Abstract
In an RNA world, RNAs would have regulated traffic through normally impermeable bilayer membranes. Using selection-amplification we previously found RNAs that bind stably and increase the ionic conductance of phospholipid membranes at high Mg2+ and Ca2+ concentrations. Now selection in reduced divalents yields RNAs that bind phosphatidylcholine liposomes under conditions closer to physiological. Such affinity for phospholipid membranes requires interactions between RNAs. In fact, we detected no functional monomeric membrane-binding RNAs. A membrane-active end-to-end heterotrimer consisting of 2 RNA 9 and 1 RNA 10 is defined by nucleotide protection, oligonucleotide competition, and mutant analysis. Oligomers of the heterotrimer bind stably, cause release of liposome-encapsulated solutes, and disrupt model black membranes. Individual RNA molecules do not show any of these activities. This novel mechanism of RNA binding to lipid membranes may not only regulate membrane permeability, but suggests that arrays of catalytic or structural RNAs on membranes are plausible. Finally, a selection met only by RNA complexes evokes new possibilities for selection-amplification itself.
The RNA world hypothesis (1) suggests that ancestral RNAs played roles presently taken by both nucleic acids and proteins. To show that RNA might serve essential membrane functions, we used selection-amplification to isolate RNA molecules with affinity for phospholipid liposomes (2). Some RNAs bound stably and increased the ionic permeability of liposomes, as well as the plasma membranes of cultured human cells. Analysis of individual isolated RNAs suggested that specific RNA sequences and folds are needed for membrane activity. In fact, one RNA appeared to bind in only one of two prevalent conformers. Earlier reports detected weaker, nonspecific interactions between membranes and nucleic acids (3), but these selected RNAs were the first stably bound nucleic acids with measurable membrane effects.
The goal of the present study was to elucidate the mechanism of RNA binding to lipid membranes at moderate concentrations of divalent cations, closer to those found in cells and tissues. We have selected (4, 5) RNAs capable of efficiently binding to pure phosphatidyl choline liposomes, and we investigated RNA structure and interactions with liposomes and black membranes. Surprisingly, binding to the liposome surface requires formation of complexes between RNAs. Active multimer formation occurs via pairing of varied selected complementary sequences in weakly structured terminal regions, including “kissing loops” (6).
Experimental Procedures
Selection.
Incubation of internally 32P-labeled RNA [1,000 pmol (cycles 1–4) and 100 pmol (cycles 5–11), 30 μl] with liposomes (20 μl, 20 mg/ml) was performed in 50 mM Hepes (pH 7.0), 50 mM NaCl, 5 mM MgCl2, and 2 mM CaCl2 at room temperature for 5 min, followed by gel filtration on a Sephacryl S-1000 (Amersham Pharmacia) 1-ml column. To decrease nonspecific sorption, the column was presaturated with liposomes (1,000 μg) and total yeast RNA (100 μg). Fifty-microliter fractions were collected, and the first two fractions containing the leading edge of the liposome peak (detected by OD320) were pooled. Liposomes were disrupted by 5-min incubation at room temperature with 0.1% Triton X-100, and RNA was precipitated with ethanol and processed as described (7). Triton X-100 was used because the chloroform extraction used previously (2) leads to loss of RNA into the interphase.
Randomized RNA.
The RNA pool with 80-mer random region was generated from a T7 promoter sequence by in vitro transcription (8) of a DNA template strand of the sequence: 5′-TGG TCA TGT GAT CGG CGT ATG - N80 - TAT CGT GTC ATC GTC GTC CCT ATA GTG AGT CGT ATT A-3′. Approximately 8 × 1014 molecules of 121-nt RNA transcribed from 2 × 1014 independently synthesized DNA templates were heated in water at 65°C for 3 min, then 10× buffer was added, and solution was cooled to room temperature over 10 min.
Liposome Preparation.
Unilamellar liposomes were prepared from phosphatidylcholine (1,2-dioleoyl-sn-glycero-3-phosphocholine; Avanti Polar Lipids) at a final concentration 20 mg/ml, by using the Avanti MiniExtruder with pore filter diameter of 100 nm, according to manufacturer's protocol.
[α-32P]GTP Efflux Experiments.
Liposomes loaded with [α-32P]GTP were prepared as described above at a final concentration 20–40 mg/ml, in the presence of [α-32P]GTP (5–10 μCi/ml). Unincorporated [α-32P]GTP was removed by using a Micro Bio-Spin P30 Column (Bio-Rad) according to manufacturer's protocol. The liposomes were incubated with RNA (6 μM) for 0–4 h at room temperature, and applied to a second P30 column. Released [α-32P]GTP remains in the column; radioactivity retained in intact liposomes appears in the void.
