Abstract
The hierarchical models of stem cell biology have been based on work first demonstrating pluripotental spleen-colony-forming units, then showing progenitors with many differentiation fates assayed in in vitro culture; there followed the definition and separation of “stem cells” using monoclonal antibodies to surface epitopes and fluorescent-activated cell characterization and sorting (FACS). These studies led to an elegant model of stem cell biology in which primitive dormant G0 stem cells with tremendous proliferative and differentiative potential gave rise to progressively more restricted and differentiated classes of stem/progenitor cells, and finally differentiated marrow hematopoietic cells. The holy grail of hematopoietic stem cell biology became the purification of the stem cell and the clonal definition of this cell. Most recently, the long-term repopulating hematopoietic stem cell (LT-HSC) has been believed to be a lineage negative sca-1+C-kit+ Flk3- and CD150+ cell. However, a series of studies over the past 10 years has indicated that murine marrow stem cells continuously change phenotype with cell cycle passage. We present here studies using tritiated thymidine suicide and pyronin-Hoechst FACS separations indicating that the murine hematopoietic stem cell is a cycling cell. This would indicate that the hematopoietic stem cell must be continuously changing in phenotype and, thus, could not be purified. The extant data indicate that murine marrow stem cells are continually transiting cell cycle and that the purification has discarded these cycling cells. Further in vivo BrdU studies indicate that the “quiescent” LT-HSC in G0 rapidly transits cycle.
Further complexity of the marrow stem cell system is indicated by studies on cell-derived microvesicles showing that they enter marrow cells and transcriptionally alter their cell fate and phenotype. Thus, the stem cell model is a model of continuing changing potential tied to cell cycle and microvesicle exposure. The challenge of the future is to define the stem cell population, not purify the stem cell. We are at the beginning of elucidation of quantum stemomics.
INTRODUCTION
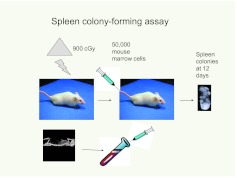
The stability of cell phenotypes underlies much of extant biology. However, this stability, at least in part, must be maintained by extracellular influences because most cells contain equivalent genomic DNA. That cell types can be altered by genetic modifications has been established by cloning, embryonic stem cells, and recent work on induced pluripotent stem cells. Assumptions of cell type stability are still predominant. The hematopoietic stem cell system is a prime example of this. Early work using morphologic approaches established a reasonable hierarchy at the level of relatively differentiated bone marrow cells. The first descriptions of a clonable functional stem cell in vivo were those of the spleen-colony-forming unit (CFU-S). Till and McCulloch (1) described an assay in which infusion of murine marrow cells into lethally irradiated mice resulted in the formation of lumps on the spleen at 8 to 12 days and these lumps were found to be clones of hematopoietic cells (Figure 1).
Fig. 1.
Spleen colony-forming assay. Colony-forming unit spleen (CFU-S) were represented by colonies or “lumps” on the surface of the spleen after infusion of marrow cells into lethally irradiated mice. Spleen colony counting was also performed at earlier time spans, 9 days or more. (Abbreviation: cGy, centigray.)
These were believed to represent pluripotent myeloid stem cells because they differentiated into all myeloid lineages. The system, however, showed interesting heterogeneity with regard to lineage choices and renewal potential. When individual colonies were dissected from the spleens, their capacity to form additional colonies in the assay was totally heterogeneous. Till et al(2) proposed that their studies of CFU-S indicated that “relevant control mechanisms were operative at the level of populations rather then single cells.” They further suggested that the behavior of individual stem cells was analogous to that of individual radioactive nuclei. Populations of nuclei give decay with a highly predictable half-life, but it was impossible to predict exactly when an individual nucleus would undergo radioactive decay. This prescient work provides a good summary of the stem cell field today. This is developed further below.
The next phase of discovery in this field was the elucidation of assays for lineage specific progenitors. Bradley and Metcalf (3) and Pluznik and Sachs (4) described the cloning of granulocyte-macrophage progenitors in soft agar in the presence of colony-stimulating activities (Figure 2).
Fig. 2.
Progenitor assay in semisolid matrices. (Abbreviations: CSFs, colony stimulation factors; GM-CFC, granulocyte-macrophage colony-forming cell.)
