Abstract
The intake of multiple medications in patients with various medical conditions challenges the delivery of medical care. Initial empirical studies and pilot implementations seem to indicate that generic safe and effective multi-drug prescription principles could be defined and reused to reduce adverse drug events and to support compliance with medical guidelines and drug formularies. Given that ontologies are known to provide well-principled, sharable, setting-independent and machine-interpretable declarative specification frameworks for modeling and reasoning on biomedical problems, we explore here their use in the context of multi-drug prescription. We propose an ontology for modeling drug-related knowledge and a repository of safe and effective generic prescription principles. To test the usability and the level of granularity of the developed ontology-based specification models and heuristic we implemented a tool that computes the complexity of multi-drug treatments, and a decision aid to check the safeness and effectiveness of prescribed multi-drug treatments.
Introduction
The increasing prevalence of chronic diseases in an aging population poses challenges to the delivery of medical care. More than one in four Americans has multiple chronic conditions (MCC)[1]. The resource implications for addressing MCC are immense: 65% of total health care spending is for 27% of Americans with MCC[2]. Polypharmacy, the intake of several medications, exponentially increases the risk of adverse drug events (ADE)[3] and non-compliance[4]. Furthermore it has been reported that missing drug treatment for active problems, or under treatment for diagnosis, is common in patients with MCC[5]. In addition the management of MCC poses challenges unaddressed in a single disease context, like the overall treatment effectiveness, safety, and complexity.
Unfortunately, clinical practice guidelines mostly focus on providing advice for a single condition. Although the development of disease-specific guidelines has led to improvements in outcomes for patients with individual conditions, the application of these guidelines to persons with MCC is less clear. In addition, as the number of chronic conditions in an individual increases, the multi-drug treatment complexity increases too, while compliance exponentially decreases. Moreover individual medications that impart disease-specific benefits may be less beneficial, or even harmful, when taken along with other medications. This is a reason why polypharmacy exponentially increases the risk of ADEs [3]. Drug therapy problems occur every day and cost money; drug-related morbidity and mortality cost the US almost $200 billion annually, exceeding the amount spent on the medications themselves [6].
The prevalent approaches to clinical decision-support related to medications are to provide drug-drug [7] and drug-disease interaction alerts[8], to prevent adverse events. Less commonly, decision aid systems recommend drugs based on efficacy or best practice recommendations[9]. Further, in many cases the knowledge is expressed in the form of rules[10] that are not generalizable and reusable outside the context for which they were developed.
Because optimizing care for patients with MCC is a high priority, in December 2010 the US Department of Health and Human Services (HHS) presented a strategic framework[11] to catalyze change in how MCC are addressed in the US. According to this report there should be a cultural shift in how chronic illnesses are addressed: from individual disease to MCC-based decision approach. In accordance with the HHS framework, we propose here an ontology-based approach to tackle the management of drug prescriptions for MCC in a principled, generalizable, reusable way. We believe that developing an ontology to support the domain knowledge needed to advise on safe and effective polypharmacy prescriptions constitutes a fundamental step towards facilitating the future creation and sharing of application-independent and reusable decision aid modules for polypharmacy prescription.
The first aim of this study is to apply formal ontology creation methods to model the domain of polypharmacy treatments. An ontology is the formal representation of domain knowledge through the specification of relevant concepts and the semantic relationships between those concepts. Ontologies can be used to represent and share knowledge that is machine-interpretable. Ontologies have been used in computer-based applications and systems for data annotation, natural language processing, decision support and information retrieval. Ontologies could enhance the reusability and interoperability of knowledge, facilitating the development of clinical decision aids. Although there is no consensus on how to develop ontologies, ontological development principles [12] could be followed to facilitate interoperability and reusability of ontologies.
The second aim is to evaluate the resulting ontology through the implementation of two simple tools with practical use in the context of polypharmacy prescription.
The first tool is related to the problem of medication complexity, closely related to the field of polypharmacy prescription. To address this problem, a taxonomy was proposed[13] to classify information related to medication regimen that have proved to influence patient outcomes, like number of medications in a regimen, number of doses per day, the number of drug units per dose, the total number of units per day, drug restrictions. For example, it is logical to think that using 4 different dosage forms, each with different frequencies and complex additional instructions for their administration, would be more difficult than using 4 agents with the same dosage form, all to be taken at the same time, with no additional instructions. George et al. [7] proposed a way to quantify the complexity of a regimen by assigning weights to drug prescriptions based on dosage form (e.g. capsules have weight of 1 point while topical dressings have weight of 3), dosing frequency (e.g. a daily dose have weight of 1 while taking a drug each 4 hours have weight 6.5), and additional directions (e.g. breaking or crushing a tablet adds 1 to the complexity index). Since this taxonomy was based on real medication regimens of high complexity extracted from clinical studies, we wanted to test our ontology with the same prescription cases. Therefore our drug ontology was provided with mechanisms to specify drug dosage forms, dosing frequency and additional drug directions as required by the medication regimen complexity index presented in [13]. In addition, the ontology was extended with mechanisms to specify polypharmacy prescriptions. To complete our study, we implemented a tool that, based on the resulting drug ontology, computes the complexity index of a polypharmacy treatment.