Black Lipid Membrane Experiments.
Black lipid membranes were formed as described (9). Lipid and buffer were identical to that used in RNA selection experiments. A stable bilayer was formed across a 25-μm diameter aperture, and then RNA was added to a final concentration of 1–10 μM on the cis side of the bilayer and 100 mV potential was applied, cis side negative. Ionic current was recorded by using an Axopatch 200B integrating patch clamp amplifier (Axon Instruments, Foster City, CA) in voltage-clamp mode.
Native Gel Electrophoresis.
RNA (30–60 pmol) alone or with deoxyoligonucleotides (30–120 pmol) in a final volume 10 μl was folded in selection buffer. Then 2 μl of 6× loading buffer (0.25% bromophenol blue/0.25% xylene cyanol/30% glycerol in water) was added, and samples were loaded on a 2% NuSieve 3:1 agarose gel. The gel was run in selection buffer at 4°C by using a 4 V/cm field for 1 h, stained with ethidium bromide, and photographed under UV light.
Probing of RNA Structure.
RNA secondary structure was probed by lead hydrolysis and S1 nuclease digestion as described (10).
Results
Selection of Liposome Binding RNA.
To select RNA molecules that adhere to lipid bilayers, RNA molecules with randomized sequences were incubated with liposomes. Bound RNA was captured by fractionation of liposomes into the void volume of a Sephacryl S-1000 column (2). In this column unbound RNA is retarded and resolved.
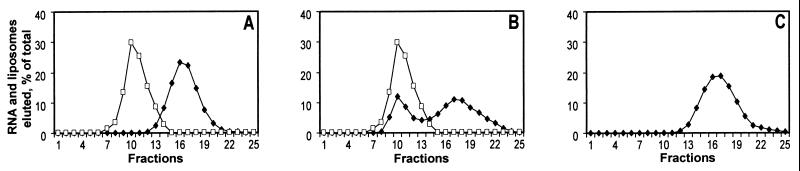
Initial randomized RNAs demonstrated no affinity for liposomes (Fig. 1A); of the initial pool, only 0.03% of RNA migrates with the liposome peak. The first signal—RNA (0.4%) comigrating with liposomes—was detected at the fifth cycle of selection. After 11 cycles, approximately 35% of RNA comigrates (Fig. 1B). Such RNA in the column void was not caused by RNA aggregation. The RNA pool tested after each cycle of selection without liposomes eluted as a single included peak indistinguishable from the initial random RNA (e.g., Fig. 1C). Nevertheless, although not detected by gel filtration, free RNAs do aggregate moderately and this is essential for membrane affinity.
Figure 1.
Chromatographic selection for liposome-binding RNAs. (A) Random RNA (⧫) and liposomes (□). (B) RNA after 11 cycles of selection (⧫) and liposomes (□). (C) RNA after 11 cycles of selection (⧫) without liposomes.
Liposome Binding Is a Result of Intermolecular Interactions.
Liposome binding of mixed RNAs after 11 rounds of selection was concentration dependent. Yields of liposome-RNA complexes were 35% and 16% for this RNA pool tested at 2 μM and 0.2 μM, respectively. Moderately more effective binding at higher RNA concentration suggested that binding requires complexes (see Fig. 3B below).
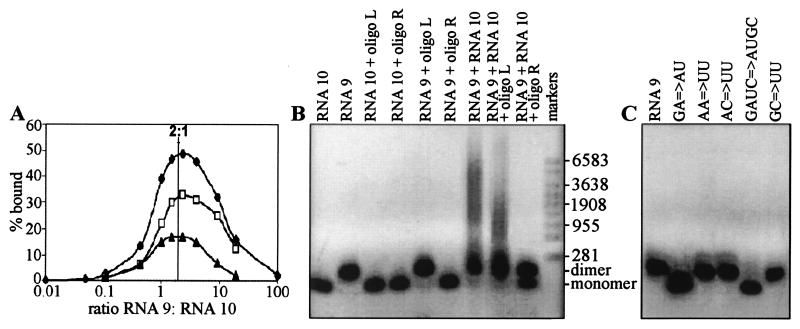
Figure 3.
