Abstract
Using vital records and census data representing 165,136 singleton births from 2003–2006, geospatial filtering and density estimates enabled the calculation of preterm birth rates at each geographical point within three urban Ohio counties. Adjusted attributable risk calculations were used to identify risk factors associated with regions of high and low rates of preterm birth. Among the three counties, affected populations varied in size as well as in demographic composition. Variation in the risk factors from one region to another suggests that a single one size fits all intervention strategy would be unlikely to efficiently or effectively impact the complex preterm birth problem. Although more useful in areas with a heterogeneous distribution of preterm birth, application of the presented approach supports the development of efficient community-level health intervention strategies by identifying communities with the highest potential impact and allowing for the prioritization of efforts on specific risk factors within those communities.
Introduction
Preterm birth (<37 weeks gestation) is the leading contributor to neonatal morbidity and infant mortality in the United States. 1 According to the National Center for Health Statistics, 68.6% of all infant deaths in 2005 occurred among preterm infants. Additionally, while just 2% of infant births occurred prior to 32 weeks gestation these very preterm babies accounted for over half of all infant deaths. 2 The over one-half million infants born prematurely each year in the United States accrue an associated annual cost exceeding $26 billion. 3 Notwithstanding recent subtle declines in the rate of late preterm births (34–37 weeks gestation), the current overall rate of prematurity in the United States remains over 20% elevated from 1981 rates. 4 Clearly, gaps remain in the understanding, management, and prevention of preterm birth requiring the development of new strategies to combat the problem.
Rather than a singular disease, preterm birth can best be described as the end result of multiple disease processes. Preterm birth results from the convergence of etiologic causes related to biology, genetics, behavior, and socioeconomics. 5–8 Although a number of studies have demonstrated a disproportional occurrence of preterm birth among racial minorities and the socioeconomically disadvantaged, it is a problem that transcends race and socioeconomic status. 9 As a consequence, prematurity cannot be solved with a single approach at the care provider or community level. To combat preterm birth at a population level, strategies for intervention must be devised to address multiple contributors to the problem. Effective intervention strategies will vary from region to region or from one community to another depending on the prevalence and impact of risk factors in each setting.
We previously demonstrated regional variation in preterm birth risk factors within a single urban county using geographic analysis. 10 Although the risk factors identified were consistent with previous findings, the distributions of risk factors and cases attributed to risk exposure were not geographically uniform. Primary risk factors also differed when comparing areas with high and low proportions of preterm birth.
This study was designed to demonstrate that the approach used previously to target high-impact regions in a single county could be generalized and reproduced in other urban settings. 10 The current effort was also intended to explore potential variation in the constellation of preterm birth risk factors identified in three geographically distinct but demographically similar urban Ohio counties. We hypothesized that as in our previous analysis, preterm birth risk factors would vary within and across the three counties containing metropolitan centers. We applied the data selection criteria and Geographic Information System (GIS) implementation used previously in a single county analysis to three county data sets in the current study. The resulting analysis was intended to support informed community-based public health initiatives to combat preterm birth. This approach was applied to Ohio’s Cuyahoga, Franklin, and Hamilton Counties containing the cities Cleveland, Columbus, and Cincinnati respectively.
Methods
Data Selection
Preterm birth risk factors identified in the literature and confirmed by clinical experts were selected for inclusion in the analysis.5 Vital statistics data, including latitude and longitude coordinates corresponding to each mother’s residential address, were obtained from the Ohio Department of Health for infants born to mothers residing in Ohio (Cuyahoga, Franklin, and Hamilton Counties) on the date of the infant’s birth during 2003 through 2006. For each subject, elements obtained from the vital statistics data set included infant gestational age, maternal race and ethnicity (Black non-Hispanic, White non-Hispanic, Other non-Hispanic, and Hispanic), maternal age at delivery (< 18 years, 18 to 34 years, or ≥35 years), marital status (married or unmarried), education level (less than completed high school or equivalent, completed high school or equivalent, any post-graduate education), interpregnancy interval (<18 months or ≥18 months between the birth of the current infant and a previous delivery), first-time mother status, the occurrence of a previous preterm birth, chronic hypertension, pregnancy related hypertension, diabetes (gestational or pre-gestational), smoking during gestation, and prepregnancy weight classification (≤10th percentile, ≥90th percentile, or midrange). Additionally, socioeconomic data representing the median household income of each census block within each county were obtained from the 2000 decennial census database11 and linked to individual records according to census block of mother’s residence.
