihood of patient identification errors will improve patient safety by increasing awareness, improving decision support, and changing hospital procedures.
In this study, email surveys were utilized to determine potential risk factors, a trigger approach was developed and validated to detect and estimate the incidence Orders On Misidentified Patient (OOMP) events, and a case-control study was conducted to establish risk factors for misidentification.
Methods
Setting:
The study was conducted at the Children’s Hospital of Pittsburgh of UPMC (CHP), a 296 bed pediatric academic medical center in western Pennsylvania. Since October, 2002, all CHP inpatient orders have been entered directly into the Cerner Millennium® EMR.14 Less than 5% of these orders are entered by the staff as “verbal” or “phone” orders. This study was approved as a quality improvement project by the University of Pittsburgh Medical Center Quality Improvement project review board.
Email Surveys:
To collect qualitative data, email surveys were sent to the members of AMDIS, a national organization of Chief Medical Information Officers (CMIOs)15, and 100 randomly selected CHP physicians who had placed at least 50 orders between January and April, 2011. A second reminder was sent to CHP physicians who did not initially respond to the survey. The survey included five open-ended questions about patient misidentification at the time of ordering: “How frequent is this type of error”, “how do these errors happen”, “what are the contributing factors”, “how are they caught”, and “what would prevent these errors”. Themes were identified by a single researcher using a modified cutting and sorting technique.16
Data Source:
Every inpatient medication order between May 1, 2006 and April 30, 2011 was included in the analysis. Medication order details, including exact order time, were obtained from the hospital’s EMR through the CHP Clinical Data Warehouse. Additional patient details (demographics, location at the time of the order, and discharge diagnoses) were also extracted and linked to the medication order through a unique encounter identification number. Excluded from analysis were: a) Any verbal or written orders, b) Orders for intravenous fluids and three common drugs (acetaminophen, albuterol, and diphenhydramine), c) Prescriptions to be filled after hospital discharge, d) Orders entered by nurse practitioners or physician assistants. Intravenous fluids and the three common drugs were excluded after a preliminary validation study showed that it was often impossible to independently determine that these were wrong for the patient or condition.
Case-Control Identification:
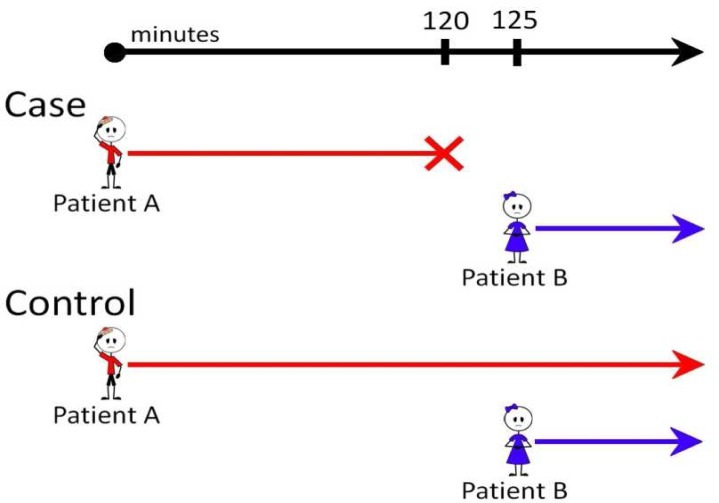
OOMP case and control dyads were identified according to the schema shown in the Figure. Case orders were all medications cancelled within 120 minutes12 of their initial order. The same drug (“Drug D”) was then reordered on a different patient within five minutes of cancellation. The original order, the cancellation, and the reorder were all entered by the same provider (“Provider P”). Cases were excluded if the same drug or another drug from the same class was reordered for Patient A within 180 minutes of cancellation. For each case dyad (Patient A and Patient B), five randomly selected control dyads were identified. Control dyad did not include a cancellation of the first order. Controls dyads were matched by study year, and the time from the first order to the second order (within 10 seconds).
Figure.