This was followed by descriptions of erythroid and megakaryocytic colony-forming cells in semi-solid matrices (5–9)—these included soft agar, methyl cellulose, and plasma clot. Subsequently, virtually all combinations of hematopoietic cells, including lymphoid cells, were shown to clone in vitro with various stimulators (10–13). This came together as a very logical model in which the pluripotent stem cell gave rise to the progenitor with varying degrees of differentiation and proliferative potential, which then in turn gave rise to morphologically recognizable differentiated marrow lineages. This naturally led to a hierarchical model of hematopoiesis in which CFU-S gave rise to progressively restricted populations of progenitors, which in turn gave rise to morphologically recognizable hematopoietic marrow cells.
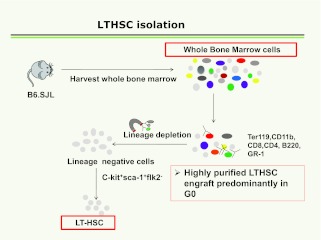
There followed a large body of work on purification of bone marrow stem cells (14–34). These studies continue to evolve, but in essence they involve depletion of differentiated lineage- positive marrow cells using antibodies to B cells, T cells, granulocytes, erythroid and monocyte/macrophage surface epitopes with an iron tag, followed by magnetic depletion. These lineage-negative cells were then labeled with what was believed to be stem-cell specific monoclonal antibodies, c-kit, and Sca-1, and negatively selected for FLT3. More recently, CD150 or Slam has been added as a powerful separative epitope (35). Cells were followed for engraftment and differentiation in lethally irradiated mice and they were termed long-term repopulating hematopoetic stem cells (LT-HSC). The general scheme is outlined in Figure 3.
Fig. 3.
Sequence of separative steps for isolation of LT-HSC.
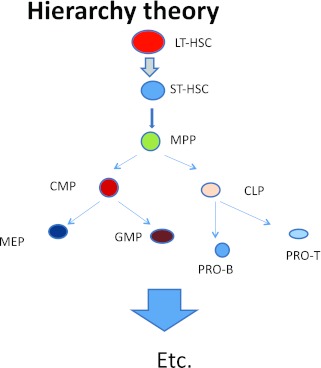
Studies on the potential of these cells to engraft and differentiate then led to an elegant model of hierarchical hematopoiesis (Figure 4).
Fig. 4.
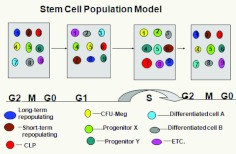
Hierarchical model of hematopoiesis.
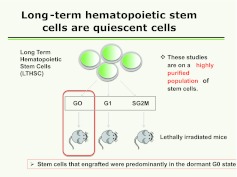
Studies indicated that the most primitive long-term multi-lineage repopulating marrow stem cell was a dormant non-cycling cell, and work by Passegue et al (14) using Hoechst and pyronin supravital dyes to separate lineage-negative C-kit+ Sca-1+Flt3- cells into G0, G1, and S/G2/M phases of cell cycle indicated that all of the stem cells potential resided in the G0 LT-HSC (Figure 5).
Fig. 5.
LT-HSC separated into into G0, G1, and SG2M phases by pyronin and Hoechst staining and fluorescent-activated cell sorting. These fractions were then engrafted competitively into lethally irradiated mice and engraftment outcomes determined.
These studies underlie the current standard tenets of stem cell biology. The gold standard for a long-term multi-lineage marrow renewal stem cell or LT-HSC is long-term multi-lineage engraftment into a lethally irradiated mouse. General tenets of the biology of the LT-HSC are that it has tremendous renewal, proliferative, and differentiative capacity; is dormant in G0; must be defined on a clonal basis; and its phenotype is stable. There have been a large number of published articles defining aspects of the regulation of this cell, almost all focused on highly purified stem cells; receptor-ligand interactions, second messenger signaling, and genomic and post-genomic regulation have all been extensively characterized on highly purified stem cells. In most cases, the phenotype of these cells has been assumed, based on surface epitope characteristics, even though it has been established that the surface epitope profile may change with different cellular inputs.