Study population
183,437 infants were born to residents of Cuyahoga, Franklin, and Hamilton Counties from 2003 through 2006. Analysis was limited to singleton births, resulting in exclusion of 6,690 infants. Every infant had a documented gestational age, though 1,957 infants were excluded because documented gestational age fell outside of the range of 23 to 44 weeks. An additional 9,654 infants were excluded due to missing spatial coordinates. Data from infants suffering neonatal or infant death were included; however, data from stillborn infants was not. No consideration for exclusion was made for any congenital anomaly. 165,136 infants were included in the final analysis.
Spatial Methodologies
Within each of the three urban counties, spatial density estimates were generated using established spatial filtering techniques.12–15 As previously reported, continuous preterm birth proportions were calculated within a sliding 6,000 foot radius window surrounding square kernel regions with side length of 1,000 feet.10 Regions of preterm birth activity greater than or less than one standard deviation from the mean Ohio county preterm birth rate of 12.8% were identified as hot or cold spots. The preterm birth rate in hotspots exceeded 14.5% and the proportion of preterm births in cold spots fell below 11.0%. Geographic features including surface road access, highways and railroads, location of residential areas, topography and waterways were used to further define each region and to ensure that each area of interest was distinct.
Statistical analysis
Statistical analyses (other than spatial analysis) were performed using SAS™ version 9.2 (SAS Institute Inc., Cary, NC) software. A multivariable logistic regression model was constructed to fit at the county level in order to identify predictors for preterm birth. For each selected geographical region we calculated adjusted attributable risks representing the impact of modifiable risk factors on the occurrence of preterm birth in the region. 16 Next, using attributable risk calculations we were able to estimate the number of preterm births potentially prevented within a region by eliminating a given risk factor by deploying an intervention program (indicated in parentheses below). 17 Risk factors considered amenable to management or intervention included: interpregnancy interval (education), previous preterm (17–hydroxy progesterone), chronic hypertension (medical management), diabetes (medical management), smoking (cessation), maternal age of 35 or older (education), and low prepregnancy weight (education).