Case-Control Study Matching Strategy
Case Validation:
Two hundred randomly selected potential cases were individually reviewed by one of us (HIL) after completing the standard physician CPOE training and additional training in error investigation from CHP’s Patient Safety Officer. A standardized data abstraction tool was used. Information collected included order details, previous and future orders of the same drug, the condition and diagnosis, and the presence of any pop-up decision support alerts. After discussion of each potential case with a CHP Attending Physician (JEL), the case was classified according to predetermined definitions. A case was “Definite” if the error was recorded in medical record or through hospital safety reporting system. An example of this would be dexamethasone ordered on a wrong patient, with the error discovered by a nurse, and reported to the hospital reporting system. A case was “Yes” if the medication is wrong for the patient or condition. An example would be insulin ordered on a patient who does not have diabetes or high blood sugar. Cases were classified as “Uncertain” if the medication could be used for the patient’s condition but there is not enough information to determine if it was wrong for the patient. An example would be morphine ordered on a patient who was in a bike accident and was currently on morphine. A case was “Unlikely” if the medication cancellation was mentioned in the medical record as intentional. An example would be potassium chloride ordered for a patient where progress notes state that it was ordered due to a lab result, but then cancelled after repeat result did not show hypokalemia.
Study Variables:
Characteristics with the potential to be associated with patient misidentification were collected through literature review, expert interview, and analysis of the email responses. Bed location, length of stay at the time of the order, and medical service were obtained from the hospital admission, discharge, and transfer (ADT) data. Medical services were grouped into Medical, Surgical, ICU, and Other. Patient age at the time of the event was classified as: Newborn (<30 days), Infant (≥30 days and <1year), Preschool (≥1 year and <5 years), School Age (≥5 years and <13 years), Teen (≥13 years and <18 years), and Adult (≥18 years). Calculation of sound-alike names, edit distance, and two-letter overlap was performed using the Perl programming language modules Text::Soundex, Text::LevenshteinXS, and String::Trigram respectively.17 Patient names were classified as “bookend” matching if the first and last letters of the names were identical. Patient bed proximity at the time of order entry was classified according to the room and bed numbering system of the specific hospital unit and classified as Same Room, Nearby, Same Unit, or Different Unit. Diagnosis overlap was determined based on the number of The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) discharge diagnosis codes that the two patients have in common. Codes were truncated at 3 digits. Ordering intensity variables were determined through analysis of the medication orders in the 240 minutes prior to the first order entered for Patient A. As a proxy measure for physician fatigue, a work block score was calculated based on the number of 4 hour blocks of time in the previous 24 hours that the physician had entered at least one order. For example, a score of 3 would indicate 3, possibly non-consecutive, 4 hour blocks in the preceding 24 hours. Thus, the maximal score would be 6. The proxy measure for distraction was the number of orders the provider had placed in the four hours prior to the initial order.
Data analysis:
Descriptive statistics were generated and the matched case-control analysis was performed using univariate conditional logistic regression. Variables found to be associated with an increased likelihood of OOMP events at a level of statistical significance of p<0.20 were then included in a multivariate conditional logistic-regression model. Because these events are relatively rare, odds ratios were considered to approximate risk ratios. All analyses were performed using the R programming language18 and the R survival package.17
Results
Email Surveys:
The response rate for the CHP physicians was 39% and 13 individual CMIOs replied to the email survey. The answers within each group varied slightly, but for the most part were consistent.
CMIO respondents believe that when they first launched their respective EMR there was a high frequency of patient misidentification. However, after changes were made to the system, they believe that most errors were eliminated and patient misidentification had become a rare event. CMIO respondents acknowledged that they are only informed of these events with significant errors. They attribute the errors to the ability to view multiple charts on one computer simultaneously and poor screen design. The contributing factors are believed to be patients with similar names and conditions. Very few of the CMIO respondents postulated how the errors are caught before reaching the patient. Some believe the errors are caught by the ordering physician. To prevent patient identification errors, they believe the ability to simultaneously view multiple charts should be deactivated.
