Abstract
In 1888, Pierre Ménétrier first described the disease that bears his name. Many of the findings he reported then remain accepted features of the disease. Based on studies performed in our laboratory over the past 20 years, we have implicated increased transforming growth factor-α (TGFα) expression and heightened epidermal growth factor receptor (EGFR) activity in the pathogenesis of Ménétrier's disease. Herein, we provide a historical perspective of this rare disorder, review our experience with Ménétrier's disease, and discuss future challenges and opportunities posed by this disorder.
HISTORICAL PERSPECTIVE
The French pathologist Pierre Ménétrier (1859–1935) first described the disease that bears his name in Archives Physiologie Normale et Pathologique in 1888. The editors of this prestigious French journal were Charles-Edouard Brown-Sequard (1817–1894) and Jean-Martin Charcot (1825–1893). These were eponymous times; if one described a disease or identified a syndrome, it was commonplace to append one's name to that entity.
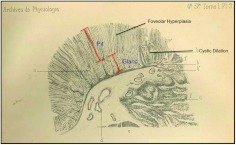
In two separate autopsy reports in that year in that journal (1), Ménétrier described seven individuals exhibiting two macroscopically distinct patterns of gastric hypertrophy: polypoid adenomas and sheet-like polyadenomas. He aptly likened the thickened gastric mucosa to cerebral convolutions. Four of the seven had the latter condition, which came to be referred to as Ménétrier's disease. Two of these four individuals had gastric cancer. He reported that the disorder affected the proximal portion of the stomach (body and fundus) and spared the distal stomach (antrum); a link to gastric cancer and antral sparing continue to be recognized features of the disease. Ménétrier's original drawing captured many of the features of the disease (Figure 1). There is marked expansion of the surface mucous cells (histologically referred to as foveolar hyperplasia) and reduced numbers of acid-producing parietal cells and pepsinogen-producing chief cells (referred to as glandular or oxyntic atrophy). The foveolae (small pits) are often tortuous (not shown) and undergo cystic dilatation. Normally, the pit to gland ratio is 1:4, but in Ménétrier's disease this ratio is often reversed, as depicted by Dr. Ménétrier. If Ménétrier had been able to perform pre-mortem gastroscopy on these patients, he would also have noted thick tenacious gastric fluid with reduced gastric acidity (gastric juice pH is often 4–7 rather than the normal 1–3), reflecting the reduced parietal cell mass.
Fig. 1.
Pierre Ménétrier's original drawing of Ménétrier's disease. See text for details.
Ménétrier's disease is also known by several other names, including giant hypertrophic gastritis and hypoproteinemic hypertrophic gastropathy. There are no pathognomonic features to diagnose Ménétrier's disease, and it continues to be a clinicopathological diagnosis. Patients, more often males than females, usually between the ages of 30 and 60 years old, typically present with abdominal pain, nausea, vomiting, and edema of peripheral tissues (due to leakage of protein selectively across the gastric lining). The disease tends to be progressive in adults. It is essential to obtain a full-thickness biopsy of the involved gastric mucosa when entertaining the diagnosis of Ménétrier's disease (2). Foveolar hyperplasia, often massive, is a histological sine qua non; a histological variant, hypertrophic lymphocytic gastritis, has been described (3) but is much less common in our experience. As mentioned above, there are usually reduced numbers of parietal cells and chief cells. Additional histological features include retention of overall mucosal architecture with prominent eosinophils and/or plasma cells in the lamina propria, along with smooth muscle hyperplasia and edema in the lamina propria. Serum gastrin tends to be normal despite the reduced gastric acidity. Until recently, there has been no effective medical therapy and patients often undergo partial or total gastrectomy.
In addition to being a pathologist, Pierre Ménétrier was a medical historian who specialized in Byzantine and Greco-Roman medicine. In considering the underlying pathogenesis of this disorder, Ménétrier, ever the historian, lamented, “we regret very much not having been able to investigate the mode of multiplication of these epithelial elements due to the conditions in which we find ourselves.” However, he presciently noted that “the glandular epithelial coat loses its highly differentiated functional character to acquire instead a new proliferative power, rather similar to that with which embryonal elements are endowed.”
EGFR IN THE PATHOGENESIS OF MÉNÉTRIER'S DISEASE
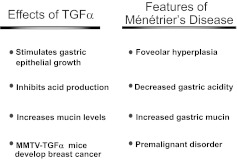
Our involvement in this disorder stemmed from observing TGFα production in normal epithelial cells, initially keratinocytes, but later gastric epithelial cells (4, 5). We went on to show that TGFα stimulates growth of gastric epithelial cells (6) and that it inhibits gastric acid production (7, 8). Systemic administration of TGFα in rats induced a rapid and marked increase in insoluble gastric mucin, and this increase correlated with TGFα's ability to protect the gastric mucosa against ethanol- and acid-induced gastric injury (9). In separate studies, we reported that targeted overexpression of TGFα in the mouse mammary gland resulted in breast cancer (10–12). Taken together, these observations led us to propose that overproduction of TGFα in the stomach might contribute to the pathogenesis of Ménétrier's disease (Table 1).