Institutional review board approval
The Ohio Department of Health and Cincinnati Children’s Hospital Medical Center Institutional Review Boards approved this study.
Results
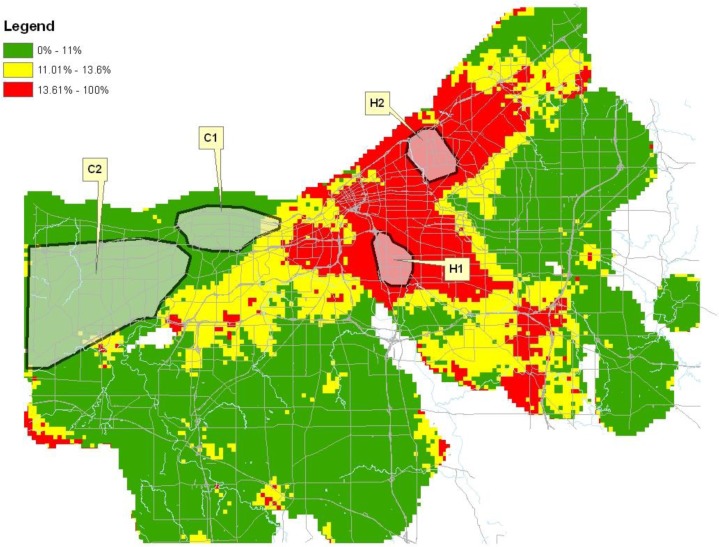
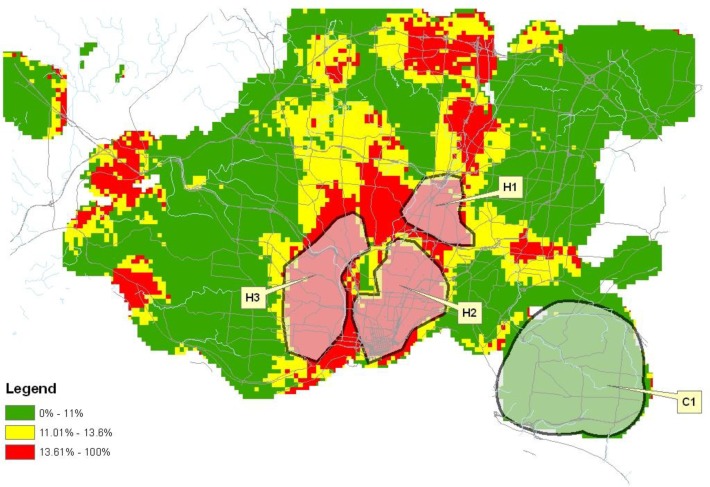
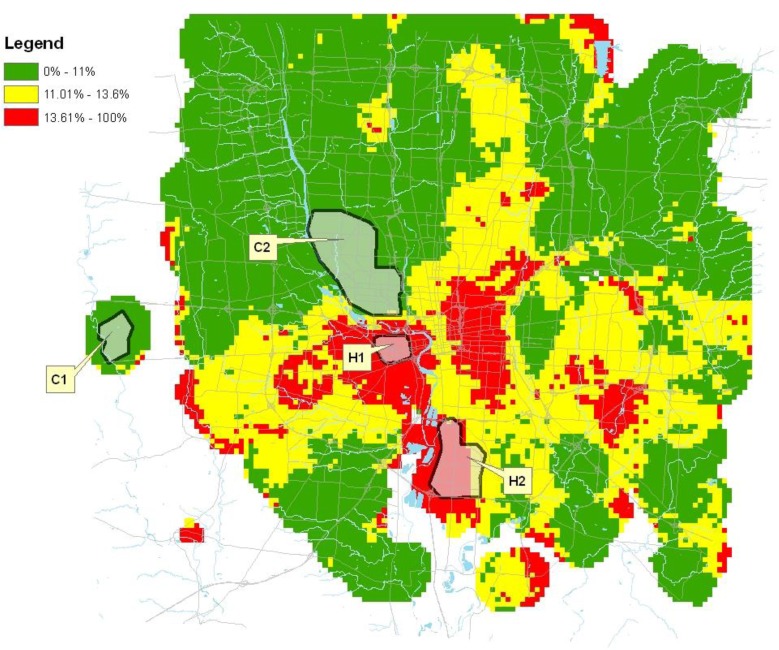
The distribution of preterm birth for each county is represented on the maps in Figures 1–3. As denoted on the maps, 12 distinct geographic areas of interest were selected for investigation, four from each of the three counties. In each of Cuyahoga and Franklin Counties, two hotspots and two cold spots were identified. In Hamilton County, three hotspots and a single cold spot were isolated for analysis. Variation in the impact of risk factors from one geographic region to another was demonstrated among these selected regions.
Figure 1.
Proportion of preterm births in Cuyahoga County, Ohio 2003–2006.
Figure 3.
Proportion of preterm births in Hamilton County, Ohio 2003–2006.
Multivariable logistic regression was conducted for each county using occurrence of premature birth as the dependent variable. At the county level, the regression confirmed the presence of expected risk factors for premature birth consistent with published literature. Advanced maternal age, diabetes, less than high school education, chronic hypertension, pregnancy induced hypertension, previous preterm, short interpregnancy interval, low income, low maternal weight, smoking, and race (African American) were each significantly correlated with preterm birth (P < 0.05) in all three counties analyzed.
Tables 1 and 2 illustrate the sample characteristics of hotspots and cold spots respectively for each county. Despite similarities in the proportion of preterm births occurring within hotspots, significant demographic differences were identified among hotspots, even when comparing hotspots that were selected from the same county. In all three counties, significant differences in the compositions of women according to race, marital status, education, income, smoking status, and length of interpregnancy interval (P <0.001) were identified among the counties’ hotspot populations. In Hamilton and Cuyahoga Counties, the hotspots also contained significant differences in the number of women with a low prepregnancy weight (P < 0.02). Within Hamilton County, there was a significant difference in the distribution of mother’s aged 35 years or older among the hotspots (P = 0.002).
Table 1.