The CHP physicians have two different beliefs for the frequency of patient identification errors. Almost half stated that it happens frequently and the other half says it is infrequent. However, the majority of CHP physicians confirmed that they have made this type of error. Some mentioned that the medication is usually cancelled before reaching the patient so it must occur more frequently than reported. It was also mentioned that the rate of patient misidentification is higher in the Emergency Department than in inpatient settings. The CHP physicians believe this type of error is due to the multiple simultaneous chart open feature. One CHP physician described how easy it is to click on the wrong patient when entering orders. The biggest contributing factors to entering an order on the wrong patient, according to the CHP physicians, are distraction and fatigue. A commonly cited risk event occurs when staff interrupt physicians placing orders to ask questions about different patients. One CHP physician said nurses and other physicians take advantage of the fact that she is sitting down at a computer to ask her questions. Another risk factor is similarities between patients. The most frequently mentioned similarities were names, condition, and location. A CHP physician reported a patient identification error was made when there were two patients next door to each other, both with flu-like symptoms. The CHP physicians said that errors are usually caught when reviewing the order after signing. The errors are also sometimes caught and intercepted by the nurses who typically realize an order is wrong when it does not correspond with the patient’s condition or when a task they know needs to be completed does not appear in their task list. Less frequently, the error is caught by the pharmacist. One CHP physician said the pharmacy is not as helpful as the nurses because they are not aware of the patient’s whole story and background. It was mentioned that CPOE does not prevent patient identification errors. The CHP physicians suggested that only allowing one chart open and creating a verification alert will reduce the errors. The CHP physicians also mentioned that they believe risk factors for misidentification include lack of sleep and/or lack of experience.
Case Identification:
In this study, 2,466,798 consecutive inpatient medication orders were analyzed. Of these, 908,942 (36.8%) were excluded as IV fluids or common drugs, an additional 408,165 (16.5%) were excluded as order modifications, and another 146,790 (5.9%) were excluded as verbal orders or non-physician CPOE. Of the remaining 1,002,901 orders, 40,079 (4.0%) were discontinued by the original ordering physician within 120 minutes, and 12,670 (1.3%) were not reordered, nor was a similar drug reordered within 180 minutes. Finally, 644 OOMP events were identified when the same drug was rapidly reordered on a different patient. The incidence of OOMP events at the order level was 0.064%. Only four of these events had previously been reported to the CHP risk management reporting system. At the encounter level, OOMP events pertained to 529 unique encounters out of a total of 61,324 encounters. The incidence of OOMP events at the encounter level was 0.9%. The sequence of order actions was rapid. The median time from order entry to cancellation was 1 minute (Range 0–116; IQR 0–11.25). The median time from cancellation to reorder for Patient B was also 1 minute (Range 0–5; IQR 1–2). For the matched control dyads, the median time from order for Patient A to order for Patient B was 5 minutes (Range 0–120; IQR 2–12.75).
Case Validation:
Of the 200 randomly selected charts reviewed, 2 (1%) were classified as “Definite”, 121 (60%) were “Likely”, 77 (39%) were “Uncertain”, and 0 (0%) were “Unlikely”. Thus, the specificity was 61 to 100 percent.
Case-Control Analysis:
According to univariate analyses, 25 variables showed a significant difference between case dyads and control dyads (Table 1). Every grouping of characteristics showed significant differences, including demographics, patient names, location/service, physician characteristics, and order characteristics.
Table 1.
Univariate analysis. Comparison of cases and controls.