The second tool constitutes a decision aid, which can be incorporated into prescribing programs to inform when a prescribed polypharmacy treatment is safe and effective, based on compliance with medical guidelines. For the evaluation of the decision module, we built a repository of disease-specific recommendations from clinical practice guidelines for chronic obstructive pulmonary disease (COPD) [14], type 2 diabetes[15], osteoporosis[16], hypertension[17], osteoarthritis[18], aspirin-based therapies for patients with diabetes[19], and adult preventive treatments supported by the US Preventive Services Task Force [20]. Provided with those medical recommendations, the decision system was tested with the subpopulation of patients 65 years old or older with mild diagnosis for all the mentioned conditions. The outcomes of the decision module were compared with results of a similar clinical study presented by Boyd et al[21].
To develop our decision support system, we extended the drug ontology resulting from the first tool implementation with drug contraindications, drug-drug interactions, drug formularies, and an abstraction of a patient’s medical records. While our ontological representation of patient’s medical records is not associated with any specific electronic health record or hospital information system, others have already worked on this problem, for instance [22]. A major contribution of the developed decision system is an initial repository of generic, reusable and scalable rules for safe and effective polypharmacy prescription.
This work summarizes a pilot study for developing and testing reusable ontology-based methodologies in concrete
This paper is organized as follows: In the Methods section we explain the ontological development approach that we followed to build the drug ontology. Then, in the Results section we explain in detail the two evaluation scenarios that we used to test the developed ontology and to generate repositories of generic safe and effective prescription principles. Finally, in the Discussion section we propose our future plans for further testing the proposed ontology-based representations providing mechanisms for optimizing polypharmacy prescriptions.
Methods
We followed a 4-step approach to develop the ontology:
1. Define the ontology domain and scope
Given the dimension of the problem of polypharmacy prescription, we decided to limit the scope of the drug ontology to the specification of information related to generic drugs (Metformin), drug interactions (Metformin has a critical drug interaction with Iodixanol, a contract agent used during coronary angiography), drug contraindications, drug formularies, drug prices and drug insurance coverage (Metformin is covered by Medicare). We have not considered: drug brand names (Actoplus Met), combination drugs (Actoplus Met has metformin, glipizide and glyburide as ingredients), mechanism of action (Metformin works as an insulin receptor agonists), drug pharmacokinetics (Metformin is excreted in the urine) or drug physiologic effects (Metformin increases glucose transport into cells).
The initial scope of the ontology was based on the following set of competency questions that we aimed to answer:
Following the method explained in George et al. [13], what is the complexity index of a polypharmacy treatment?
Is a drug safe when considered in combination with a set of drugs?
Is a drug contraindicated for any of the patient’s medical conditions?
Do the prescribed drugs adhere to the medical recommendations applicable to the patient? Is there any prescribed drug not directly linked to any recommended prescription? Are there missing drug prescriptions (no drug has been prescribed for a medical condition)?
Is a drug prescription within the formulary constraints in terms of frequency of intake, drug concentration, dosing form, recommended drug units and drug instructions (take with food, at a certain time, with liquids, etc.)?
Is a drug covered by the patient’s health insurance?
What is the cost of a polypharmacy treatment, based on patient’s insurance, intake frequency range, drug concentration, dosing form and recommended drug units?
2. Review existing drug-based knowledge repositories
Prior to constructing the ontology, we reviewed the biomedical literature, drug-based knowledge repositories and ontology repositories including RxNorm (http://www.nlm.nih.gov/research/umls/rxnorm/), maintained by the US National Library of Medicine (NLM). RxNorm provides normalized names for clinical drugs and links its names to many of the drug vocabularies commonly used in pharmacy management and drug interaction software, including First Databank, Micromedex, MediSpan, Gold Standard, and Multum. RxNorm includes the National Drug File - Reference Terminology (NDF-RT) from the Veterans Health Administration. NDF-RT is a terminology used to code clinical drug properties, including mechanism of action, physiologic effect, and therapeutic category. We used from RxNorm the representations of drug forms, and drug-drug interactions. We adopted from RxNorm the categorization of drug-drug interactions as critical and significant interactions.
We reviewed UMLS, also maintained by the NLM, as the source for the medical terminology and taxonomy used for representing medical findings and observations that could be linked to patient’s medical record to determine the disease-specific guideline compliance (within the patient’s findings there is a recent diagnosis of mild COPD, therefore COPD guideline [14] suggests prescribing a long acting anticholinergic or beta agonist).
