Abstract
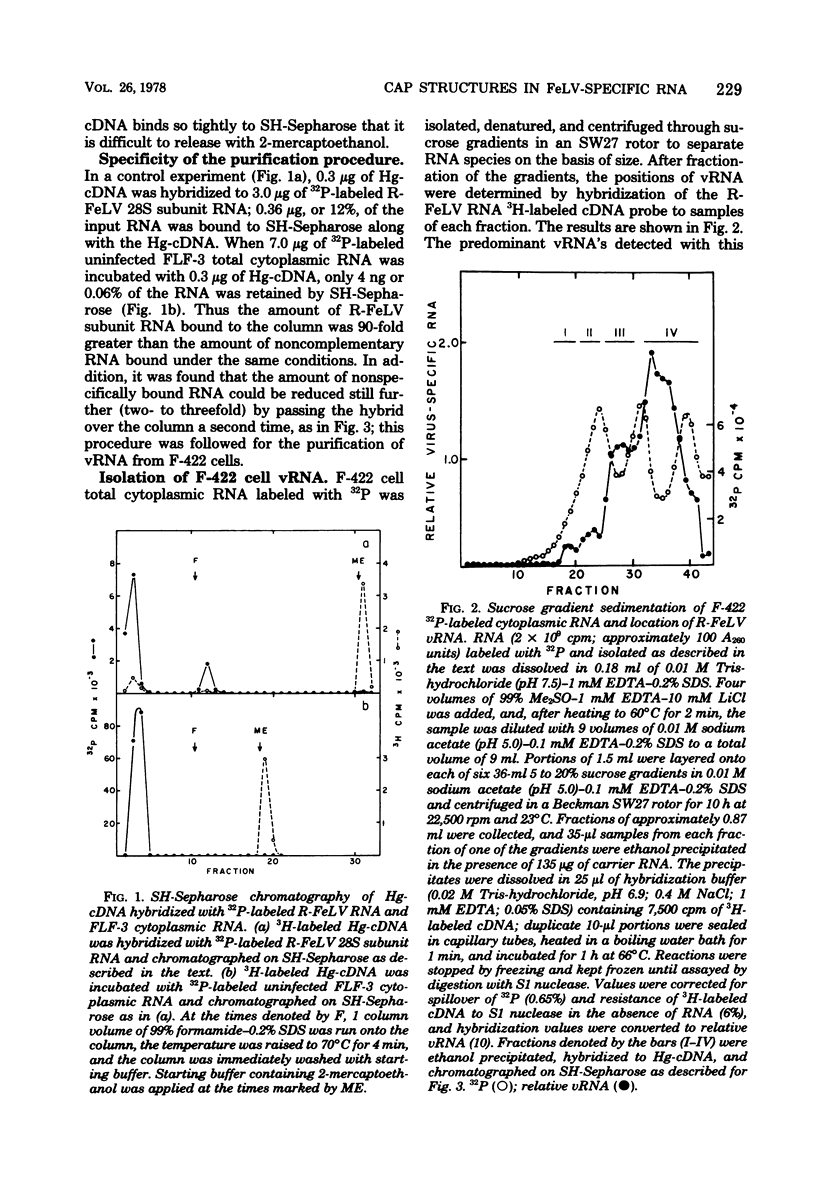
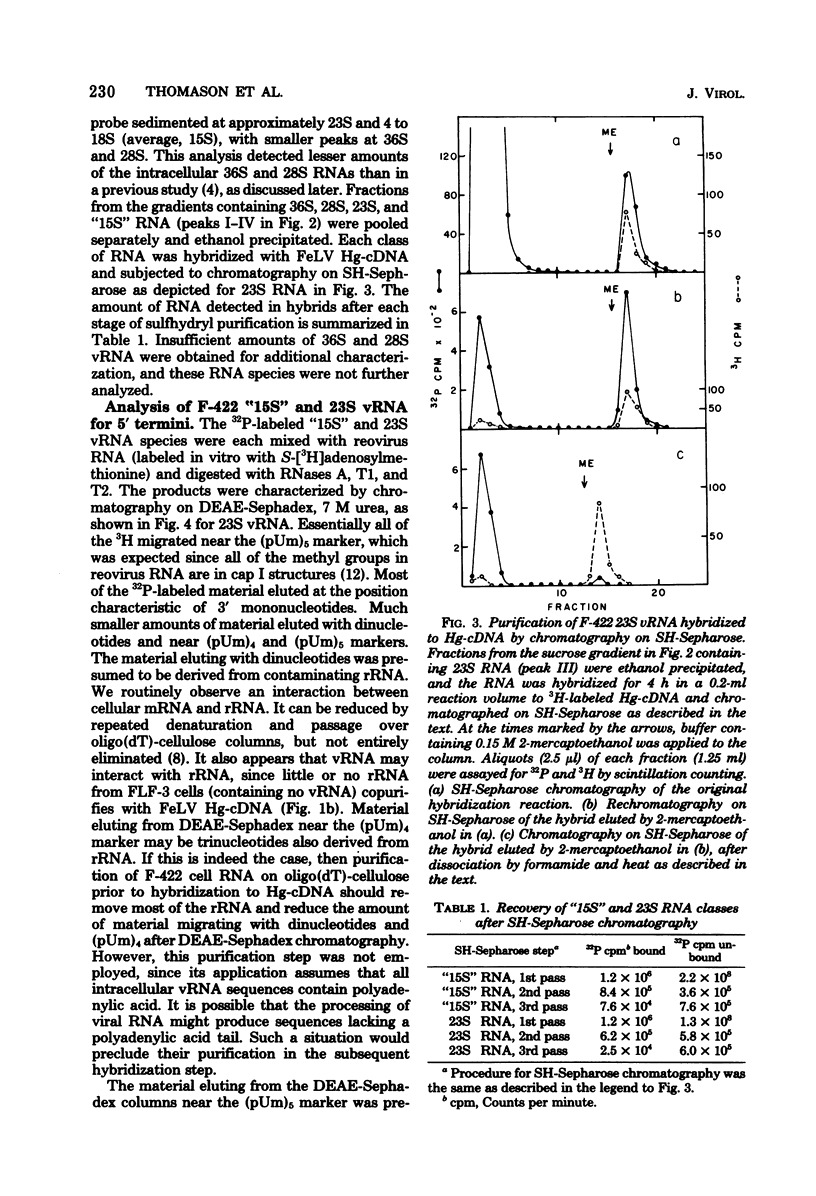
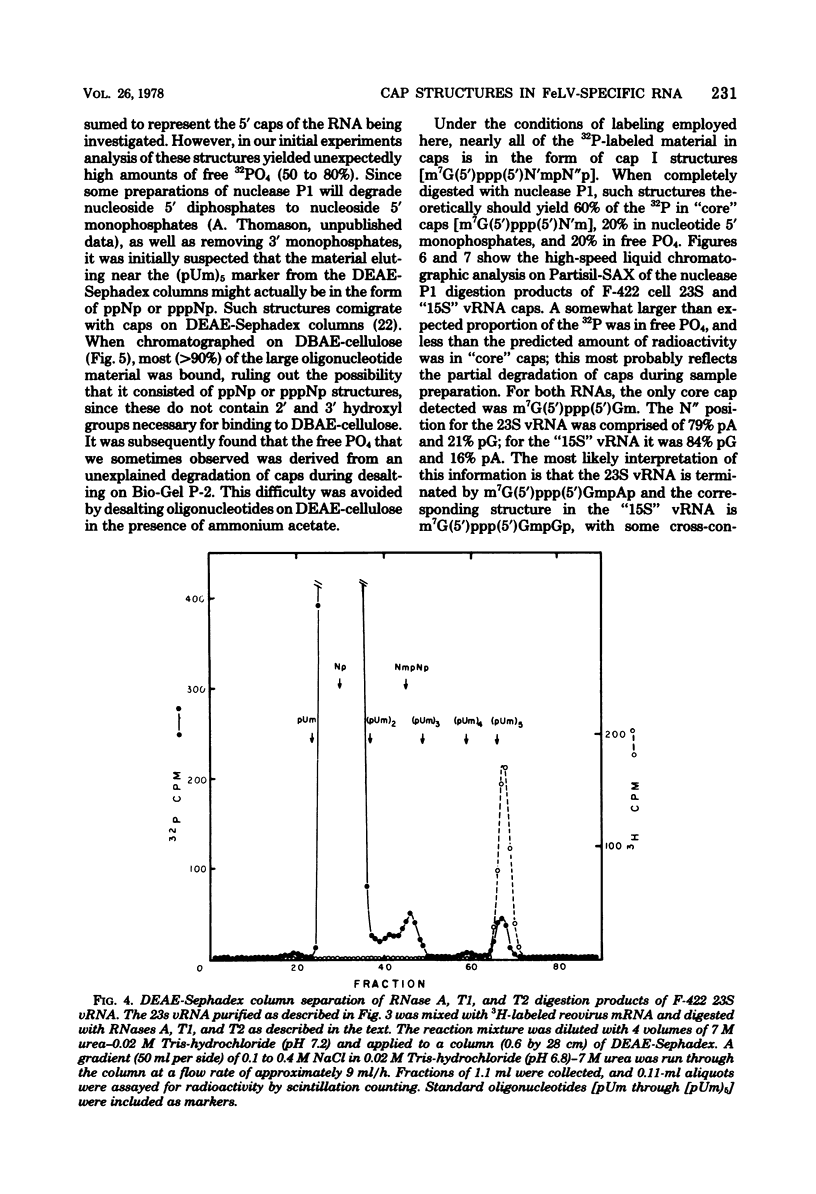
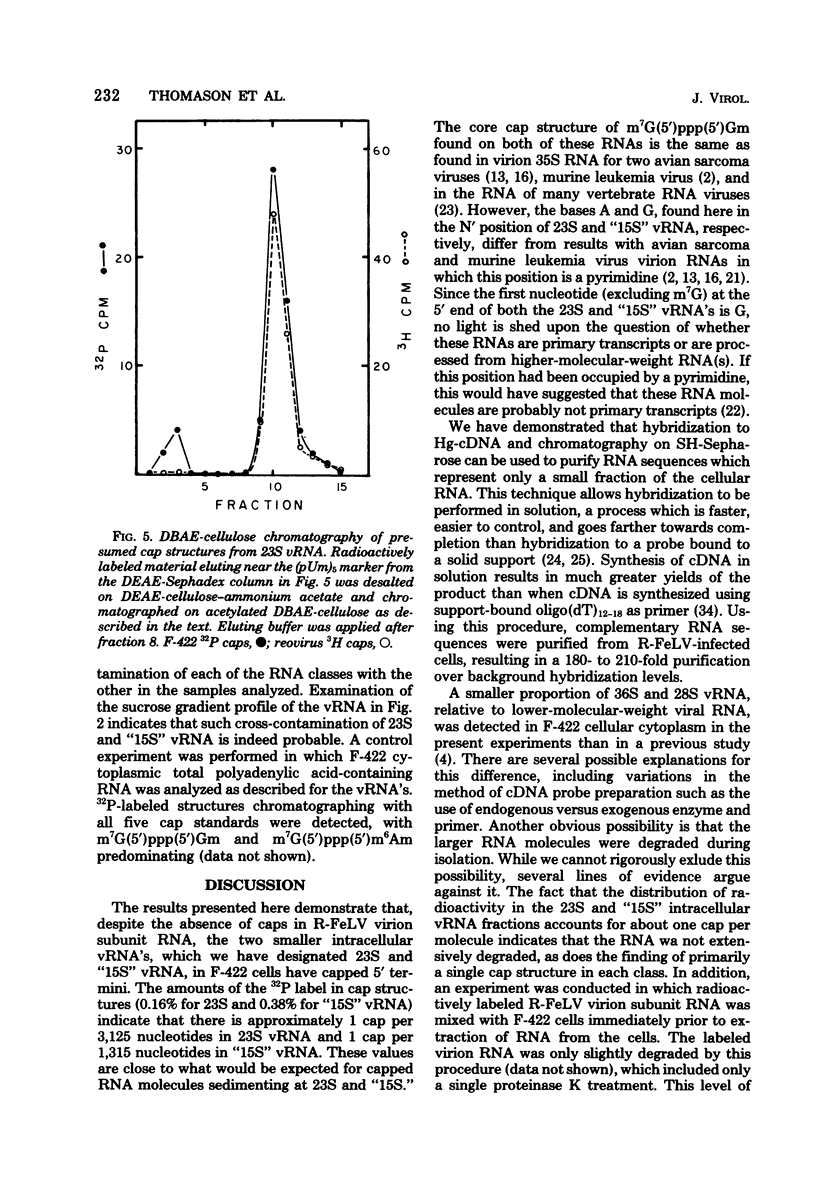
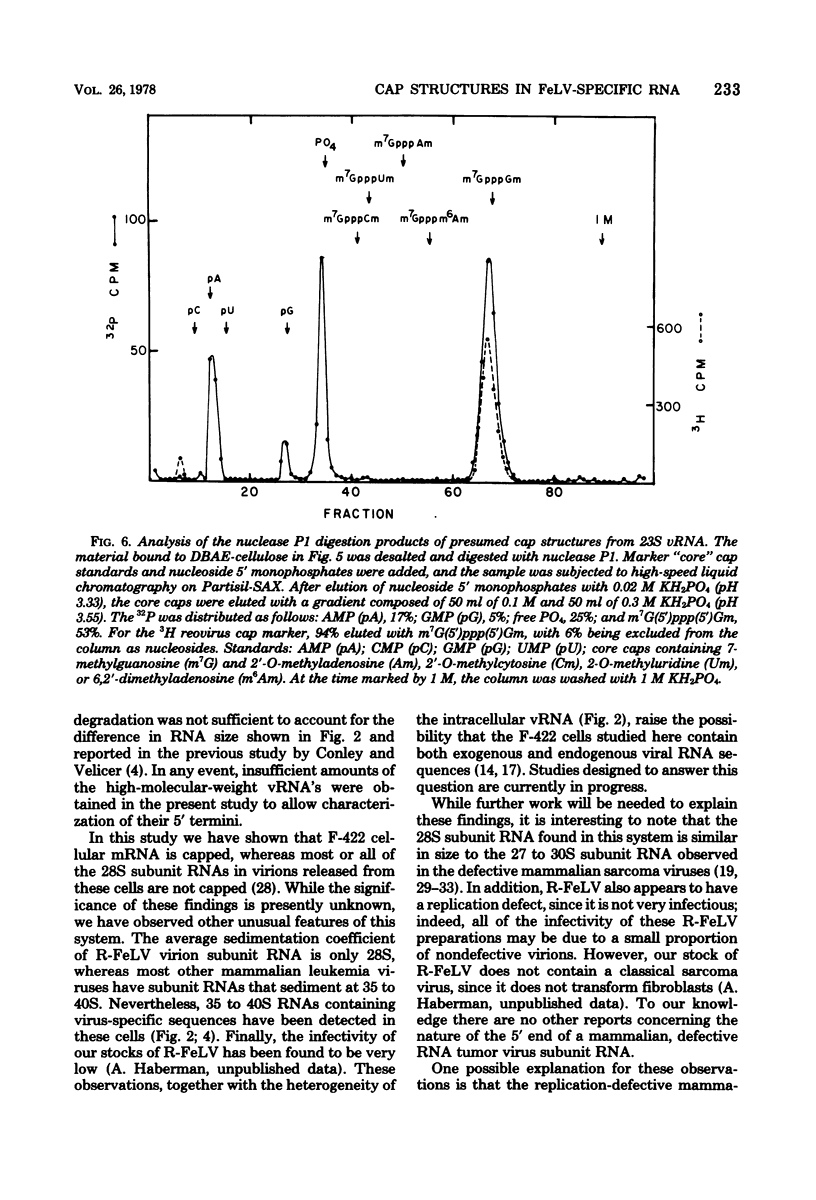
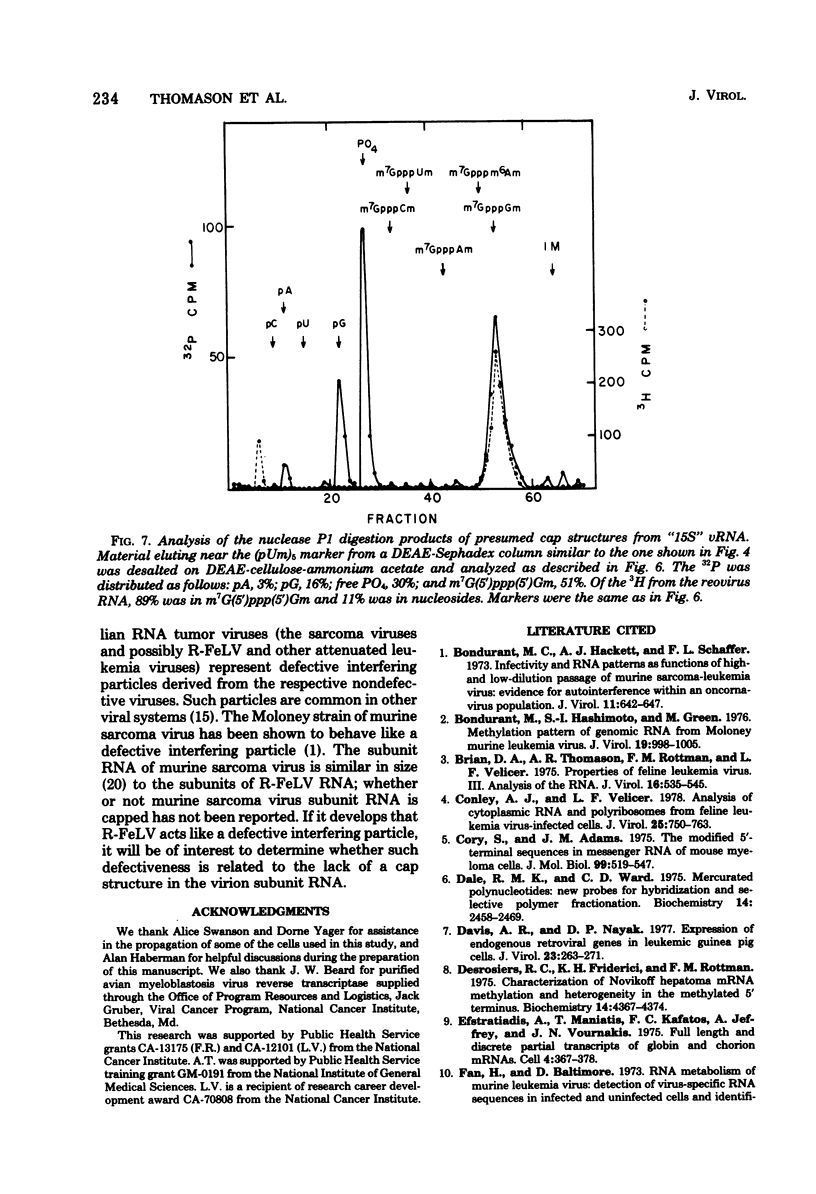
The F-422 line of feline thymus tumor cells, chronically infected with the Rickard strain of feline leukemia virus (R-FeLV), was labeled with 32P, and the total cytoplasmic RNA was isolated. The RNA was centrifuged through sucrose gradients, and R-FeLV virus-specific RNA (vRNA) was located by hybridization of portions of the gradient fractions to R-FeLV complementary DNA. vRNA classes with average sedimentation coefficients of approximately 36S, 28S, 23S, and 15S were identified. Each class of RNA was recovered by hybridized with mercurated R-FeLV complementary DNA, and the hybrids were chromatographed on columns of sulfhydryl-Sepharose