Hotspot Demographics
| Cuyahoga H1 | Cuyahoga H2 | Franklin H1 | Franklin H2 | Hamilton H1 | Hamilton H2 | Hamilton H3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preterm Births (%) | 208 | 16.6% | 307 | 18.6% | 115 | 16.9% | 101 | 16.0% | 210 | 17.5% | 696 | 16.9% | 830 | 17.6% |
|
| ||||||||||||||
| Age, y | ||||||||||||||
| <18 | 113 | 9.0% | 172 | 10.4% | 77 | 11.3% | 32 | 5.1% | 85 | 7.1% | 399 | 9.7% | 347 | 7.4% |
| 18 to 34 | 1044 | 83.2% | 1354 | 82.0% | 571 | 84.0% | 564 | 89.1% | 1015 | 84.4% | 3404 | 82.7% | 4079 | 86.5% |
| ≥35 | 98 | 7.8% | 125 | 7.6% | 32 | 4.7% | 37 | 5.8% | 102 | 8.5% | 315 | 7.6% | 290 | 6.1% |
| Diabetes | 55 | 4.4% | 55 | 3.3% | 24 | 3.5% | 16 | 2.5% | 48 | 4.0% | 124 | 3.0% | 176 | 3.7% |
| Education | ||||||||||||||
| < High School | 514 | 41.0% | 570 | 34.5% | 409 | 60.1% | 189 | 29.9% | 337 | 28.0% | 1441 | 35.0% | 1910 | 40.5% |
| High School | 598 | 47.6% | 816 | 49.4% | 218 | 32.1% | 339 | 53.6% | 567 | 47.2% | 1710 | 41.5% | 2022 | 42.9% |
| College | 116 | 9.2% | 195 | 11.8% | 26 | 3.8% | 88 | 13.9% | 250 | 20.8% | 759 | 18.4% | 507 | 10.8% |
| First Time Mother | 449 | 35.8% | 601 | 36.4% | 231 | 34.0% | 228 | 36.0% | 473 | 39.4% | 1585 | 38.5% | 1541 | 32.7% |
| Hypertension | ||||||||||||||
| Chronic | 37 | 2.9% | 48 | 2.9% | 16 | 2.4% | 8 | 1.3% | 19 | 1.6% | 49 | 1.2% | 69 | 1.5% |
| Pregnancy Related | 58 | 4.6% | 53 | 3.2% | 16 | 2.4% | 18 | 2.8% | 29 | 2.4% | 100 | 2.4% | 107 | 2.3% |
| Income Quartile | ||||||||||||||
| <25% | 1060 | 84.5% | 1474 | 89.3% | 573 | 84.3% | 128 | 20.2% | 460 | 38.3% | 3293 | 80.0% | 3256 | 69.0% |
| 25%–50.0% | 195 | 15.5% | 132 | 8.0% | 107 | 15.7% | 250 | 39.5% | 646 | 53.7% | 584 | 14.2% | 1347 | 28.6% |
| 50.1%–75.0% | 0 | 0.0% | 45 | 2.7% | 0 | 0.0% | 210 | 33.2% | 96 | 8.0% | 226 | 5.5% | 113 | 2.4% |
| >75% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 45 | 7.1% | 0 | 0.0% | 15 | 0.4% | 0 | 0.0% |
| Interpregnancy Interval <18 mo. | 115 | 9.2% | 99 | 6.0% | 96 | 14.1% | 36 | 5.7% | 72 | 6.0% | 385 | 9.3% | 480 | 10.2% |
| Prepregnancy Weight | ||||||||||||||
| Lower 10% | 147 | 11.7% | 128 | 7.8% | 112 | 16.5% | 98 | 15.5% | 165 | 13.7% | 429 | 10.4% | 534 | 11.3% |
| Upper 10% | 130 | 10.4% | 176 | 10.7% | 74 | 10.9% | 72 | 11.4% | 141 | 11.7% | 475 | 11.5% | 542 | 11.5% |
| Previous Preterm | 34 | 2.7% | 37 | 2.2% | 10 | 1.5% | 9 | 1.4% | 20 | 1.7% | 105 | 2.5% | 115 | 2.4% |
| Race | ||||||||||||||
| Non-Hispanic Black | 662 | 52.7% | 1610 | 97.5% | 97 | 14.3% | 123 | 19.4% | 598 | 49.8% | 3160 | 76.7% | 2891 | 61.3% |
| Non-Hispanic White | 510 | 40.6% | 25 | 1.5% | 544 | 80.0% | 489 | 77.3% | 500 | 41.6% | 794 | 19.3% | 1533 | 32.5% |
| Hispanic | 73 | 5.8% | 7 | 0.4% | 21 | 3.1% | 10 | 1.6% | 89 | 7.4% | 58 | 1.4% | 242 | 5.1% |
| Smoking | 338 | 26.9% | 226 | 13.7% | 269 | 39.6% | 166 | 26.2% | 228 | 19.0% | 682 | 16.6% | 953 | 20.2% |
| Unmarried | 850 | 67.7% | 1357 | 82.2% | 443 | 65.1% | 298 | 47.1% | 710 | 59.1% | 2992 | 72.7% | 3326 | 70.5% |
Table 2.