We analyzed the taxonomy proposed in George et al. [13] to quantify the medication regimen index. We checked that the classification of dosage forms, dosing frequencies and additional directions proposed in that work was consistent with the terminology used in UMLS.
We also inspected the drug-based ontologies proposed in [7] [8][9] [23] for the specification of drug treatments. When comparing those ontologies with the drug regimens selected by domain experts from clinical studies concerning the treatment for COPD, we found out that the ontologies were not expressive enough. They did not provide mechanisms to specify dosing frequency ranges (take drug each 4–6 hours), variable drug dosage (take 1–2 tablets), or additional directions (take in the morning, with food).
Finally we reviewed Epocrates (www.epocrates.com), a free access tool with wide spread use as mobile drug reference within US physicians. Statistics (http://www.epocrates.com/who) indicate that this software is used by 50% of physicians in the US. We found out that this drug database was a good source of information for learning how to specify adverse drug-drug interactions, drug contraindications, pricing, and US healthcare insurance formularies. With respect to drug formularies the tool differentiates between recommended, alternative, maximum recommended and extended release formularies. The alternative doses are used for various reasons, to include decreasing complexity of dosing or to decrease the side-effects of a drug. The extended release forms are formulated so that they are released into the body over a specified period of time to decrease dosing frequency and thus decrease the complexity of dosing. For instance, for diclofenac the recommended dose is 50 mg, 2–3 times per day, the alternative dose is 75mg, 2 times per day, the maximum dose is 150mg per day, and the extended release recommendation is 100 mg, 2 times per day. We found out that none of the ontologies proposed in [7] [8][9] [23] provided specific mechanisms to differentiate between the different types of formularies, nor did they consider those differences when reasoning about formulary compliance. With respect to drug interactions and contraindications Epocrates organizes them into categories, to help clinicians to determine the appropriate action: avoid/use alternative and monitor/modify therapeutics. For this work, we decided to adopt a combination of RxNorm and Epocrates category systems for drug interactions and contraindications (critical, significant/use alternatives, monitor), but in the future a different category system could be considered.
3. Create classes and properties
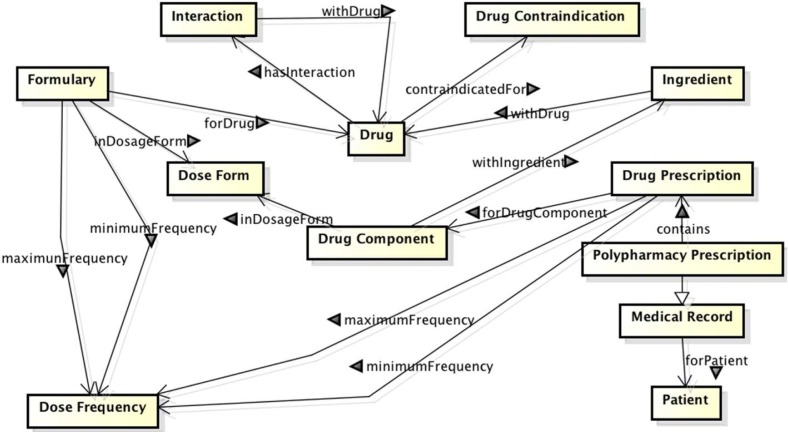
The initial list of classes and properties were derived from the explained competency questions, and then refined based on the review of the state of the art. We proposed six classes directly related to the drug ontology: disease, drug, drug component, ingredient, drug cost, drug dosage form, dose frequency, drug contraindications and drug interactions. Additionally, we included classes for modeling patient’s medical records (diagnostic procedures, finding, laboratory procedures, drug prescriptions), medical observations and measurement (moderate pain, LDL Cholesterol test equal to 120 mg/dL), and UMLS clinical terms (LDL Cholesterol is the UMLS concept C0850994 with semantic type laboratory procedure). In Figure 1 we provide a diagram showing the main class interactions.
Figure 1:
Interactions between main ontology classes. Implement the ontology in a formal representation
For the ontology representation we used the Web Ontology Language (OWL)[24], created by the World Wide Web Consortium as an ontology description language for formally describing the meaning of concepts. OWL allows a user to model concepts that are machine understandable while facilitating sharing, reusing and interchanging. We chose OWL over the Open Biomedical Ontologies (OBO) format because OWL provides a rigorously defined formal semantic that facilitates ontology development and maintenance. Furthermore, OWL-based knowledge representations support the inference of complex properties based on rule-based engines.