Characterization of RNA 9–10 complexes. (A) Optimization of the RNA 9/10 ratio by gel filtration. Total RNA for each trial was fixed at 100 pmol and the ratio RNA 9–10 was varied. Binding to liposomes was measured three ways. In the first way, both RNAs were labeled ⧫, in the second only RNA 9 was labeled □, and in the third only RNA 10 was labeled ▴. (B) Detection of complexes formed by RNA 9 and RNA 10 and effect of deoxyoligonucleotides 5′-CTGCCCT-3′ (oligo L) and 5′-GGTCATGTGATCGGCGTATG-3′ (oligo R) on complex formation. RNA 9 (30 pmol), RNA 10 (30 pmol), or RNA 9 + RNA 10 (20 + 10 pmol) were folded with or without deoxyoligonucleotide L or R (90 pmol), fractionated on a 2% nondenaturing agarose gel, and visualized under UV light as described in Experimental Procedures. (C) Test of RNA 9 and RNA 9 mutants for dimer formation. All procedures are as for B.
RNAs from the 11th cycle were reverse-transcribed, cloned, and sequenced. The pool was very heterogeneous (Fig. 2A). Of 88 RNAs only five pairs of identical molecules were found. Sequence comparison of the cloned RNAs did not reveal any common sequence motif among the 83 independent RNAs with affinity for liposomes. Such varied RNA sequences that meet a liposome-binding selection resemble our previous findings (2).
Figure 2.
Analysis of the RNA pool after 11 cycles of selection. (A) RNA sequences for the 11 (of 88 initial) RNAs designated in bold at the bottom right of B. The sequences present only initially randomized nucleotides. (B) Arbitrary division of equimolar mixtures comprising 88 individual sequences. The nonoverlapping smaller groups were assayed for liposome binding by gel filtration. Percentage of liposome binding is shown for each group of RNAs.
Surprisingly, when 20 isolated individual RNAs were transcribed and assayed for liposome binding, all 20 bound poorly, with 0.5–2% of RNA in the column void with liposomes. Under the same conditions, binding of an equimolar mixture of the 88 RNAs was ≈23%. Therefore, efficient RNA binding to liposomes may occur by collaboration of different RNA species.
RNAs 9 and 10 Are One Minimal Membrane-Binding Group.
To identify a set of cooperating RNAs, the 88 individual species were arbitrarily and progressively divided into nonoverlapping smaller groups assayed for membrane binding (summarized in Fig. 2B). Ultimately a mixture of five isolates, RNAs 2, 5, 6, 9, and 10 (sequences in Fig. 2A; rightward group in 2B), was found, which exhibited 20% binding to liposomes. When equimolar RNA 9 was mixed with each of four other individual RNAs and tested for liposome binding, it was found that with RNAs 2, 5, and 6 binding is the same as for individuals (0.5–2%). In contrast, mixed with RNA 10 it is as high as 39%. Thus RNAs 9 and 10 cooperate to bind liposomes with high efficiency. Renaturation is not crucial: similar high-level binding was obtained whether the RNAs were folded together or folded separately and then mixed.
In support of the specificity of the RNA 9–10 complex, RNAs 2, 5, and 6 mixed together displayed only 5% binding. Individually, RNAs 9 and 10 under similar conditions form liposome complexes with yields 2% and 0.5%, respectively. However, if time of gel filtration for RNA 9 was decreased from 20–25 min (standard conditions) to 15 min, 4–7% of the RNA eluted earlier as an unresolved shoulder leading the major nonbound RNA peak. These data suggest formation of a weak RNA 9 homo-complex with liposomes that dissociates in the course of gel filtration; this effect was not observed for RNA 10.
An Optimal Ratio Between RNA 9 and RNA 10.
We determined the optimal binding ratio between RNA 9 and RNA 10 by fixing total RNA at 100 pmol and varying the molar ratio of RNA 9 to RNA 10. In three independent but parallel experiments, binding to phospholipid liposomes was measured. In the first series, both RNA 9 and RNA 10 were labeled to determine the optimal molar ratio for total binding. In the second experiment, RNA 9 was labeled and RNA 10 was cold to determine the optimal ratio for RNA 9 binding. In the third RNA 10 was labeled and RNA 9 was cold, to find the optimum for RNA 10 binding. Data in Fig. 3A indicate that the most efficient binding is achieved in mixtures containing RNA 9 and RNA 10 near the proportion 2:1. Total RNA binding to liposomes is maximized at 2:1 RNA 9/RNA 10. In addition, parallel experiments with only RNA 9 or RNA 10 labeled showed that approximately two molecules of RNA 9 are bound to liposomes for each molecule of RNA 10. Consistent results were obtained if total RNA was fixed at a lower level (50 pmol), and the molar ratio 9:10 was similarly varied (data not shown). Thus both input and bound product reflect an optimal 2:1 stoichiometry, suggesting that a 9:9:10 trimer is the foundation of the minimal active membrane-binding RNA. Of course, hexamers, nonamers or higher oligomers of the same composition also could be the active agents.
Effect of Salts on RNA 9–10 Binding to Liposomes.