CAUTIONARY NOTES
There were indications in the literature that the stem cell hierarchy as envisioned above may not be an appropriate model. The comments on the heterogeneity of CFU-S were noted above. In the 1980s, Ogawa described daughter cell experiments that showed that within one cell cycle passage, totally different lineages could be pursued by two daughter cells derived from a blast colony (10). These data were inconsistent with a straightforward hierarchical model of hematopoiesis. Other work has suggested the presence of multiple early stem cells with different lineage predilections (36). Our own work cloning highly purified lineage-negative rhodamine-low Hoechst-low stem cells at different points in a cytokine-induced cell cycle transit indicated that each cell was functionally different as to gross colony morphology and differentiation profile (37).
THE CONTINUUM
In a series of publications over the past 10 years we have described studies indicating that murine marrow stem cells reversibly change phenotype as they transit cell cycle under cytokine stimulation (38–50). However, studies by Passegue et al (14) indicated that all long-term multi-lineage repopulating stem cells were in the G0 phase of cell cycle. They separated Lin-Sca-1+c-Kit+ FLk3 – cells into G0, G1, and S/G2/M phases using the supravital dyes pyronin and Hoechst and found that all long-term repopulating cells resided in the G0 fraction. This prompted us to examine the same model for purified LT-HSC and unseparated whole marrow. We essentially reproduced the data of Passegue et al (14) on LT-HSC, but found very different results when we applied this approach to whole unseparated bone marrow cells. Here, we found that more than 50% of the long-term multi-lineage repopulating cells were in the S or S/G2/M phases of the cell cycle. This was an instantaneous look at cycle, and it suggested that most of the stem cells were in active cell cycle. Further work using tritiated thymidine suicide, which kills cells selectively in the S phase, indicated that more than 70% of the cells were in S phase. This approach gives a half-hour window on cycle status and once again suggested that most, if not all, of the long-term repopulating stem cells were in active cell cycle. We also addressed the progression of G0 LT-HSC through cell cycle in vivo using repeated injections of BrdU into C57BL mice followed by FACS of LT-HSC labeled with pyronin and Hoechst dye. We found that after 48 hours of injections, over 65% of G0 LT-HSC were labeled and thus had progressed through cycle. Separate studies showed that BrdU injections were not inducing the LT-HSC into cycle. The implications of these studies are profound. If the long-term multi-lineage marrow repopulating cells are a cycling population, it means that the phenotype must be continually changing and that any purification will give a misleading picture of the phenotype of this cell. There is now a huge amount of literature on purified stem cells including studies on second-messenger signaling, receptor-ligand binding, and genetic regulation. Most of these are probably studying a non-representative cell. This form the basis for a new set on tenets for stem cell biology: 1) the stem cell is on a continuum of change, 2) the phenotype is not stable, 3) clonal studies only address the heterogeneity of the population, 4) stem cells need to be studied on a population basis, and 5) the stem cell is a cycling cell.
Why the Discrepancy With Purified Marrow Stem Cells and Whole Marrow?
It appears clear that the purification, which is not random, discards the cycling stem cells that make up the predominant population. Although the stem cell capacity of the lineage-positive and lineage-negative Sca-1- c-kit- populations are quite low on a percentage basis, most of the stem cells are in these populations. This was indicated by previous studies on lineage-negative, rhodamine-low, Hoechst-low stem cells showing that well over 90% of the stem cell capacity is lost with the separation (51). A continuum model is shown with the hierarchical cells included on the continuum (Figure 6).
Fig. 6.
The phenotype of the “stem” cell continually and reversibly changes with passage through cell cycle. (Abbreviations: CLP, common lymphoid progenitor; CMP, common multipotent progenitor; LT-HSC, long-term hematopoetic stem cell; ST-HSC, short-term hematopeotic stem cell; GMP, granulocyte-macrophage progenitor; ERMegPP, megakaryocye erythroid progenitor; MP, multipotent progenitor.)
Put into another context, a model of stem-cell cycle progression is shown in Figure 7. Here, the population is stable but the individual cells are constantly changing in phenotype as they traverse cycle. This is analogous to the prescient analogy of Till et al (2) that CFU-Ss are similar to radioactive nuclei, the population half-life being stable and accurate, but the radioactive decay of individual nuclei unpredictable (Figure 7).
Fig. 7.
Cell cycle continuum model. Each numbered circle is the same cell followed through all cycles. They show reversible changes in phenotype of marrow cells.