Cold Spot Demographics
| Cuyahoga C1 | Cuyahoga C2 | Franklin C1 | Franklin C2 | Hamilton C1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preterm Births (%) | 262 | 9.5% | 233 | 7.4% | 23 | 6.6% | 106 | 7.6% | 184 | 7.4% |
|
| ||||||||||
| Age, y | ||||||||||
| <18 | 51 | 1.8% | 20 | 0.6% | 2 | 0.6% | 7 | 0.5% | 23 | 0.9% |
| 18 to 34 | 2230 | 80.7% | 2434 | 77.8% | 310 | 89.1% | 975 | 70.0% | 1878 | 75.7% |
| ≥35 | 481 | 17.4% | 675 | 21.6% | 36 | 10.3% | 411 | 29.5% | 580 | 23.4% |
| Diabetes | 92 | 3.3% | 164 | 5.2% | 13 | 3.7% | 25 | 1.8% | 62 | 2.5% |
| Education | ||||||||||
| < High School | 264 | 9.6% | 178 | 5.7% | 13 | 3.7% | 29 | 2.1% | 111 | 4.5% |
| High School | 1057 | 38.3% | 946 | 30.2% | 128 | 36.8% | 182 | 13.1% | 608 | 24.5% |
| College | 1402 | 50.8% | 1967 | 62.9% | 206 | 59.2% | 1164 | 83.6% | 1737 | 70.0% |
| First Time Mother | 1320 | 47.8% | 1262 | 40.3% | 123 | 35.3% | 663 | 47.6% | 962 | 38.8% |
| Hypertension | ||||||||||
| Chronic | 34 | 1.2% | 40 | 1.3% | 12 | 3.4% | 17 | 1.2% | 21 | 0.8% |
| Pregnancy Related | 91 | 3.3% | 122 | 3.9% | 8 | 2.3% | 37 | 2.7% | 47 | 1.9% |
| Income Quartile | ||||||||||
| <25% | 235 | 8.5% | 0 | 0.0% | 0 | 0.0% | 38 | 2.7% | 87 | 3.5% |
| 25%–50.0% | 908 | 32.9% | 150 | 4.8% | 0 | 0.0% | 164 | 11.8% | 398 | 16.0% |
| 50.1%–75.0% | 1284 | 46.5% | 1080 | 34.5% | 0 | 0.0% | 299 | 21.5% | 529 | 21.3% |
| >75% | 335 | 12.1% | 1899 | 60.7% | 348 | 100.0% | 892 | 64.0% | 1467 | 59.1% |
| Interpregnancy Interval <18 mo. | 153 | 5.5% | 194 | 6.2% | 17 | 4.9% | 35 | 2.5% | 125 | 5.0% |
| Prepregnancy Weight | ||||||||||
| Lower 10% | 290 | 10.5% | 342 | 10.9% | 39 | 11.2% | 194 | 13.9% | 268 | 10.8% |
| Upper 10% | 176 | 6.4% | 156 | 5.0% | 28 | 8.0% | 118 | 8.5% | 144 | 5.8% |
| Previous Preterm | 59 | 2.1% | 57 | 1.8% | 5 | 1.4% | 1 | 0.1% | 19 | 0.8% |
| Race | ||||||||||
| Non-Hispanic Black | 201 | 7.3% | 55 | 1.8% | 4 | 1.1% | 22 | 1.6% | 71 | 2.9% |
| Non-Hispanic White | 2392 | 86.6% | 2850 | 91.1% | 332 | 95.4% | 1275 | 93.4% | 2296 | 92.5% |
| Hispanic | 93 | 3.4% | 89 | 2.8% | 5 | 1.4% | 29 | 2.1% | 59 | 2.4% |
| Smoking | 365 | 13.2% | 205 | 6.6% | 20 | 5.7% | 43 | 3.1% | 174 | 7.0% |
| Unmarried | 676 | 24.5% | 316 | 10.1% | 39 | 11.2% | 133 | 9.5% | 314 | 12.7% |
Comparing aggregated hotspot to aggregated cold spot regions from all three counties revealed significant differences in the demographic composition with regards to race, age, marital status, education, income, and smoking status. Differences were also found in the prevalence of chronic hypertension, short interpregnancy interval, and previous preterm birth (P <0.003).
Among mothers who delivered preterm in Hamilton County, 50.5% had at least one of the modifiable risk factors identified. In Cuyahoga and Franklin Counties, the percentages of women who delivered preterm with at least one modifiable risk factor were 51.5% and 57.1% respectively. Potentially preventable cases attributable to each risk factor are listed by county in Tables 3–5. In Hamilton County, the greatest number of potentially preventable preterm births was attributable to risk associated with the occurrence of a previous preterm birth. In Cuyahoga County, advanced maternal age contributed to the greatest number of preterm births; in Franklin, the greatest number of preterm birth cases were attributable to the short interpregnancy interval risk. While smoking was associated with the second most cases of preterm birth in Cuyahoga and Franklin Counties, low prepregnancy weight was the second most impactful risk in Hamilton County. Considering individual hotspots, the risk factor associated with the greatest number of attributable cases varied from region to region. The most cases of preterm birth were attributed to smoking in three of seven hotspots. In four of the seven hotspots, the second most number of cases were attributable to risk from a previous preterm birth. The only risk factor not associated with either the largest or second largest number of preterm birth cases in any hotspot was diabetes.