| Characteristic | Cases n = 644 | Controls n = 3220 | P Value |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Biological Sex | |||
| Pt A Male | 45.7% | 44.8% | 0.662 |
| Pt B Male | 43.9% | 44.2% | 0.910 |
| Same Sex | 60.4% | 51.6% | <0.001 |
|
| |||
| Age Group Pt A | <0.001 | ||
| Adult | 4.7% | 5.8% | |
| Teen | 14.6% | 27.7% | |
| School Age | 14.9% | 27.3% | |
| Preschool | 20.5% | 20.2% | |
| Infant | 23.4% | 11.1% | |
| Newborn | 21.9% | 8.0% | |
|
| |||
| Age Difference | <0.001 | ||
| Pt A >3 years older | 17.4% | 28.1% | |
| Pt A 1 month to 3 years older | 20.0% | 17.5% | |
| Within 1 month | 18.6% | 8.3% | |
| Pt B 1 month to 3 years older | 17.7% | 17.7% | |
| Pt B >3 years older | 26.2% | 28.4% | |
|
| |||
| Similar Name | |||
|
| |||
| Sound Alike Names | |||
| First Name | 1.6% | 0.5% | 0.003 |
| Last Name | 1.7% | 1.2% | 0.310 |
| First to Last Name | 0.0% | 0.2% | 0.994 |
|
| |||
| “Bookend” Letter Match | |||
| First Name | 2.2% | 1.0% | 0.018 |
| Last Name | 2.0% | 1.6% | 0.468 |
|
| |||
| Edit Distance | |||
| First Name – mean | 0.9706 | 0.9972 | 0.042 |
| Last Name – mean | 1.0157 | 1.0386 | 0.136 |
|
| |||
| Two Letter Overlap | |||
| First Name – mean | 0.0646 | 0.0644 | 0.971 |
| Last Name – mean | 0.0727 | 0.0542 | 0.001 |
| Full Name – mean | 0.0850 | 0.0769 | 0.037 |
|
| |||
| Bed Location/Service | |||
|
| |||
| Same Service | 57.3% | 62.6% | 0.005 |
|
| |||
| Length of Stay | |||
| Pt A (hours) – mean | 406.58 | 321.36 | 0.017 |
| Pt B (hours) – mean | 311.63 | 317.50 | 0.849 |
|
| |||
| Diagnosis codes in common – mean | 2.2065 | 1.9637 | 0.008 |
|
| |||
| Service Pt A | <0.001 | ||
| Surgical | 32.6% | 38.2% | |
| Medical | 55.7% | 55.5% | |
| ICU | 5.3% | 1.3% | |
| Other | 6.1% | 3.4% | |
|
| |||
| Service Pt B | <0.001 | ||
| Surgical | 31.5% | 37.3% | |
| Medical | 57.6% | 56.4% | |
| ICU | 4.8% | 1.3% | |
| Other | 5.6% | 3.4% | |
|
| |||
| Proximity | <0.001 | ||
| Different Unit | 15.8% | 22.6% | |
| Same Unit | 61.5% | 59.5% | |
| Nearby | 16.6% | 12.5% | |
| Same Room | 6.1% | 5.3% | |
|
| |||
| Order Characteristics | |||
|
| |||
| STAT Priority | 38.2% | 33.4% | 0.019 |
|
| |||
| Day of Week | 0.030 | ||
| Monday | 9.9% | 14.4% | |
| Tuesday | 28.3% | 16.3% | |
| Wednesday | 16.5% | 15.5% | |
| Thursday | 17.1% | 14.8% | |
| Friday | 16.9% | 14.1% | |
| Saturday | 11.0% | 12.5% | |
| Sunday | 12.7% | 12.5% | |
|
| |||
| Hour | <0.001 | ||
| 6:01PM to Midnight | 23.1% | 21.9% | |
| 12:01AM to 6AM | 14.6% | 7.3% | |
| 6:01AM to Noon | 31.2% | 35.5% | |
| 12:01PM to 6PM | 31.1% | 35.3% | |
|
| |||
| Physician characteristics | |||
|
| |||
| Work Block Score | 3.0544 | 3.0562 | 0.973 |
| Unique physicians entering Pt A orders* – mean | 0.5233 | 0.4566 | 0.020 |
| Unique physicians entering Pt B orders* – mean | 1.0062 | 0.6363 | <0.001 |
|
| |||
| Order Intensity* | |||
|
| |||
| All orders by Provider A – mean | 3.4783 | 2.8740 | <0.001 |
| Orders by Provider A for Drug A – mean | 0.1770 | 0.5909 | <0.001 |
| Orders by all MDs for Pt A – mean | 0.9783 | 0.7976 | 0.007 |
| Orders by all MDs for Pt B – mean | 1.1553 | 0.7173 | <0.001 |
| Orders by Provider A for Pt A – mean | 0.5342 | 0.3659 | <0.001 |
| Orders by Provider A for Pt B – mean | 0.5947 | 0.2784 | <0.001 |
Mean count of orders in the 240 minutes prior to the study order for Pt A.
The 14 variables that remained significantly associated with OOMP events after multivariate analysis are shown in Table 2. In the case dyads as compared to the control dyads, Patient A was more likely to be a newborn or infant, or to be the same sex as Patient B. Patient A is also more likely to be more than one month younger than Patient B. Patient A and Patient B are more likely to have a greater number of two letter overlaps in their last names. Patient B is more likely to be on a medical service and less likely to be on the same service as Patient A. Patient A and Patient B are most likely to be in a nearby room.