Selection buffer contained 50 mM Na+, 5 mM Mg2+, and 2 mM Ca2+, and liposome binding at the optimal RNA ratio was 49%. In buffer with no Mg2+ (but 7 mM Ca2+) or no Ca2+ (but 7 mM Mg2+), binding was 64% and 35%, respectively (not shown). Thus either divalent metal ion supports binding, although Ca2+ is more active. Upon increase of Na+ up to 150 mM, binding dropped to 30%; decreasing Na+ to 10 mM yields 58% bound (data not shown). Monovalents are therefore not critical and may compete with divalent ions.
Analysis of Complex Formation by Native Gel.
Formation of RNA complexes could be followed by native gel electrophoresis, in the absence of phospholipid. Gel results in Fig. 3B show that RNA 10 by itself is monomeric (lane 1), whereas the abnormally low electrophoretic mobilities of RNA 9 alone (lane 2) and mixed RNA 9 + RNA 10 (lane 7) suggest stable complexes in solution. Complex size suggests a unique dimer for free RNA 9 complexes and larger and more varied complexes for RNA 9 + RNA 10. A notable absence of density in the RNA 9 + 10 lane at the point where trimers would be suggests that RNA 9–10 heterotrimers are entirely in complexes (9:9:10)2 and above. Thus active RNA 9–10 complexes might be varied and contain many RNAs. Actual sizes are uncertain because of possible differences in shape between markers and RNA 9–10 complexes.
The large RNA 9–10 complexes, formed from pure RNAs, are included in our Sephacryl columns and do not appear in the void (data not shown) unless liposomes are present. If RNA 9 + RNA 10 are mixed and liposomes added, the RNA in the void volume is nearly all in form of complexes on a native gel (liposomes disrupted by using non-ionic detergent) whereas retarded RNA was mainly monomeric (data not shown). These observations taken together suggest that RNA 9–9–10 oligomers form in solution, nearly quantitatively bind to liposomes, and then persist in the membrane at the same overall stoichiometry.
Secondary Structure Models for RNA 9 and RNA 10, Free and in Complex.
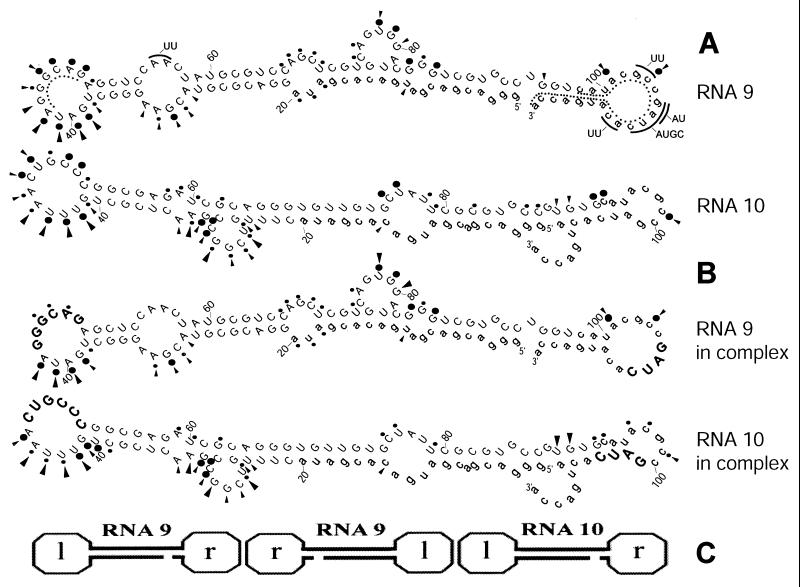
Lead and S1 nuclease probing of RNA 9 and RNA 10 (free of liposomes) was consistent with calculated stable secondary structures (11) (Fig. 4A). In fact, every major predicted loop is confirmed, except for part of a small internal loop in RNA 9 (nucleotides 54–57), and a part of the right terminal loop in RNA 9 (nucleotides 105–111).
Figure 4.
Characterization of RNA 9–10 complexes. (A and B) Secondary structures predicted for RNA 9 and RNA 10, free and in complex, with lead cleavage sites (circles) and S1 nuclease cleavage sites (arrows) on 5′ [32P]RNA. Larger circles and arrows indicate more frequent cuts. Constant sequences are shown in lowercase; randomized nucleotides are capitalized. Deoxyoligonucleotide L and R target sequences are shown by curved dotted lines. Mutated positions and changes are shown on arcs outside the sequences. Regions important for complex formation are shown in larger bold type. (C) The heterotrimer-building block for RNA 9–10 complexes. Two RNA 9 molecules interact via right-hand loops (r), and the left-hand loop (l) of the middle RNA 9 molecule interacts with the left-hand loop of RNA 10. The diagram is schematic and does not attempt to show realistic molecular or complex shapes.