TABLE 1.
Actions of TGFα and Corresponding Features of Ménétrier's Disease That Led to Consideration of a Role for TGFα and EGFR in the Pathogenesis of Ménétrier's Disease
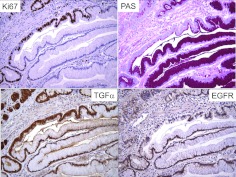
We observed increased immunoreactivity for TGFα in the involved gastric mucosa of 15 patients with Ménétrier's disease (13, 14). Moreover, in support of this circumstantial evidence, transgenic mice that overexpress TGFα in the stomach exhibit many of the features of Ménétrier's disease, including foveolar hyperplasia with increased mucin staining, reduced numbers of chief cells and parietal cells, and reduced basal and histamine-stimulated rates of acid production (14). In both patients and these TGFα transgenic mice, serum gastrin levels were normal despite the low gastric acidity, which is a potent stimulus to increase serum gastrin. From careful study of these TGFα transgenic mice using a panel of proliferative, apoptotic and lineage-specific markers, we concluded that the reduction in parietal cells and chief cells was not due to enhanced destruction but rather to a selective increase in surface mucus cells with a markedly expanded, highly proliferative progenitor compartment that is repositioned toward the base of the gland from its normal location at the isthmus. We have speculated that increased EGFR activity, in the setting of inappropriately normal levels of serum gastrin (known to be trophic for parietal cells and chief cells) expanded and redirected gastric progenitor cells towards a surface mucous cell lineage at the expense of the glandular lineages (15). Figure 2 displays an expanded progenitor zone within a region of foveolar hyperplasia. One section of the gland is shorter, hyperproliferative (increased Ki67 staining), and overexpresses TGFα compared to the adjacent section that is taller, more differentiated (increased PAS staining), and appears to exhibit more EGFR immunoreactivity.
Fig. 2.
Expanded proliferative zone within a region of foveolar hyperplasia. See text for details.
Based on this evidence, we received compassionate-use approval from the US Food and Drug Administration (FDA) to treat an individual with Ménétrier's disease with cetuximab, a chimeric, immunoglobulin G1 (IgG1) monoclonal antibody that binds to the extracellular portion of the EGFR and inhibits binding of ligands such as TGFα. The patient had unremitting vomiting (70 times per week) and co-existent primary pulmonary hypertension, which made him a poor candidate for gastrectomy. He experienced rapid and dramatic clinical improvement to cetuximab administration (16). Notably, 1 day after the first dose of cetuximab, there was a marked decrease in proliferation in the involved fundic mucosa with Ki67 staining reduced from 45 to 5 positive cells per glandular unit. After 4 weekly doses of cetuximab, his vomiting decreased to five episodes per month, his serum albumin increased, and parietal cells returned, as evidenced by HK-ATPase staining. In addition, there was a prompt and sustained increase in serum gastrin levels.
This promising result led us to conduct a single-arm clinical trial to test the efficacy of 1 month of cetuximab in a larger patient population. To enter the trial, patients needed to have clinically and histologically documented Ménétrier's disease that had been present for more than 6 months, and impaired quality-of-life to the extent that gastrectomy was being considered. All of the seven patients who completed the one-month treatment course showed statistically significant improvement in both clinical and biochemical parameters (17). A striking feature was that there was a marked increase in parietal cells, as determined by HK-ATPase immunoreactivity after 24 hours of the first dose of cetuximab. All of these patients elected to continue treatment beyond this initial one-month duration, and four ultimately showed near or complete histological remission.
During the course of the cetuximab trial, we evaluated 48 individuals for possible Ménétrier's disease. Of these, 25 were confirmed to have the disease, and 23 had an assorted number of mimics. We recently described this experience, and proposed an algorithm to help clinicians to distinguish Ménétrier's disease from its mimics (2). Individuals with Ménétrier's disease were more likely to exhibit low serum albumin and high gastric pH, and less likely to be anemic than those with disorders mimicking Ménétrier's disease.