Tissue Microvesicles: Cell Fate Changers
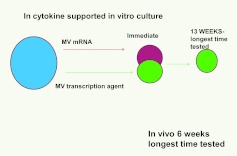
Recently, there was much controversy over the topic of stem-cell plasticity. There were a number of observations that marrow cells engrafted into lethally irradiated mice, and tracked using green fluorescent protein expression or the presence of the Y chromosome in female hosts, could give rise to non-hematopoietic cells in a number of tissues. There was a rather mindless debate centered around whether the phenomena was due to cell fusion, was clonal, or was functional or robust (52). Trans- and de-differentiation were put forward as explanations. We performed a series of experiments in which normal or irradiated lung tissue was co-cultured opposite murine marrow but separated from it by a 0.4-micron cell impermeable membrane (53, 54). We showed that the marrow cells expressed high levels of the lung-specific mRNA: surfactants A–D, clara cell-specific protein, and aquaporin 5, but only if they took up the microvesicles. These microvesicle exposed marrow cells expressed lung-specific protein and showed enhanced conversion to epithelial pneumocytes when transplanted into irradiated mice. For experiments in which rat lung was co-cultured across from mouse marrow, and surfactants B and C were determined with species-specific primers, the mouse marrow expressed high levels of both rat and mouse surfactants B and C mRNA immediately after co-culture; however, after 13 weeks in cytokine-supported culture, only mouse mRNA was detectable. The rat mRNA was quickly degraded in culture. This indicated that a stable, transcriptionally mediated genetic change had occurred in the mouse marrow cells. This is shown in Figure 8.
Fig. 8.
Mouse marrow cells exposed to lung microvesicles were evaluated immediately after exposure and after 13 weeks in cytokine supported culture only mouse surfactant B&C was demonstrable, indicating a transcriptional mechanism underlying the genetic changes.
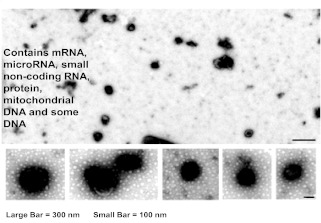
Lung-derived microvesicles are shown in Figure 9. They were found to contain protein, a variety of mRNA species, and some DNA.
Fig. 9.
Lung-derived microvesicles.
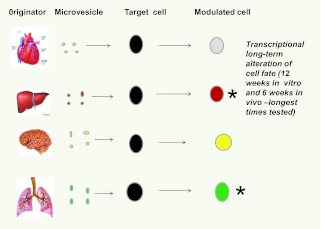
Other studies indicated that tissue-specific mRNA was induced in murine marrow cells exposed to murine heart, liver, and brain tissue (Figure 10).
Fig. 10.
Tissue specificity of microvesicle cell fate modulation. Microvesicles from liver, lung, brain and heart all modulated target marrow cells to express mRNA specific for the originator tissues.
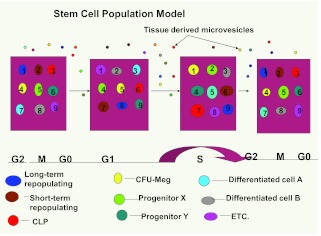
Recent studies also indicated that lung-derived microvesicles entered murine marrow stem/progenitor cells at selected times during cell cycle transit, and that this changed depending on whether the lung tissue was irradiated or not. Thus, a modified hematopoietic model now includes microvesicle modulation of phenotype (Figure 11).
Fig. 11.
Microvesicle modulation of marrow cells on a continuum of cell cycle– related change. A modification of Figure 7.
To the Last Place of Decimals
In the late 1800s, an American physicist, Albert Michelson, echoed the views of many that almost all fundamental facts about physics were now known and that “our future discoveries must be looked for in the sixth place of decimals.” Earlier, James Clerk Maxwell had warned that the real reward for the labor of careful measurement “was the development of new scientific ideas.” It seems that many in the hematopoietic stem cell field think, that with progressive advances in stem cell purification, we have approached the point where we may have to look to the last place of decimals. However, after Michelson's comments, quantum mechanics changed everything. We would propose that the current work on the cell cycle nature of the stem cell and microvesicle cell fate alteration could lead us to quantum stemomics.
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103468. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Benz, Boston: I have just a quick one for you, Peter, on the relative efficiency of repopulating with unfractionated versus purified cells. An alternate explanation might be that it is the long-term cell but that in the presence of later progenitors that we establish a more efficient microenvironment, et cetera, you might get a higher long-term repopulating assay. Have you come up with ways to control for that?