Analysis of the structures revealed complementary antiparallel sequences in RNA 9 (GGGCAG, nucleotides 43–48) and RNA 10 (CUGCCC, nucleotides 47–52). These leftward (Fig. 4) hexanucleotide loop sequences, including five GC pairs, could efficiently hybridize leading to formation of a “kissing loop complex” (6). In addition, the rightward loop of RNA 9 contains a self-complementary tetranucleotide sequence GAUC (nucleotides 106–109) that could nucleate the interaction observed between two RNA 9 molecules (Fig. 3B; see below). This latter interaction would explain the probing results; i.e., this sequence is resistant to our probing in all conditions, as would be predicted if it were paired in the 9:9 dimer.
Probing of mixtures of RNAs 9 and 10 revealed changed structures accompanying complex formation (Fig. 4B). The most dramatic changes are observed exactly within the one-half of the leftward terminal hairpin loop regions where kissing loop complex formation is expected. In RNA 9 complex formation interferes with S1 and lead cuts at positions 43–49; in RNA 10 the complex formation suppressed S1 and lead cuts at positions 47–52. These protections are centered on the loop–loop complementarities evident in the sequences.
Complex formation also affects reactivity of some sequences within the 3′ terminal part of RNA 10: increase of reactivity at UG (nucleotides 89–90) and AC (nucleotides 96–97), and decrease in reactivity at sequence GC (nucleotides 92–93). As a result of shared constant regions, a GAUC sequence is present in the 3′ end of RNA 10 (nucleotides 101–104), within an imperfect hairpin stem. Results of the probing experiments suggest that RNA 9 and RNA 10 can interact in the region of these sequences because complex formation results in a change of reactivities in the 3′ end of RNA 10. To summarize at this point, secondary structure probing moderately supports RNA 9 dimerization and RNA 9–10 interaction via the right loop region (Fig. 4C) and very specifically supports RNA 9–10 interaction via leftward kissing loops.
Confirmation of the RNA 9–10 Left Hand Loop–Loop Complex.
The importance of the left loop region (nucleotides 43–48) in RNA 9 for complex formation was confirmed in competition experiments with the complementary deoxyoligonucleotide 5′-CTGCCCT-3′ (oligo L) targeted to the predicted pairing sequence in the left-hand RNA 9 loop. Three-fold molar excess of the deoxyoligonucleotide had a strong inhibitory effect on complex formation on gels (Fig. 3B, compare lanes 7 and 8). The percentage of RNA in monomer form increases, remaining complexes become substantially smaller, and measured liposome binding dropped from 49% to 1–2% (not shown). A control oligonucleotide 5′-GCCTTCC-3′ with the same nucleotide composition, but a scrambled sequence, had no detectable effect on complex formation. Thus RNA 9–10 loop–loop interaction is disrupted by a competitor for the RNA 9 loop.
Persistence of complexes that appear much larger than dimers in the presence of oligo L suggests that hybridization of the two left-hand loops is not the only interaction between the two RNAs, even though it is essential for liposome binding. The 3′ (right end; Fig. 4) of the molecules also may be involved in RNA 9–10 interactions.
The Right Hand Loop Is Important for RNA 9 Dimerization and RNA 9:10 Complex Formation.
5′-TGGTCATGTGATCGGCGTATG-3′ (oligo R), is complementary to constant nucleotides 98–118. When added in 2-fold molar excess over RNA, it quantitatively converted RNA 9 to monomers (Fig. 3B, compare lanes 2 and 6). Control 10-mer deoxyoligonucleotides, complementary to all other RNA 9 sequences, had no effect on complex formation (not shown). Thus RNA 9–9 dimer is apparently forming by using right-hand, near-terminal sequences, perhaps exclusively. The fact that RNA 9 forms these homo-oligomers may explain its unstable liposome complexes (described above).
To define more closely the 3′ sequence of RNA 9 responsible for dimer formation, a series of mutant RNA 9 molecules has been synthesized. GAUC ⇒ AUGC (nucleotides 106–109), and GA ⇒ AU (nucleotides 106–107) are mutant in the self-complementary loop sequence, and GC ⇒ UU (nucleotides 103–104) and AC ⇒ UU (nucleotides 110–111) are mutated in adjacent 3′-loop sequences. AA ⇒ UU (nucleotides 55–56) also was tested because the internal loop (nucleotides 54–57) in RNA 9 was resistant to probing and thus possibly involved in complex formation. Predicted structures for these mutants were either unchanged or only slightly altered. In Fig. 3C, dimerization of the GC ⇒ UU, AC ⇒ UU, and AA ⇒ UU mutants is normal (compare lanes 1 and 3, 4, and 6). At the same time, GAUC ⇒ AUGC and GA ⇒ AU mutants in the 3′ loop self-complementary GAUC migrate as monomers (lanes 2 and 5). Changing as few as 2 nt in the right hand loop therefore disrupts RNA 9 dimerization.