DISCUSSION
Rationale for Studying a Rare Disease
Paradoxically, rare diseases affect a lot of people. In the United States, a rare disease is defined as one that affects fewer than 200,000 Americans. By this criterion, there are 6,000 such disorders in this country affecting 25 million individuals. Furthermore, studying a rare disease may provide insight into disease pathogenesis, and even normal physiology, which extends far beyond the disease being studied. For example, Al Knudsen, a pediatrician in Philadelphia studying retinoblastoma, put forth the hypothesis that children affected with this disorder would have a germline mutation in one allele and tumors would result from a “hit” to the second. This proved to be correct, and fueled the still-dominant view in cancer biology that cancer is a disorder of mutated genes. Perhaps the 17th century medical pioneer William Harvery said it best: “Nature is nowhere accustomed more openly to display her secret mysteries than in cases where she shows traces of her workings apart from the beaten path.” For those interested in this topic, we recommend the monograph Exceptional Matters by Sir Keith Peters (18).
Challenges and Opportunities
Our work has implicated enhanced EGFR signaling in the pathogenesis of Ménétrier's disease, and the clinical and biochemical response to cetuximab in these patients supports this contention. However, many questions remain unanswered. Despite the molecular and genetic tools presently available, we, in some ways, share Ménétrier's earlier lament. We still do not know the precise cause(s) of the disorder, especially in adults. Although we observe increased TGFα immunoreactivity in the involved gastric mucosa and transgenic mice that overexpress TGFα in the stomach phenocopy many of the patients with features of Ménétrier's disease, we have not systematically examined the other six EGFR ligands. Such an analysis is complicated by the knowledge that at least in vitro there is auto- and cross-induction of EGFR ligands (4, 19).
What is the inciting stimulus? The link to an underlying inflammatory condition is intriguing. Four of the nine individuals in our cetuximab trial had ulcerative colitis and another had ankylosis spondylitis. There is also an epidemiological link to CMV infection in adolescents with Ménétrier's disease, and increased TGFα immunoreactivity has been observed in the involved gastric mucosa of these adolescents. Is it a spectrum of disorders? What is the incidence and prevalence of the disorder? What is the true natural history of the disorder? What is the precise relationship to gastric cancer in these patients? These and other questions about this fascinating disorder await answers.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Nathan, Boston: I find this absolutely fascinating. This is an acquired disease, is that correct?
Coffey, Nashville: Yes, it is generally believed that Ménétrier's disease is an acquired disorder, although there are case reports of familial Ménétrier's disease. As I mentioned, we recently reported our experience at Vanderbilt evaluating 48 individuals for possible Ménétrier's disease (2). Three patients turned out to have juvenile polyposis due to germline SMAD4 mutations. These patients had what we believe to be secondary foveolar hyperplasia due to impaired TGFβ signaling, leading to excess EGFR signaling. The typical histological changes of juvenile polyposis had been overlooked by the referring physicians.
Nathan, Boston: So, is this a form of say, I hate to use the word, autoimmune disease? Is there is an invasion into the stomach of macrophages or T cells that may induce this?
Coffey, Nashville: Your suggestion that Ménétrier's disease may be an autoimmune disorder is an intriguing one. Four of the nine patients in the cetuximab trial had ulcerative colitis and a fifth patient had ankylosing spondylitis. We have not investigated whether there is invasion into the stomach of macrophages or T cells. Related to your question, there is an adolescent form of this disorder that has been linked to CMV infection. Young adults present with all of the clinical manifestations (including peripheral edema) and, within a month, there is spontaneous and complete resolution of the disease. Those affected display acute and convalescent titers for CMV. There is biological plausibility for this link to CMV infection in that glycoprotein B, the principal envelope glycoprotein of CMV, binds EGFR, which, in turn, heterodimerizes with ErbB3. This may occur in adults. A 45-year-old immunocompetent gentleman came to Vanderbilt to be evaluated for entry into the cetuximab trial. Two months earlier, he had abrupt onset of nausea, vomiting, and near anasarca (generalized edema) along with histological features consistent with Ménétrier's disease from a gastric biopsy. During the process of evaluation, he had complete resolution of all of the clinical manifestations of the disease. We documented acute and convalescent titers for CMV, as well distinctive CMV intranuclear inclusions in gastric epithelial cells from his outside gastric biopsy. We recommend that patients with Ménétrier's disease be tested for Helicobacter pylori and active CMV infection, and have symptoms for 3 to 6 months before considering cetuximab or gastrectomy. We also think it would be worthwhile to search for other infectious agents that might be causing the disease.
Boyer, New Haven: Bob, I enjoyed your beautiful presentation very much. What about the hypoalbuminemia? Do you have any thoughts as to the mechanism? Do the transgenic mice develop this problem and is it a defect in the junction between the cells?