Table 3.
Cuyahoga County Attributable Cases
| Cuyahoga County | Cuyahoga H1 | Cuyahoga H2 | Cuyahoga C1 | Cuyahoga C2 | |
|---|---|---|---|---|---|
|
| |||||
| (Preterm Births/ Total Births) | (7212/ 60934) | (208 / 1255) | (307 /1651) | (262/ 2762) | (233 / 3129) |
|
| |||||
| Interpregnancy Interval <18 mo. | 177 | 5 | 10 | 3 | 0 |
| Previous Preterm | 251 | 7 | 13 | 10 | 6 |
| Hypertension, Chronic | 168 | 4 | 2 | 7 | 7 |
| Diabetes | 163 | 2 | 8 | 17* | 9 |
| Smoking | 278 | 28* | 4 | 2 | 1 |
| Prepregnancy Weight, Lower 10% | 197 | 0 | 7 | 3 | 8 |
| Age, 35 Years or Older | 306* | 4 | 22* | 17* | 21* |
Indicates the risk factor contributing to the greatest number of preventable cases within each region.
Table 5.
Hamilton County Attributable Cases.
| Hamilton County | Hamilton H1 | Hamilton H2 | Hamilton H3 | Hamilton C1 | |
|---|---|---|---|---|---|
|
| |||||
| (Preterm Births/ Total Births) | (4948/ 41724) | (210/ 1202) | (696/ 4118) | (830/ 4716) | (184/ 2481) |
|
| |||||
| Interpregnancy Interval <18 mo. | 161 | 4 | 27 | 32 | 3 |
| Previous Preterm | 194* | 7 | 34 | 27 | 6 |
| Hypertension, Chronic | 68 | 2 | 10 | 7 | 0 |
| Diabetes | 85 | 2 | 0 | 13 | 5 |
| Smoking | 168 | 0 | 42* | 35* | 14 |
| Prepregnancy Weight, Lower 10% | 185 | 24* | 1 | 15 | 14 |
| Age, 35 Years or Older | 108 | 8 | 2 | 19 | 15* |
Indicates the risk factor contributing to the greatest number of preventable cases within each region.
Variation was also present in the cold spots. Advanced maternal age contributed to the first or second most number of cases in four of the five cold spots. Low prepregnancy weight and diabetes were each identified as contributing to the most or second most number of cases in two of the five cold spots. Neither short interpregnancy interval nor smoking was associated with the most or second most number of cases of preterm birth in any cold spot.
Discussion
Using the described methods, we successfully identified variations in the preterm birth distribution patterns within all three urban Ohio counties despite their dissimilar geographies. The geographic analysis demonstrated the practicality of identifying regions within urban counties where reduction in the preterm birth rate would have the greatest impact toward reducing the overall preterm birth rate within the county. The approach also highlighted risk factors that made the most substantial contribution to the preterm birth problem within each identified area.
The initial analysis revealing the preterm birth distribution within a particular county is a vital first step in the development of intervention strategies. In both Cuyahoga and Hamilton Counties, large pockets of high preterm birth rates were identified. Within these areas, attributable case analysis offers substantial utility as the implementation of interventions within the affected neighborhoods has the potential to provide substantial impact upon the country preterm birth rate while improving the efficiency with which resources are expended. Our analysis revealed homogeneity in the distribution of preterm birth within Franklin County suggesting that it may not be possible to improve efficiency of resource expenditure within the county by focusing on specific neighborhoods.
Although we were able to find areas of high and low preterm birth proportions in all three counties, the identified hotspots varied in area and in population characteristics. This underscores the importance of conducting this type of analysis as a starting point for developing community specific interventions rather than merely implementing a strategy that has worked elsewhere. It is interesting to note that even among regions having comparably high preterm birth rates, demographics demonstrated variability. For example, in Cuyahoga County, hotspot H1 contained a racial composition that was 52.7% Non-Hispanic Black and 40.6% Non-Hispanic White compared to hotspot H2 in which 97.5% was Non-Hispanic Black and just 1.5% Non-Hispanic White. Despite the racial differences, there was little difference in the rate of preterm birth between these two geographically distinct areas. Using another example, within four hotspots over 80% of mothers lived in census blocks characterized as belonging to the lowest income quartile. In both hotspot Franklin H2 and Hamilton H1, however, a smaller percentage of women resided in the lowest income quartile census blocks (20.2% and 38.3% respectively). The analysis demonstrates that although there are similarities, not all hotspots are the same dispelling notions that preterm birth is a problem that affects a single demographic profile. Although preterm birth has been shown to disproportionality impact the black population, these data demonstrate that preterm birth also impacts women and children from all demographic groups. Even more important than demographic differences between geographically distinct hotspots is the variation that exists in the risk factors contributing to preterm birth in each hotspot. A single one size fits all approach is unlikely to be efficient or effective in combatting the complex preterm birth problem.