to separate them from unhybridized cellular RNA. Although insufficient amount of 36S and 28S vRNA were obtained for further analysis, the 23S and 15S VRNA classes were analyzed to determine the nature of their 5' termini. Each of these vRNA classes was found to contain stoichiometric amounts of cap structures per unit length of RNA, consistent with the presence of one cap per molecule. The structure of the 23S vRNA cap was found to be m7G5'ppp5'GmpAp, whereas that of the 15S vRNA cap was m7G5'ppp5'GmpGp.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondurant M. C., Hackett A. J., Schaffer F. L. Infectivity and RNA patterns as functions of high- and low-dilution passage of murine sarcoma-leukemia virus: evidence for autointerference within an oncornavirus population. J Virol. 1973 May;11(5):642–647. doi: 10.1128/jvi.11.5.642-647.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurant M., Hashimoto S., Green M. Methylation pattern of genomic RNA from Moloney murine leukemia virus. J Virol. 1976 Sep;19(3):998–1005. doi: 10.1128/jvi.19.3.998-1005.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian D. A., Thomason A. R., Rottman F. M., Velicer L. F. Properties of feline leukemia virus. III. Analysis of the RNA. J Virol. 1975 Sep;16(3):535–545. doi: 10.1128/jvi.16.3.535-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conely A. J., Velicer L. F. Analysis of cytoplasmic RNA and polyribosmomes from feline leukemia virus-infected cells. J Virol. 1978 Mar;25(3):750–763. doi: 10.1128/jvi.25.3.750-763.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. The modified 5'-terminal sequences in messenger RNA of mouse myeloma cells. J Mol Biol. 1975 Dec 25;99(4):519–547. doi: 10.1016/s0022-2836(75)80170-7. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P. Expression of endogenous retroviral genes in leukemic guinea pig cells. J Virol. 1977 Aug;23(2):263–271. doi: 10.1128/jvi.23.2.263-271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. J., Levin R., Parks W. P., Scolnick E. M. Quantitative analysis of the rescue of RNA sequences by mammalian type C viruses. J Virol. 1975 Jan;17(1):43–50. doi: 10.1128/jvi.17.1.43-50.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith J., Fraenkel-Conrat H. Identification of the 5' end of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3347–3350. doi: 10.1073/pnas.72.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R., Ruscetti S. K., Parks W. P., Scolnick E. M. Expression of feline type-C virus in normal and tumor tissues of the domestic cat. Int J Cancer. 1976 Nov 15;18(5):661–671. doi: 10.1002/ijc.2910180515. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. A., Hollis V. W., Jr, Bassin R. H., Fischinger P. J. Characterization of RNA from noninfectious virions produced by sarcoma positive-leukemia negative transformed 3T3 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):3002–3006. doi: 10.1073/pnas.70.10.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggin C. H., Bondurant M. C., Mitchell W. M. Differences between murine leukemia virus and murine sarcoma virus: effects of virion age and multiplicity of infection on viral RNA. Intervirology. 1974;2(4):209–221. doi: 10.1159/000149426. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Haseltine W. A., Baltimore D. 5'-terminus of Moloney murine leukemia virus 35s RNA is m7G5' ppp5' GmpCp. J Virol. 1976 Oct;20(1):324–329. doi: 10.1128/jvi.20.1.324-329.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Martin M. A. Chemical linkage of nucleic acids to neutral and phosphorylated cellulose powders and isolation of specific sequences by affinity chromatography. Biochemistry. 1974 Jul 30;13(16):3411–3418. doi: 10.1021/bi00713a036. [DOI] [PubMed] [Google Scholar]
- Spiegelman G. B., Haber J. E., Halvorson H. O. Kinetics of ribonucleic acid-deoxyribonucleic acid membrane filter hybridization. Biochemistry. 1973 Mar 13;12(6):1234–1242. doi: 10.1021/bi00730a034. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Green M. Intracellular viral RNA species in mouse cells nonproductively transformed by the murine sarcoma virus. J Virol. 1974 Sep;14(3):587–591. doi: 10.1128/jvi.14.3.587-591.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Long C., Hatanaka M. Viral RNA of murine sarcoma virus produced by a hamster-mouse somatic cell hybrid. Virology. 1974 Jul;60(1):200–205. doi: 10.1016/0042-6822(74)90377-8. [DOI] [PubMed] [Google Scholar]