In the presence of oligo R, only an apparent 1:1 dimer but no larger complexes between RNA 9 and RNA 10 were observed (Fig. 3B, lane 9), and no liposome binding was detected (not shown). Similar results were obtained with GAUC ⇒ AUGC and GA ⇒ AU mutants. Thus RNA 9 homo-oligomerization via the rightward loop (Fig. 4) is essential for both oligomerization and the membrane phenotype.
Therefore blocking the right-hand loop of RNA 9 (Fig. 4) limits complex formation to the 9–10 dimer and prevents 9:9 interaction. Because of the very clear pattern in the protection data (Fig. 4 A and B), the RNA 9–10 dimer presumably relies on a kissing loop structure through the left-hand loops (Fig. 4), and this interaction is stable in isolation. Thus both right-hand and left-hand regions are important for RNA 9–9–10 complex formation and interaction with liposomes.
Note that these results, taken together, account for the measured stoichiometry of the complex (Fig. 3A) by suggesting that an RNA 9 (right–right) RNA 9 (left–left) RNA 10 heterotrimer is an essential substructure. This RNA 9–9–10 trimer is also “open” in that it offers further loop interactions to other RNA molecules, potentially accounting for the observed large heterogeneous complexes (Fig. 3).
RNA 9 + RNA 10 Disrupt Black Lipid Membrane.
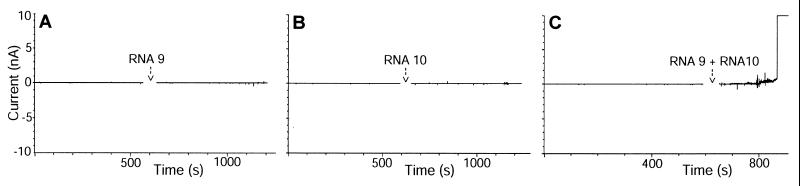
Experiments with black lipid membrane show that these liposome binding RNAs destabilize a pure phospholipid bilayer. Conductivity measurements indicated that RNAs 9 and 10 alone, at concentrations up to 10 μM, did not have a significant effect on the stability of the bilayer, even up to 30 min of incubation (Fig. 5 A and B). However, mixtures of RNAs 9 + 10 at the same total concentration first produce transient quick increases in conductance (baseline conductivity spikes), probably explained by formation of subcritical assemblies. Then they reproducibly destroy the phospholipid bilayer within 1–5 min (Fig. 5C; the rapid conductivity increase is accompanied by disappearance of a microscopically visible membrane). No effect was observed at mixed RNA concentrations lower than 1 μM, even after prolonged incubation up to 30 min. Randomized sequences or nonspecific RNA had no effect on membrane stability. However, highly heterogeneous 11th cycle pool RNAs also reproducibly cooperated to destabilize a planar phospholipid membrane (data not shown).
Figure 5.
Disruption of a black lipid membrane by RNA complexes. Black lipid membranes were formed: 10 μM RNA 9 (A), 10 μM RNA 10 (B), or RNA 9 + RNA 10 (6.6 + 3.3 μM) (C) was added to the cis side of the membrane and 100 mV potential applied with the cis side negative. Ionic current in nanoamperes was recorded at intervals of 100 μs.
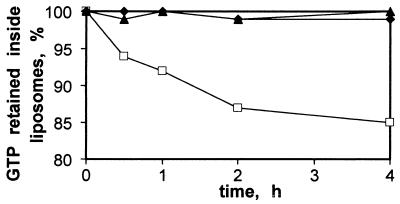
RNA 9 + RNA 10 Release Liposome-Encapsulated [α-32P]GTP.
RNAs 9 and 10 were also tested to determine whether liposome permeabilities are affected. Liposomes charged with [α-32P]GTP were incubated with mixed RNA 9 (4 μM) and RNA 10 (2 μM) and released radiolabeled GTP was separated by P30 spin column and quantitated. RNA 9–10 complexes permeabilize liposomes, although no disruption was observed in control liposome incubations without RNA or in the presence of RNA 9 or 10 alone (Fig. 6). Survival of the majority of the liposomes in the presence of RNA 9–10 is not surprising because it was required by the selection.