This analysis may assist in the prioritization and efficient use of community resources. To effect a reduction in the preterm birth rate within a hotspot it would be much more efficient to target interventions within the hotspot rather than launching a county-wide campaign. For example, elimination of smoking in Cuyahoga H1 would potentially prevent 28 preterm births corresponding to a 2.2% reduction in the Cuyahoga H1 preterm birth rate, a reduction from 16.6% to 14.4%. A similar, but much more expensive county-wide effort could prevent 278 preterm birth cases corresponding to a 0.5% reduction in the county’s preterm birth rate, a reduction from 11.8% to 11.3%. Other substantial reductions could be achieved through similar tailored interventions at the sub-county level including a potential 1.3% reduction in Cuyahoga H2 preterm birth rate associated with the risk of advanced maternal age, as well as a 2.0% and 0.9% potential reduction in Hamilton H1 and Franklin H1 respectively from the elimination of the risk associated with underweight mothers. Within Franklin County, stakeholders may determine that targeted interventions would be unlikely to yield a substantial reduction in the potential number of preterm birth cases and may opt instead for a broader regional approach.
For another practical example, within Hamilton County, previous preterm birth is a considerable contributor to subsequent preterm births. Although it is not possible to modify a woman’s preterm birth history, 17 alpha-hydroxyprogesterone caproate (17-OHPC) given as a weekly series of injections after 16 weeks of gestation has been shown to be an effective intervention to reduce preterm birth among women who have previously delivered spontaneously prior to term.18 An effort to pilot the use of 17-OHPC among high-risk women would costly to implement at a county level. Instead, the pilot could be focused within one or two clinics and expanded. Based upon this analysis, the pilot is most likely to demonstrate a benefit if introduced in H2 or H3. Even though preterm birth rates in H1 are also high, the previous preterm risk factor has less of an impact in that region Geospatial analysis does not dictate which strategy should be implemented, but allows for the development of informed strategies and improved efficiency in the use of resources in battling preterm birth.
Some limitations are introduced by the use of vital records data which are typically captured at the birth hospital following delivery. Self-reported variables include education, race, and smoking measures. Quality of the clinical measures such as hypertension and diabetes cannot be independently verified and the reliability is likely to vary. Additionally, gestational age estimates (based upon the combined estimate of gestation variable which reconciles calculated gestation from the mother’s reported last menstrual period with a clinical estimate of gestation) are potentially inaccurate. Nevertheless, previous validation studies of vital records data have found that although the sensitivity of risk factor and outcome data were of varying quality, the specificity of those data usually exceeded 95%. 19, 20 Lastly, the geocoded addresses are derived from the mother’s reported residence at the time of birth. The provided data do not indicate how long the mother lived at the reported address prior to delivery. From the perspective of developing intervention strategies, it would be potentially more useful to capture an address corresponding to where pregnant mothers lived during the majority of the pregnancy duration than at the single point of time corresponding to delivery.
Conclusions
The application of the spatial analysis approach offers promise in improving the effective prevention of preterm birth by informing efficient public health strategies. Particularly in geographic regions with patterns of uneven preterm birth distribution this approach enables the targeting of interventions within highly affected neighborhoods. Not only does this type analysis enable the prioritization of geographic locations to target, but within those locations priorities may also be set as to which risk factors should be targeted. This is significant when reflecting on current economic limitations. Historically, prevention has been underfunded. With the current landscape of budget cuts, it is imperative that public health efforts are focused and tailored due to ever decreasing resources while maintaining maximum positive impact on the at-risk populations.
Figure 2.
Proportion of preterm births in Franklin County, Ohio 2003–2006.
Table 4.