Table 2.
Multivariate analysis.
| Characteristic | Risk Ratio (95% CI) | P Value |
|---|---|---|
|
| ||
| Same Sex | 1.58 (1.28 – 1.94) | <0.001 |
|
| ||
| Age Group Pt A, ref = adult | ||
| Infant | 2.93 (1.67 – 5.17) | <0.001 |
| Newborn | 3.57 (1.93 – 6.59) | <0.001 |
|
| ||
| Age Difference, ref = A 1 month to 3 years older | ||
| Pt B 1 month to 3 years older | 0.67 (0.48 – 0.93) | 0.018 |
| Pt B > 3 years older | 0.62 (0.46 – 0.84) | 0.002 |
|
| ||
| Two Letter Overlap | ||
| Last Name | 4.44 (1.49 – 13.19) | 0.007 |
|
| ||
| Same Service | 0.72 (0.58 – 0.89) | 0.002 |
|
| ||
| Proximity, ref = Same Room | ||
| Nearby Room | 2.82 (1.24 – 6.44) | 0.014 |
|
| ||
| Service Pt B, ref = Surgical | ||
| Medical | 1.30 (1.02 - 1.65) | 0.033 |
|
| ||
| Day of Week, ref = Monday | ||
| Sunday | 1.68 (1.10 – 2.55) | 0.016 |
| Wednesday | 1.58 (1.06 - 2.35) | 0.023 |
| Thursday | 1.92 (1.31 – 2.82) | 0.001 |
| Friday | 2.02 (1.37 – 2.98) | <0.001 |
| Saturday | 1.60 (1.05 – 2.44) | 0.029 |
|
| ||
| Hour, ref = 6:01PM to Midnight | ||
| 12:01AM – 6AM | 1.67 (1.17 – 2.37) | 0.004 |
|
| ||
| Unique providers entering Pt B orders* | 1.44 (1.09 – 1.90) | 0.010 |
|
| ||
| Orders by Provider P for all patients* | 1.04 (1.01 – 1.08) | 0.010 |
|
| ||
| Orders by all Providers for Pt B* | 0.78 (0.61 – 0.98) | 0.037 |
|
| ||
| Orders by Provider P for Drug D* | 0.42 (0.34 – 0.52) | <0.001 |
|
| ||
| Orders by Provider P for Pt A* | 1.16 (1.00 – 1.34) | 0.048 |
Mean count of orders in the 240 minutes prior to the study order for Pt A.
The date and time of the initial order were also significant. The highest risk was on Friday and the lowest risk was on Monday. The highest risk time interval was 12:01AM to 6AM. It is more likely that many different providers will enter orders for Patient B in the case dyads as opposed to the control dyads. Provider P is more likely to place a greater number of orders for all patients and less likely to have placed orders of Drug D. In the case dyads, it is less likely that there will be many different providers placing orders for Patient B. Finally, Provider P is more likely to have entered a greater number of orders for Patient A.
Discussion
One commonly mentioned unintended consequence of CPOE systems is patient identification errors. However, this is the first study to quantify the frequency and analyze risk factors for this type of error. We successfully utilized email surveys to determine potential risk factors, created and validated a trigger to identify OOMP events, and completed a case control study which identified risk factors.
Several points became evident from the email surveys. The first is that there is little consensus between the CMIO respondents and the CHP physicians. Their views differ on the frequency, contributing factors, and error interception. This could be due to the fact that CMIOs may be less aware of the actual work flow and processes that occur when placing an order. Also, the CMIO respondents may be more focused on the computer and not on human limitations. Becoming aware of physician needs would allow for a computer design that is more helpful and useful to the users.
The surveys also showed that having multiple charts open simultaneously is thought to cause errors. The CMIOs and the CHP physicians both cited this feature as a cause for errors. Email surveys were helpful in establishing potential risk factors, but the wide variety of beliefs made evident the need for a rigorous and quantitative method to identify patient misidentification.