Figure 6.
Disruption of liposomes by RNA complexes. Liposomes loaded with [α-32P]GTP were incubated with 6 μM RNA 9 (⧫), 6 μM RNA 10 (▴), or RNA 9 + RNA 10 (6.6 + 3.3 μM) (□) and applied to a P30 gel-filtration column. Percentage of cpm in the void is plotted.
RNAs Are Exterior.
When the RNA 9–10 complex was probed in the presence of liposomes it, was found that RNAs remain accessible to lead and S1 nuclease and therefore are on the outside of liposomes (data not shown). Thus complexes interact with the outer membrane surface to indirectly disrupt membrane integrity.
An Earlier Isolate, RNA 13, May Bind Liposomes by the Same Mechanism.
Taking into account our present results, we now speculate that earlier membrane-binding RNAs (2), isolate 13 and others, also act by the formation of supramolecular complexes. In isolate 13, complementary sequences in positions 6–13 and 62–68 (2) are located in loop regions or in unstable regions within predicted structures, suggesting the possibility of interaction. The requirement for these sequences in conversion of the RNA to a form capable of binding to liposomes is supported by truncation experiments. Truncated 63 mer, where these sequences were preserved, retained ability to bind to liposomes, whereas the RNAs that lack one or both of the sequences demonstrate strongly decreased activity or did not bind at all. Isolate 13 and others also performed better as mixtures of species. Finally, the observed sensitivity of the membrane-binding ability of isolate 13 to the details of renaturation is consistent with formation of intermolecular complexes. Unfortunately, the essential high concentration of divalent ions complicated investigation of complex formation by nondenaturing gel electrophoresis, and therefore direct comparison of isolate 13 (2), and RNA 9–10 complexes was not possible.
Discussion
The present study and the study of Khvorova et al. (2) are examples of selection of RNA complexes capable of binding to a repetitive surface rather than to a single, specific molecule. Here lower divalent concentrations, longer randomized sequences (80 rather than 50 nt), and pure phosphatidylcholine membranes (no cholesterol) differ from earlier conditions.
The outcome of this selection was unexpected. Resolution of the final selected pool (Fig. 2B) shows that the probability of autonomous RNA binding to phospholipid bilayers under our conditions is low, likely ≤0.05. Therefore, our selection was met entirely, or nearly so, by heteromeric RNA associations. Such associations may sum affinities by forming numerous weak bonds.
A specific ensemble that has these properties is the oligomerized heterotrimer RNA (9–9–10)n where n is two or more. RNAs 9 and 10 dimerize through the left-hand loop (Fig. 4) and RNAs 9 through the right hand loops (Fig. 4). The heterotrimer can further oligomerize in solution and bind stably to the exposed face of a phospholipid bilayer. Such ensembles persist with their initial stoichiometry, and when they reach a critical size (or cover a critical area), they can disrupt a planar phospholipid bilayer.
Successful RNA associations can be of many kinds because in different mixtures of many pool RNAs roughly one-half of the RNAs bind, judged from the fact that binding is approximately one-half that of purified competent complexes (Fig. 2). Because these RNAs share little sequence homology (see the representative sequence sample in Fig. 2A), the observation that one-half of the RNAs bind implies varied complexes, bound via different sequences. Thus individual RNAs are expected to form binding associations with a minority of the other sequences in the pool. This idea is supported by isolation of the RNA 9–10 pair: Neither is complemented by other sequences in its subpool.
However, selections are constrained because RNA groups must function in the initial rounds of selection when particular RNA concentrations are low. Therefore interRNA interactions must be simple enough to be shared with many partners, so that activity is possible when there are few molecules of any particular sequence. It is likely important, for example, that the RNA 9–10 complex principally requires only 6 nt. Therefore ≈2% of molecules (≈80/46 = 0.02) may be potential partners. To say this another way, even though there will many consortia, each one of them is predicted to include many possible partners to have succeeded early in selection. Therefore we predict other membrane-binding partners for RNAs 9 and 10 among the selected population.
These results emphasize a largely unrecognized possibility: Selections might yield RNA consortia, functioning in groups. In fact, cooperation of RNAs during SELEX may be routine. Many published pools show some RNAs that do not meet the selection when tested as pure species; this includes our previous RNAs with membrane affinity (2). Different selection products are frequently not tested for interaction. Even more subtly, obligatory oligomerization of individual pure RNAs would almost always go unnoticed. Now that molecular groups have been shown to be selectable entities, selections where interactions will give some useful function can be designed. For example, the optimal size of a randomized RNA is a significant experimental problem (12–13). Conceivably, a randomized pool of small RNAs with oligomerization built into partially constant sequences (e.g., as in ref. 14) could be used to let selection itself settle on the optimal size for RNA assemblies that meet a selection criterion.