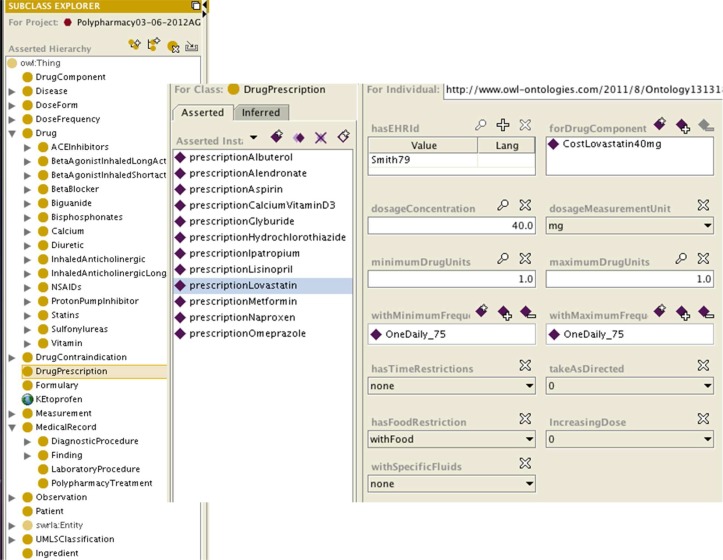
For encoding the OWL ontologies, we used the Protégé tool (http://protege.stanford.edu/). The left section of Figure 2 shows a snapshot of Protégé illustrating the class hierarchy of our ontology, while the right section depicts an instance of class DrugPrescription corresponding to a prescription for Lovastatin. Lovastatin prescribed with a dosage concentration of 1 tablet (40mg) once daily with food.
Figure 2:
Snapshot of Protégé illustrating the drug ontology. On the left, the class hierarchy is depicted, and on the right an instance of drug prescription is shown.
Results
To test the level of granularity, completeness and usefulness of the proposed ontological representations, we addressed two different problems directly related to the context of polypharmacy prescription.
Both implementations were built as Java programs accessing the OWL drug ontology through 45 queries expressed in the Semantic Web Rule Language (SWRL)[25]. SWRL is a declarative approach that has emerged as a solution for rule-based systems interoperability in the Semantic Web. SWRL allows performing queries by enacting Horn-clause rules (antecedent implies consequent) over OWL ontologies. The combined used of OWL and SWRL have already gained adoption in Health Informatics [7] [8] [9] [23]. We share this interest in reusing existing ontologies and combining OWL and SWRL for generating reusable, interoperable, safe prescription heuristics. The execution of SWRL rules are performed through the Jess reasoner[26]. An Appendix contains the SWRL rules that are not explained in detail through this paper.
For building the tools, we created a repository of setting-independent rules for reasoning on the context of polypharmacy treatments. The rules operate over the ontologies and are independent of specific drugs and diseases, to make the approach and implementation generalizable. Therefore we believe that the prescription rules should be reusable and scalable to other clinical contexts related to the polypharmacy prescription for patients, different from the ones considered here.
Recently, there have been few ontology-based approaches to address similar problems. In Bright et al. [23] an ontology for guiding safe antibiotic prescribing was proposed and evaluated, but the problem of reusing and extending the ontological representation to other types of medications was not considered. Like us, in Kawazoe and Ohe [8] a decision module was built based on generic, reusable, drug and disease independent ontology-based rules to monitor and inform on drug overdoses, contraindications and drug-drug interactions. Closely related to our approach is the work presented in Sheth et al [7] where an ontology was built to support formulary compliance in the context of polypharmacy prescription for patients with multiple conditions. Our work extends [7] by checking that the prescribed drug treatment complies with multiple guidelines. Similar to research by Rung-Ching et al. [9] our approach for supporting adherence to guideline is supported by the use of semantic-based, disease-specific rules.
Below, we explain in detail, the two evaluation scenarios used:
1. Implementation of a medication regimen complexity index for polypharmacy prescriptions
The tool consists on a set of 16 SWRL rules that compute a regimen’s complexity based on the taxonomy of 65 items considered in George et al. [13]. To each dosage form, frequency and additional prescription directions George et al. assigns a weight. For instance: tablet dosage form has weight 1, inhaler dosage form has weight 3, if a drug has to be taken with specific liquid the weight is 1, if a drug requires multiple units at one time the weight is 1, if a drug has to be taken at a specific time the weight is 1, if a drug has to be taken in relation with food the weight is 1, if a drug has to be taken once daily the weight is 1, if a drug has to be taken twice per day the weight is 2, if a drug has to be taken there times per day the weight is 3, if a drug is taken when needed the weigh is 0.5, and if a drug is taken once per week, the is weight 0.13. During this implementation we found out that the methodology proposed in George et al. [13] did not assign any complexity index to the weekly intake of a drug. Therefore, we chose to assign the weight 0.14 (daily frequency with complexity 1 divided by 7 days) to the weekly drug intake, extending George et al. approach.