Coffey, Nashville: Thank you for your kind comments. We do think there is loosening of junctions between the gastric epithelial cells. In a case report (Clin Gastroenterol Hepatol 2005;3:654–9), we obtained a generous piece of involved gastric mucosa from a snare biopsy. This was incubated with ruthenium red, a radiopaque dye, and then processed for electron microscopy. Prior to treatment, the dye penetrated deep along the pericellular route. However, in biopsies obtained 24 hours and 1 month after treatment with cetuximab, the dye was confined to the luminal surface, suggesting that EGFR blockade was able to tighten the junctions. We have not performed this analysis in TGFα transgenic mice, but it would certainly be worth doing so.
REFERENCES
- 1.Menetrier P. Des polyadenomes gastriques et leur rapport avecle cancer de l'estomac. Arch Physiol Norm Pathol. 1888;1:32–55. 236–62. [Google Scholar]
- 2.Rich A, Toro TZ, Tanksley J, et al. Distinguishing Menetrier's disease from its mimics. Gut. 2010;59:1617–24. doi: 10.1136/gut.2010.220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfsen HC, Carpenter HA, Talley NJ. Menetrier's disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology. 1993;104:1310–9. doi: 10.1016/0016-5085(93)90339-e. [DOI] [PubMed] [Google Scholar]
- 4.Coffey RJ, Jr, Derynck R, Wilcox JN, et al. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817–20. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989;84:1017–23. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutten MJ, Dempsey PJ, Solomon TE, Coffey RJ., Jr TGF-alpha is a potent mitogen for primary cultures of guinea pig gastric mucous epithelial cells. Am J Physiol. 1993;265:G361–9. doi: 10.1152/ajpgi.1993.265.2.G361. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JJ, Goldenring JR, Modlin IM, Coffey RJ. Inhibition of parietal cell H+ secretion by transforming growth factor alpha: a possible autocrine regulatory mechanism. Surgery. 1990;108:220–6. discussion 6–7. [PubMed] [Google Scholar]
- 8.Guglietta A, Lesch CA, Romano M, McClure RW, Coffey RJ. Effect of transforming growth factor-alpha on gastric acid secretion in rats and monkeys. Dig Dis Sci. 1994;39:177–82. doi: 10.1007/BF02090079. [DOI] [PubMed] [Google Scholar]
- 9.Romano M, Polk WH, Awad JA, et al. Transforming growth factor alpha protection against drug-induced injury to the rat gastric mucosa in vivo. J Clin Invest. 1992;90:2409–21. doi: 10.1172/JCI116132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui Y, Halter SA, Holt JT, Hogan BL, Coffey RJ. Development of mammary hyperplasia and neoplasia in MMTV-TGF alpha transgenic mice. Cell. 1990;61:1147–55. doi: 10.1016/0092-8674(90)90077-r. [DOI] [PubMed] [Google Scholar]
- 11.Halter SA, Dempsey P, Matsui Y, et al. Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-alpha. Characterization of mammary gland and skin proliferations. Am J Pathol. 1992;140:1131–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Coffey RJ, Jr, Meise KS, Matsui Y, Hogan BL, Dempsey PJ, Halter SA. Acceleration of mammary neoplasia in transforming growth factor alpha transgenic mice by 7,12-dimethylbenzanthracene. Cancer Res. 1994;54:1678–83. [PubMed] [Google Scholar]
- 13.Bluth RF, Carpenter HA, Pittelkow MR, Page DL, Coffey RJ. Immunolocalization of transforming growth factor-alpha in normal and diseased human gastric mucosa. Hum Pathol. 1995;26:1333–40. doi: 10.1016/0046-8177(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey PJ, Goldenring JR, Soroka CJ, et al. Possible role of transforming growth factor alpha in the pathogenesis of Ménétrier's disease: supportive evidence form humans and transgenic mice. Gastroenterology. 1992;103:1950–63. doi: 10.1016/0016-5085(92)91455-d. [DOI] [PubMed] [Google Scholar]
- 15.Coffey RJ, Washington MK, Corless CL, Heinrich MC. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdick JS, Chung E, Tanner G, et al. Treatment of Menetrier's disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med. 2000;343:1697–701. doi: 10.1056/NEJM200012073432305. [DOI] [PubMed] [Google Scholar]
- 17.Fiske WH, Tanksley J, Nam KT, et al. Efficacy of cetuximab in the treatment of Menetrier's disease. Sci Transl Med. 2009;1:8ra18. doi: 10.1126/scitranslmed.3000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters K. London: Royal College of Physicians; 2004. Exceptional Matters. [Google Scholar]
- 19.Barnard JA, Graves-Deal R, Pittelkow MR, et al. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994;269:22817–22. [PubMed] [Google Scholar]