Darwinian evolution in an RNA world may require catalysts encapsulated in membranes. Superior catalysts then can benefit from the products of their own reactions. However, an impermeable membrane prevents the uptake of external nutrients and the excretion of waste products. Thus even RNA-based cells had to have mechanisms for transportation of polar and complex molecules. Given that ancient RNAs were likely built of monomers similar to contemporary RNAs, their hydrophilicity would probably not allow them to function as transmembrane shuttles capable of crossing the lipid bilayer. Thus regulated systems of molecules capable of transiently opening lipid membranes by action from one side may have been selected, as here. RNAs that resemble those selected here also might help reorganize membranes, e.g., during cell division. They might even initiate such events. Such RNA complexes also could be useful as portable pathways because of their intrinsic nature as ordered, membrane-bound, multifunctional systems.
Therefore RNA–phospholipid membrane interactions are more varied than we would have expected on the basis of earlier results. Not only is stable modification of permeability an easily selected RNA activity (2), but overall destabilization of membrane systems by hetero-oligomeric RNAs also. The variety of known RNA-membrane phenomena seems likely to increase as experimentation expands. Further, there are modern RNAs that show unexplained association with membranes (15, 16) and modern phenotypes that suggest RNA involvement in membranes. The latter include a role for RNase activity, and therefore probably RNA, in the osmotic integrity of some Saccharomyces (17). Accordingly, RNA-membrane phenomena like those studied here may extend to present-day cells.
Acknowledgments
We thank the members of our laboratory and also Valentin Vlassov, Vladimir Budker, Sergey Kazakov, and Alexey Wolfson for helpful discussions. We are particularly grateful for the hospitality of David Deamer and members of his laboratory who hosted A.V.'s black lipid membrane experiments. This work was supported by National Institutes of Health Grants RG 30881 and RG 48080.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gilbert W. Nature (London) 1986;319:618. [Google Scholar]
- 2.Khvorova A, Kwak Y-G, Tamkun M, Majerfeld I, Yarus M. Proc Natl Acad Sci USA. 1999;96:10649–10654. doi: 10.1073/pnas.96.19.10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budker V G, Godovikov A A, Naumova L P, Slepneva I A. Nucleic Acids Res. 1980;8:2499–2515. doi: 10.1093/nar/8.11.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuerk C, Gold L. Science. 1990;3:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 5.Ellington A D, Szostak J W. Nature (London) 1990;30:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 6.Tomizawa J. Cell. 1984;38:861–870. doi: 10.1016/0092-8674(84)90281-2. [DOI] [PubMed] [Google Scholar]
- 7.Ciesiolka J, Illangasekare M, Majerfeld I, Nickles T, Welch M, Yarus M, Zinnen S. Methods Enzymol. 1996;267:315–335. doi: 10.1016/s0076-6879(96)67021-9. [DOI] [PubMed] [Google Scholar]
- 8.Milligan J F, Uhlenbeck O C. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- 9.Akeson M, Branton D, Kasianowicz J J, Brandin E, Deamer D. Biophys J. 1999;77:3227–3233. doi: 10.1016/S0006-3495(99)77153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Illangasekare M, Yarus M. Proc Natl Acad Sci USA. 1999;96:5470–5475. doi: 10.1073/pnas.96.10.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuker M, Mathews D H, Turner D H. In: RNA Biochemistry and Biotechnology. Barciszewski J, Clark B F C, editors. Dordrecht, The Netherlands: Kluwer; 1999. pp. 11–43. [Google Scholar]
- 12.Sabeti P C, Unrau P J, Bartel D P. Chem Biol. 1997;10:767–774. doi: 10.1016/s1074-5521(97)90315-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang F, Bugg C W, Yarus M. Biochemistry. 2000;39:15548–15555. doi: 10.1021/bi002061f. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger L, Westhof E, Leontis N B. Nucleic Acids Res. 2001;29:455–463. doi: 10.1093/nar/29.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter P, Keenan R, Schmitz U. Science. 2000;287:1212–1213. doi: 10.1126/science.287.5456.1212. [DOI] [PubMed] [Google Scholar]
- 16.Batey R T, Rambo R P, Lucast L, Rha B, Doudna J A. Science. 2000;287:1232–1239. doi: 10.1126/science.287.5456.1232. [DOI] [PubMed] [Google Scholar]
- 17.MacIntosh G C, Bariola P A, Newbigin E, Green P. Proc Natl Acad Sci USA. 2001;98:1018–1023. doi: 10.1073/pnas.98.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]