For example, if we take the polypharmacy treatment shown in Table 1, our tool computed a complexity of 31.64 based on frequency, drug units and additional directions. Take for instance the second row prescription in Table 1: the complexity associated is 3.14 because tablets have to be taken once per week (weight=0.14 because of frequency), in the morning (weight=1 due to additional directions), before food (weight=1 because of additional directions), with water (weight=1 also for additional directions). Or in the case of the fifth row the associated complexity is 5 because 2 units (weight=2 because of multiple drug units) of tablets are prescribed once daily (weight=1 because of frequency) at breakfast (weight=1 due to additional directions) with food (weight=1 given additional directions).
Table 1:
polypharmacy treatment prescribed to a patient with mild diagnosis of osteoporosis, osteoarthritis, type 2 diabetes mellitus, hypertension and chronic obstructive pulmonary disease.
| Drug | Dosage form | Dosage concentration | Drug units | Frequency | Additional directions | Complexity |
|---|---|---|---|---|---|---|
| Albuterol | Inhaler | 2 mg | 1 | As needed | 0.5 | |
| Alendronate | Tablet | 70 mg | 1 | Once per week | In the morning, before food, with water | 3.14= 0.14+3 |
| Aspirin | Tablet | 81 mg | 1 | Once daily | With food | 1 |
| Calcium and Vitamin D3 | Tablet | 500 mg and 200 IU | 1 | Three times daily | With food | 4= 3+1 |
| Glyburide | Tablet | 5 mg | 2 | Once daily | At breakfast, with food | 5= 2+1+2 |
| Hydrochloro thiazide | Tablet | 12.5 mg | 1 | Once daily | 1 | |
| Lisinopril | Tablet | 40 mg | 1 | Once daily | 1 | |
| Lovastatin | Tablet | 40 mg | 1 | Once daily | With food | 2=1+1 |
| Metformin | Tablet | 850 mg | 1 | Twice daily | With food | 3=2+1 |
| Naproxen | Tablet | 250 mg | 1 | Twice daily | With food | 3=2+1 |
| Omeprazole | Tablet | 20 mg | 1 | Once daily | With food | 2=1+1 |
| Ipatropium | Inhaler | 17 mcg | 2 | Four times daily | 5=1+4 |
Once for each individual drug prescription the complexity is calculated, based on the frequency, drug units and additional directions, George et al. method also considers the different variants of dosage forms prescribed in the polypharmacy treatment. In our example the polypharmacy treatment contains two types of dosage forms, tablets and inhalers, so the complexity is increased by 4 (1 due to tablet dosage form, and 3 because of inhaler dosage form). Therefore the polypharmacy treatment shown in Table 1 has a final complexity of 35.64.
The following is an example of a SWRL rule for computing complexity: “if the prescription has variable dose (different minimum and maximum dose, like 1–2 tables per day) then assign weight 1” :
DrugPrescription(?dp) ^ hasEHRId(?dp,?patient) ^ maximumDrugUnits(?dp, ?maxu) ^ minimumDrugUnits(?dp, ?minu)^ swrlb:greaterThan(?maxu,?minu)-> sqwrl:select(1)
2. Implementation of a decision support system for safe and effective polypharmacy prescriptions
The developed decision support system provides independent modules for checking safety and effectiveness properties considering a drug in isolation, or all the drugs in the regimen.
First the decision system checks the following for each drug in the regimen:
1). There is no critical drug contraindication
As in [8], [9] we have defined 3 critical contraindications based on patient’s clinical state: pregnancy, kidney and liver failure. Take for instance the following SWRL rule that checks if a drug has a critical contraindication for kidney failure:
contraindicatedFor(?drug, ?contraind) ^ DrugDiseaseContraindication(?contraind, ?diseasecontraind) ^ severityLevel(?diseasecontraind, “critical”) ^ disease(?diseasecontraind, ?disease) ^ KidneyFailure(?disease) -> sqwrl:select(?contraind)
Remains as future work to add SWRL rules to detected dosage-related contraindications. For instance dosage not recommended for patient’s age or weight.
2). If the drug is covered by the patient’s insurance, or it is an out-of-pocket drug
For this we check the patient’s record to know their insurance, and we check that the drug is covered by the insurance’s formulary.
3). If the drug prescription complies with formularies
We defined three SWRL rules. Firstly, a SWRL rule checks that the drug’s dosage form and unit of measure belongs to the set of recommended ones. Secondly, if it is not an extended release drug, the SWRL rule checks that the product of the clinician’s choice of drug’s minimum unit, dosage concentration, and minimum frequency is greater than or equal to the product of the recommended drug’s minimum units, dosage concentration, and minimum frequency. Thirdly, if it is not an extended release drug, the SWRL rule checks that the product of the clinician’s choice of drug’s maximum unit, dosage concentration, and maximum frequency is less than or equal to the drug’s maximum recommended units, dosage concentration, and maximum frequency, as expressed in the maximum recommended formulary. The formula we use for checking this is the following:
PrDosageForm.equalsIgnoreCase(FRDosageForm)&
PrMeasurementUnit.equalsIgnoreCase(FRDosageMeasurementUnit)
FRMinUnit*FRMinDosageConcentration*toMonthlyFreq(FRMinFreq)≥
PrMinDosageConcentration*toMonthlyFreq(PrMinFreq) &&
PrMaxUnit*PrMaxDosageConcentration*toMonthlyFreq(PrMaxFreq)≤
FMaxMaxDosageConcentration*toMonthlyFreq(FNaxMaxFreq),
where function toMonthlyFreq takes as its input parameter a dosing frequency (for instance three times daily) and returns the numeric value corresponding to the number of doses per month (for three times daily frequency the monthly number of doses is 90).