To determine potential cases of patient misidentification, we created a trigger approach. This method was used to avoid contacting and relying on providers to identify their own errors. Self-report would be problematic because of recall bias, unwillingness to admit mistakes, and the burden of contacting the providers. A previously used trigger is the abrupt cancellation of a medication. To apply it to patient identification errors, we extended the trigger to include events in which the cancellation is followed by a quick reorder on a different patient. The case validation shows that our trigger was sufficiently specific in detecting OOMP events to proceed with the case-control study. Our specificity of 61–100% compares favorably with typical trigger specificities.19
The reported incidence at the encounter level for adverse drug reactions for hospitalized children is 0.6% to 16.8%.20 Our incidence rate for OOMP events at the encounter level is within this range. When additional events that are not caught are considered, this indicates that patient identification errors may be as common as adverse drug reactions. Our rate at the order level is comparable to the reported incidence rate of mislabeled laboratory specimens.7 Both lab results and closed loop medication administration are highly automated processes that depend on initially picking the correct patient by the clinician at the bedside.
The most commonly mentioned risk factors in previous qualitative research have been similar name, similar condition, and distraction. In the present study, ten variables were used to measure similar names (both by sound and spelling). Only the spelling of the last name proved significant. It is possible that errors could occur when the provider is selecting a name from a list sorted alphabetically by last name. To measure similar conditions, we created measures for diagnosis overlap and same service. The diagnosis overlap was not significant. The same service measure indicates that Patient A is more likely to be on a different service from Patient B. These results do not support the notion that similar condition increases the chance of an OOMP event.
Consistent with previous research, our proxy measure for distraction was significant in the multivariate analysis. The email survey suggested fatigue as a cause of patient misidentification. In this study, fatigue was measured by number of four hour blocks in the previous 24 hours in which the provider had placed orders. Our results do not demonstrate fatigue contributing to patient misidentification. The other most commonly mentioned risk factor in the email surveys was having two charts open. Data were not available to measure this.
The results of our case-control study indicate additional characteristics that are strongly associated with the case dyads. High risk situations are based on day of the week, time, patient age, and room location. Provider familiarity with the drug and patient affect the likelihood of error.
Overall, this study demonstrates that it is the context of the order entry process, more than the characteristics of the patient names themselves, which are associated with increased risk of patient identification errors.
Certain limitations must be considered in interpreting the results of our study. First, the qualitative analysis is limited by the response rate of the informal email survey; however, it fulfilled its intended purpose of generating suggestions of risk factors. There are several limitations regarding the trigger. The validation was limited to a retrospective analysis of what was documented in the EMR at the time of the event and there were no examples of providers documenting their error. Only four of 644 events had been reported through the hospital reporting system.
Second, the sensitivity of the trigger was not evaluated. The algorithm required the same provider for all order actions. If a different provider or a nurse cancelled or reordered, the trigger did not detect it. If the cancel/reorder sequence was reversed the OOMP would not be detected. Medications that actually reached the wrong patient were not detected. These limitations would suggest that the true incidence of OOMP events is greater than reported here.
Third, the variables created did not always fully measure the suggested risk factor. For example, distraction was measured by quantifying the number of orders placed in the previous four hours. This is an imperfect representation of distraction as a provider could be distracted without having many orders to place. Fatigue was also an imperfect measure because data were not available for the provider’s amount of sleep. The data for similar name are based on the patient’s current name in the EMR. When the error occurred, we do not know if their name was the same as it is now. For example, in hospital information systems newborns are often named “Baby Boy Smith” or “Baby Girl Garcia.” Thus, at the time of the OOMP event the patient names may have been more similar than reported here.
Finally, the data analyzed were only collected from inpatient medication orders from a single institution, so may not be generalizable to other nonpediatric hospitals or CPOE contexts.
Future research will be required to determine if not allowing multiple charts to be open simultaneously will decrease the rate of errors. Research should also be conducted to identify the sensitivity of our patient misidentification trigger. Implementing the trigger in real time would allow for risk factors to be investigated immediately after the patient identification error. Ultimately, to reduce OOMP events and improve safety, future research should focus on creating a pop-up alert at the time of order for Patient A warning the provider of a high risk for error. This study does not confirm that a sound-alike name alert alone would be a fruitful intervention.
Conclusion
Through an automated approach, we were able to successfully detect orders entered on misidentified patients. These events are frequent and underreported. A number of significant risk factors were identified and, with additional study, could help hospitals understand, recognize, and prevent these errors and improve patient safety.