Franklin County Attributable Cases.
| Franklin County | Franklin H1 | Franklin H2 | Franklin C1 | Franklin C2 | |
|---|---|---|---|---|---|
|
| |||||
| (Preterm Births/ Total Births) | (6915/ 62476) | (115/ 680) | (101/ 633) | (23/ 348) | (106/1393) |
|
| |||||
| Interpregnancy Interval <18 mo. | 250* | 0 | 1 | 0 | 0 |
| Previous Preterm | 149 | 1 | 3* | 2* | 0 |
| Hypertension, Chronic | 162 | 4 | 3* | 1 | 4 |
| Diabetes | 112 | 2 | 3* | 0 | 0 |
| Smoking | 212 | 2 | 2 | 1 | 0 |
| Prepregnancy Weight, Lower 10% | 192 | 6* | 0 | 0 | 7* |
| Age, 35 Years or Older | 115 | 2 | 0 | 0 | 6 |
Indicates the risk factor contributing to the greatest number of preventable cases within each region.
References
- 1.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010 Feb 11;362(6):529–35. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 2.Macdorman MF, Mathews TJ. Recent trends in infant mortality in the United States. NCHS Data Brief. 2008 Oct;(9):1–8. [PubMed] [Google Scholar]
- 3.National Center for Health Statistics Final natality data. Retrieved October 29, 2010: www.marchofdimes.com/peristats.
- 4.Martin JA, Osterman MJ, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS Data Brief. 2010 May;(39):1–8. [PubMed] [Google Scholar]
- 5.Behrman RE, Butler AS, Institute of Medicine (U.S.) Committee on Understanding Premature Birth and Assuring Healthy Outcomes Preterm birth : causes, consequences, and prevention. Washington, D.C: National Academies Press; 2007. [PubMed] [Google Scholar]
- 6.DeFranco EA, Lian M, Muglia LA, Schootman M. Area-level poverty and preterm birth risk: a population-based multilevel analysis. BMC Public Health. 2008;8:316. doi: 10.1186/1471-2458-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med. 2007 Jan;25(1):40–51. doi: 10.1055/s-2006-956774. [DOI] [PubMed] [Google Scholar]
- 8.Kogan MD. Social causes of low birth weight. J R Soc Med. [Review] 1995 Nov;88(11):611–5. doi: 10.1177/014107689508801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. [Review] 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.South AP, Jones DE, Hall ES, Huo S, Meinzen-Derr J, Liu L, et al. Spatial Analysis of Preterm Birth Demonstrates Opportunities for Targeted Intervention. Maternal and Child Health Journal. 2011 Feb 3; doi: 10.1007/s10995-011-0748-2. [DOI] [PubMed] [Google Scholar]
- 11.Bureau of the Census . Census 2000 summary file 3 (sf 3)-sample data. Hamilton County; Ohio: 2000. [Google Scholar]
- 12.English PB, Kharrazi M, Davies S, Scalf R, Waller L, Neutra R. Changes in the spatial pattern of low birth weight in a southern California county: the role of individual and neighborhood level factors. Soc Sci Med. [Research Support, U.S. Gov’t, P.H.S.] 2003 May;56(10):2073–88. doi: 10.1016/s0277-9536(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 13.Rushton G, Krishnamurthy R, Krishnamurti D, Lolonis P, Song H. The spatial relationship between infant mortality and birth defect rates in a U.S. city. Stat Med. 1996 Sep 15–30;15(17–18):1907–19. doi: 10.1002/(sici)1097-0258(19960930)15:18<1907::aid-sim402>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Rushton G, Lolonis P. Exploratory spatial analysis of birth defect rates in an urban population. Stat Med. 1996 Apr-May;15(7–9):717–26. doi: 10.1002/(sici)1097-0258(19960415)15:7/9<717::aid-sim243>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhen H, Lawson AB, McDermott S, Lamichhane AP, Aelion M. A spatial analysis of mental retardation of unknown cause and maternal residence during pregnancy. Geospat Health. 2008 May;2(2):173–82. doi: 10.4081/gh.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole P, MacMahon B. Attributable risk percent in case-control studies. Br J Prev Soc Med. 1971 Nov;25(4):242–4. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordis L. Epidemiology. third edition. Elsevier Science; 2004. [Google Scholar]
- 18.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003 Jun 12;348(24):2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 19.Reichman NE, Hade EM. Validation of birth certificate data. A study of women in New Jersey’s HealthStart program. Ann Epidemiol. 2001 Apr;11(3):186–93. doi: 10.1016/s1047-2797(00)00209-x. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Validation Studies] [DOI] [PubMed] [Google Scholar]
- 20.Reichman NE, Schwartz-Soicher O. Accuracy of birth certificate data by risk factors and outcomes: analysis of data from New Jersey. Am J Obstet Gynecol. 2007 Jul;197(1):32, e1–8. doi: 10.1016/j.ajog.2007.02.026. [Clinical Trial Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]