When it is an extended release drug, the tool checks that the clinician’s choice of the drug’s dosage form, dosage concentration, dosage measurement unit, and dosing frequency is within the ranges of values recommended by the extended release formulary. For all drugs, without differentiating between extended release ones, the tool checks that all the additional drug directions have been provided to the patient.
For instance, when the decision support system checks the clinician’s choice from Table 1 for glyburide, it detects that it complies with formulary constraints. According to the recommendation, glyburide should be given with breakfast or the first meal, and the recommended dose is 1.25 to 20 mg twice per day. The maximum allowed dose for glyburide, based on the formularies, is of 20 mg per day.
Finally, the decision support system checks and computes the following for the polypharmacy treatment as a whole:
4). Critical drug-drug interactions
For detecting critical drug-drug interactions we have defined the following generic SWRL rule, which states that “if a drug prescription contains two drugs for which a critical drug-drug contraindication exists then retrieve that contraindication”:
DrugPrescription(?f1) ^ hasEHRId(?f1, ?patient) ^ forDrugComponent(?f1,?drug1) ^ DrugPrescription(?f2) ^ hasEHRId(?f2, ?patient) ^ forDrugComponent(?f2,?drug2) ^ contraindicatedFor(?drug1, ?contraind) ^ DrugDrugContraindication(?contraind) ^ severityLevel(?contraind, “critical”) ^ contraindicatedwithDrug(?drug1, ?drug2) ->sqwrl:select(?contraind)
The previous SWRL rule does not consider the drug’s form. For instance, topical propranolol administered as eye drops may not necessarily have the same systemic effect as propranolol administered parentally. The reason for that is that RxNorm and Epocrates, the knowledge bases used for learning to model in our ontology drug-drug interactions, did not consider drug’s form as an additional factor to judge drug-drug interaction severity.
5). Overall treatment’s cost
The regimen’s cost is based on patient’s health insurance, drug’s maximum frequency, drug’s dosage form, drug’s maximum dose and drug units prescribed. The following SWRL rule allows retrieving, for each prescribed drug, the concentration, maximum frequency (e.g. twice daily is maximum frequency in once to twice daily), maximum unit (e.g. 2 is maximum unit in 1–2 tablets) and cost per unit:
DrugPrescription(?f) ^ hasEHRId(?f, ?patient) ^ hasInsurance(?patient, ?insurance) ^ forDrugComponent(?f,?c)^ costSource(?c, ?insurance) ^ inDosageForm(?c, ?dform) ^ dosageConcentration(?c, ?dconcentration) ^ dosageMeasurementUnit(?c,?dMeasurementUnit) ^ withMaximumFrequency(?f,?maxFrequency) ^ maximumDrugUnits(?f, ?maxUnit) ^ costPerUnit(?c, ?costUnit) -> sqwrl:select(?dconcentration,?maxFrequency, ?maxUnit, ?costUnit)
The cost of the prescribed drug is calculated as the product of maximum prescribed units (maxUnit), cost per unit (costUnit) and maximum monthly intake (for instance if maxFrequency is each 12 hours then the maximum monthly intake is 60). For instance for 2 table units, cost per tablet $0.3 and maximum frequency 2 tablets per each 12 hours the monthly cost is 2x0.3x(2x60)=72.