Acknowledgments
The authors would like to thank CHP Patient Safety, Data Warehouse, and Nursing Informatics colleagues for helpful discussions and data extraction. HIL was supported by the Children’s Hospital of Pittsburgh of UPMC summer Student Research Training Program.
References
- 1.Bates DW, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 2.Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. Journal of the American Medical Informatics Association. 11:104–112. doi: 10.1197/jamia.M1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbloom ST, et al. Interventions to regulate ordering of serum magnesium levels: report of an unintended consequence of decision support. J Am Med Inform Assoc. 2005;12:546–553. doi: 10.1197/jamia.M1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chassin MR, Becher EC. The Wrong Patient. Annals of Internal Medicine. 2002;136:826–833. doi: 10.7326/0003-4819-136-11-200206040-00012. [DOI] [PubMed] [Google Scholar]
- 5.Franklin BD, O’Grady K, Donyai P, Jacklin A, Barber N. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: a before-and-after study. Qual Saf Health Care. 2007;16:279–284. doi: 10.1136/qshc.2006.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn EJ, Moga PJ. Patient misidentification in laboratory medicine: a qualitative analysis of 227 root cause analysis reports in the Veterans Health Administration. Arch Pathol Lab Med. 2010;134:244–255. doi: 10.5858/134.2.244. [DOI] [PubMed] [Google Scholar]
- 7.Valenstein PN, Raab SS, Walsh MK. Identification errors involving clinical laboratories: a College of American Pathologists Q-Probes study of patient and specimen identification errors at 120 institutions. Arch Pathol Lab Med. 2006;130:1106–1113. doi: 10.5858/2006-130-1106-IEICL. [DOI] [PubMed] [Google Scholar]
- 8.Koppel R, et al. Role of Computerized Physician Order Entry Systems in Facilitating Medication Errors. JAMA: The Journal of the American Medical Association. 2005;293:1197–1203. doi: 10.1001/jama.293.10.1197. [DOI] [PubMed] [Google Scholar]
- 9.Classen DC, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood) 2011;30:581–589. doi: 10.1377/hlthaff.2011.0190. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, et al. Prevalence of adverse events in pediatric intensive care units in the United States. Pediatr Crit Care Med. 2010;11:568–578. doi: 10.1097/PCC.0b013e3181d8e405. [DOI] [PubMed] [Google Scholar]
- 11.Sharek PJ, et al. Adverse events in the neonatal intensive care unit: development, testing, and findings of an NICU-focused trigger tool to identify harm in North American NICUs. Pediatrics. 2006;118:1332–1340. doi: 10.1542/peds.2006-0565. [DOI] [PubMed] [Google Scholar]
- 12.Koppel R, et al. Identifying and quantifying medication errors: evaluation of rapidly discontinued medication orders submitted to a computerized physician order entry system. J Am Med Inform Assoc. 2008;15:461–465. doi: 10.1197/jamia.M2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Classen DC, Pestotnik SL, Evans RS, Burke JP. Description of a computerized adverse drug event monitor using a hospital information system. Hosp Pharm. 1992;27:774, 776–779, 783. [PubMed] [Google Scholar]
- 14.Acute Care Electronic Medical Record : Cerner Corporation at < http://www.cerner.com/solutions/Hospitals_and_Health_Systems/Acute_Care_EMR/>.
- 15.AMDIS | Association of Medical Directors of Information Systems at < http://amdis.org/>.
- 16.Ryan GW, Bernard HR. Techniques to Identify Themes. Field Methods. 2003;15:85–109. [Google Scholar]
- 17.The Comprehensive Perl Archive Network www.cpan.org. at < http://www.cpan.org/>.
- 18.Team RDCR. A Language and Environment for Statistical Computing. Vienna, Austria: 2011. at < http://www.R-project.org>. [Google Scholar]
- 19.Matlow AG, et al. Description of the development and validation of the Canadian Paediatric Trigger Tool. BMJ Qual Saf. 2011;20:416–423. doi: 10.1136/bmjqs.2010.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth RMD, et al. Adverse drug reactions in children-a systematic review. PLoS ONE. 2012;7:e24061. doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]