6). Compliance with the guidelines that apply to the patient, based on the patient’s medical records
Take for instance the following SWRL rule, the prescription is in compliance with the guideline[15] “if a patient has a diagnosis of diabetes mellitus 2 and has been prescribed Metformin”:
Diagnosis(?diag) ^ hasEHRId(?diag,?patient) ^ hasObservation(?diag, ?obs1) ^ NoneMeasurable(?obs1) ^ UMLSConcept(?obs1, ?concept1) ^ DiabetesMellitus(?concept1) ^ DrugPrescription(?presc) ^ hasEHRId(?presc, ?patient) ^ forDrug(?presc,?drug) ^ Metformin(?drug) -> sqwrl:select(?drug)
A prescription is in compliance with the considered guideline [15] “if the patient has a diagnosis of diabetes mellitus 2, has an HbA1c level higher than 7, is already on metformin, and has been prescribed a drug from class sulfonylureas”:
Diagnosis(?diag) ^ hasEHRId(?diag, ?patient) ^ hasObservation(?diag, ?obs1) ^ NoneMeasurable(?obs1) ^ UMLSConcept(?obs1, ?concept1) ^ DiabetesMellitus(?concept1) ^ HBA1CTest(?test) ^ hasEHRId(?test, ?id) ^ hasMeasurableObservation(?test, ?obs2) ^ hasQuantity(?obs2, ?quant) ^ operator(?quant, “=”) ^ value(?quant, ?val) ^ swrlb:greaterThan(?val, 7) ^ DrugPrescription(?presc) ^ hasEHRId(?presc,?patient) ^ forDrug(?presc,?drug1) ^ Metformin(?drug1) ^ forDrug(?presc,?drug2) ^ Sulfonylureas(?drug2)-> sqwrl:select(?drug1)
The provided SWRL rules strongly resemble those specified in Rung-Ching et al. [9] for the development of an anti-diabetic drug ontology for guideline-based recommendations. This example clearly exposes the benefits of ontology-based specifications, as ways to reuse and share declarative knowledge that is implementation-free. We have assumed that we are always accessing the patient’s current diagnostic procedures, findings, laboratory procedures and drug prescriptions. When this assumption is not made, additional reasoning is required to query the patient’s current data. For the previous example, we will need to first retrieve the date of the latest HBA1c:
Diagnosis(?diag) ^ hasEHRId(?diag, ?patient) ^ hasObservation(?diag, ?obs1) ^ NoneMeasurable(?obs1) ^ UMLSConcept(?obs1, ?concept1) ^ DiabetesMellitus(?concept1) ^ HBA1CTest(?test) ^ hasEHRId(?test, ?patient) ^ hasDate(?test, ?day) ^ sqwrl:makeSet(?s, ?day) ^ sqwrl:max(?lastday, ?s) ^ swrlb:equal(?lastday,?day) -> sqwrl:select(?day)
Only after we know the date of the latest HBA1c test we can check that it returns a value greater than 7:
HBA1CTest(?test) ^ hasEHRId(?test, ?patient) ^ hasDate(?test, day) ^ hasMeasurableObservation(?test, ?obs2) ^ hasQuantity(?obs2, ?quant) ^ operator(?quant, “=”) ^ value(?quant, ?val) ^ swrlb:greaterThan(?val, 7) -> sqwrl:select(?test)
Continuing with the description of the decision support system, it explicitly links prescribed drugs with guidelines (according to diabetes mellius 2 guideline [15] the patient should have been prescribed a drug from the class Sulfonylureas, given that Glyburide is from the class Sulfonylureas, it is a potential candidate to be linked to that recommendation), and therefore can inform the clinician if there are any missing drug for the active problems (no drug from class Sulfonylureas was prescribed, although advised by the guideline). To evaluate the decision support system, we considered the same patient case analyzed in Boyd et al. [21]: a 79-years old woman covered by Medicare insurance with osteoporosis, osteoarthritis, type 2 diabetes mellitus, hypertension and chronic obstructive pulmonary disease, all of moderate severity. We defined 20 SWRL rules (see Appendix) for checking the compliance with the guidelines [14–20] for patients 65 years old or older with mild diagnosis for all the mentioned conditions.
Boyd et al. recommended the polypharmacy treatment depicted in Table 1 based on the same guidelines and the assumption that the patient was not taking any medication other than metformin and albuterol MDI. Our decision support system informed that the regimen chosen in [21] by the expert panel was safe and effective and that it complied with the considered guidelines. When we tested the decision support system with regimens in non-compliance with the guidelines, it inferred appropriately that drugs were missing or redundant (prescribed though not recommended by the guidelines), or if formulary’s restrictions were violated. It is in our plans to further test in more systematic way the proposed decision support.
Discussion
The aim of this work was to challenge the existing paradigm that focuses on management of individual chronic diseases. We proposed, instead, an ontology-based MCC-based approach for facilitating the future development of decision support systems for multi-drug prescriptions. Experience shows that existing disease-specific approaches cannot be easily generalized or extended to MCC, and that more structured and principled solutions as the one we propose here should be pursued.
As the HHS report suggests[11], the high complexity of the problem requires a progressive approach. This preliminary work should be continued and extended for addressing important existing barriers. Future problems that we would like to look at are: advising on treatment variants that preserve effectiveness (comply with guidelines and formularies) while reducing the polypharmacy cost, or reducing regimen complexity. For this purpose, we will propose and evaluate a repository of prescription rules based on previous pilot studies on polypharmacy optimization. Preliminary studies, including Boyd et al [21], show that it is possible to provide a balance of high quality of care while improving cost-efficiency by optimizing doses (reducing multiple doses with lower frequency doses with the same therapeutic effect), choosing the more effective of two or more drugs in the same class, titrating a drug to the optimal tolerable dose for an adequate duration, eliminating duplicated therapies, and combining drugs (prescribe a drug supplement that combines calcium and vitamin D3, as shown in Table 1) to reduce treatment complexity and cost, and increase adherence.
References
- [1].Robert Wood Johnson Foundation . Chronic care: making the case for ongoing care. 2010. [Google Scholar]
- [2].Anderson G, Horvath J. Chronic Conditions: Making the Case for Ongoing Care. 2002. Robert Wood Johnson Foundation’s Partnership for Solutions. [Google Scholar]
- [3].Goldberg RM, Mabee J, Chan L, Wong S. Drug-drug and drug-disease interactions in the emergency department: analysis of a high risk population. Am J Emerg Med. 1996;(14):447–50. doi: 10.1016/S0735-6757(96)90147-3. [DOI] [PubMed] [Google Scholar]
- [4].WHO | ADHERENCE TO LONG-TERM THERAPIES: EVIDENCE FOR ACTION. WHO. [Online]. Available: http://www.who.int/chp/knowledge/publications/adherence_report/en/. [Accessed: 29-Oct-2011] [PubMed]
- [5].Finkers F, Maring JG, Boersma F, Taxis K. A study of medication reviews to identify drug-related problems of polypharmacy patients in the Dutch nursing home setting. J Clin Pharm Ther. 2007 Oct;32(5):469–476. doi: 10.1111/j.1365-2710.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- [6].Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001 Apr;41(2):192–199. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- [7].Sheth AP, Agrawal S, Lathem J, Oldham N, Wingate H, Yadav P, Gallagher K. Active Semantic Electronic Medical Record. Proceedings of the 5th International Semantic Web Conference; Athens, GA. 2006; pp. 913–926. [Google Scholar]
- [8].Kawazoe Y, Ohe K. An ontology-based mediator of clinical information for decision support systems: a prototype of a clinical alert system for prescription. Methods Inf Med. 2008;47(6):549–559. doi: 10.3414/ME9126. [DOI] [PubMed] [Google Scholar]
- [9].Chen R-C, Bau C-T, Huang Y-H. 2010 IEEE International Conference on Fuzzy Systems (FUZZ) 2010. Development of anti-diabetic drugs ontology for guideline-based clinical drugs recommend system using OWL and SWRL; pp. 1–6. [Google Scholar]
- [10].Jenders RA, Corman R, Dasgupta B. Making the standard more standard: a data and query model for knowledge representation in the Arden syntax. AMIA Annu Symp Proc. 2003:323–330. [PMC free article] [PubMed] [Google Scholar]
- [11].U.S. Department of Health and Human Services . Multiple chronic conditions: a strategic framework. Optimum health and quality of life for individuals with multiple chronic conditions. Washington DC: Dec, 2010. [Google Scholar]
- [12].Laboratorio FL. Overview Of Methodologies For Building Ontologies. 1999.
- [13].George J, Phun Y-T, Bailey MJ, Kong DCM, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004 Sep;38(9):1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- [14].Pocket Guide to COPD Diagnosis, Management, and Prevention. Global Initiative for Chronic Obstructive Lung Disease 2011 Dec; [Google Scholar]
- [15].Standards of Medical Care in Diabetes--2012. Diabetes Care. 2011 Dec;35(Supplement_1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].The Clinician’s Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation. 2010.
- [17].Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. 2003. others. [DOI] [PubMed]
- [18].Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000 Sep;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [19].Aspirin Therapy in Diabetes. Diabetes Care. 2004 Jan;27(90001):72S–73. doi: 10.2337/diacare.27.2007.s72. [DOI] [PubMed] [Google Scholar]
- [20].US Preventive Services Task Force Recommendations for Adults. Mar, 2012.
- [21].Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005 Aug;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- [22].Cure O. 18th IEEE Symposium on Computer-Based Medical Systems, 2005. Proceedings. 2005. Ontology interaction with a patient electronic health record; pp. 185–190. [Google Scholar]
- [23].Bright TJ, Yoko Furuya E, Kuperman GJ, Cimino JJ, Bakken S. Development and evaluation of an ontology for guiding appropriate antibiotic prescribing. Journal of Biomedical Informatics. 2012 Feb;45(1):120–128. doi: 10.1016/j.jbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].W3C OWL Working Group OWL 2 Web Ontology Language Document Overview. W3C, W3c Recommendation. 2009.
- [25].Horrocks I, Patel-Schneider P, Boley H, Tabet S, Grosof B, Dean M. SWRL: A Semantic Web Rule Language Combining OWL and RuleML. 2004 May; [Google Scholar]
- [26].Jesss, the Rule Engine for the Java Platform. [Online]. Available: http://www.jessrules.com/jess/index.shtml. [Accessed: 12-Mar-2012].