Quesenberry, Providence: That's an interesting point and one of the things we are looking at is how can we address the population as opposed to the purified cell and what you've just described is potentially a way to do that because we have an assay that's transplantation in the lethally irradiated mouse. It's a pretty rigorous assay. So, if you modulate that assay, I think what you could begin to show is the stem cell potential of many other cells that don't show it in that assay. I think that's possible.
REFERENCES
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 2.Till JE, McCulloch EA, Siminovitch L. A Stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci U S A. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–99. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 4.Pluznik DH, Sachs L. The cloning of normal “mast” cells in tissue culture. J Cell Physiol. 1965;66:319–24. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- 5.Long MW, Gragowski LL, Heffner CH, et al. Phorbol diesters stimulate the development of an early murine progenitor cell. The burst-forming unit-megakaryocyte. J Clin Invest. 1985;76:431–8. doi: 10.1172/JCI111990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregory CJ. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976;89:289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- 7.Nakeff A, Dicke KA, Noord van MJ. Megakaryocytes in agar cultures of mouse bone marrow. Ser Haematol. 1975;8:4–21. [PubMed] [Google Scholar]
- 8.Metcalf D, MacDonald HR, Odartchenko N, et al. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci U S A. 1975;72:1744–8. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLeod DL, Shreeve MM, Axelrad AA. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974;44:517–34. [PubMed] [Google Scholar]
- 10.Suda J, Suda T, Ogawa M. Analysis of differentiation of mouse hemopoietic stem cells in culture by sequential replating of paired progenitors. Blood. 1984;64:393–9. [PubMed] [Google Scholar]
- 11.Suda T, Suda J, Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A. 1984;81:2520–4. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suda T, Suda J, Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci U S A. 1983;80:6689–93. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley TR, Hodgson GS. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979;54:1446–50. [PubMed] [Google Scholar]
- 14.Passegue E, Wagers AJ, Giuriato S, et al. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsberg EC, Serwold T, Kogan S, et al. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–26. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–46. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren L, Bryder D, Weissman IL, et al. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci U S A. 2006;103:17807–12. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsberg EC, Bhattacharya D, Weissman IL. Hematopoietic stem cells: expression profiling and beyond. Stem Cell Rev. 2006;2:23–30. doi: 10.1007/s12015-006-0005-z. [DOI] [PubMed] [Google Scholar]
- 19.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–73. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 20.Randall TD, Lund FE, Howard MC, et al. Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood. 1996;87:4057–67. [PubMed] [Google Scholar]
- 21.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SJ, Wandycz AM, Hemmati HD, et al. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–39. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 23.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 24.Cheshier SH, Morrison SJ, Liao X, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–5. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akashi K, Traver D, Miyamoto T, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–7. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 26.Kondo M, Scherer DC, Miyamoto T, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–6. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 27.Kondo M, Scherer DC, King AG, et al. Lymphocyte development from hematopoietic stem cells. Curr Opin Genet Dev. 2001;11:520–6. doi: 10.1016/s0959-437x(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 28.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–6. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto T, Iwasaki H, Reizis B, et al. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–47. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 30.Manz MG, Miyamoto T, Akashi K, et al. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A. 2002;99:11872–7. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arber C, BitMansour A, Sparer TE, et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–8. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 32.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsberg EC, Prohaska SS, Katzman S, et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 35.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Sieburg HB, Cho RH, Dykstra B, et al. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–6. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colvin GA, Berz D, Liu L, et al. Heterogeneity of non-cycling and cycling synchronized murine hematopoietic stem/progenitor cells. J Cell Physiol. 2010;222:57–65. doi: 10.1002/jcp.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dooner MS, Aliotta JM, Pimentel J, et al. Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle. Stem Cells Dev. 2008;17:207–19. doi: 10.1089/scd.2007.0195. [DOI] [PubMed] [Google Scholar]
- 39.Quesenberry PJ, Dooner GJ, Tatto MD, et al. Expression of cell cycle-related genes with cytokine-induced cell cycle progression of primitive hematopoietic stem cells. Stem Cells Dev. 2010;19:453–60. doi: 10.1089/scd.2009.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy GP, McAuliffe CI, Pang L, et al. Cytokine receptor repertoire and cytokine responsiveness of Ho(dull)/Rh(dull) stem cells with differing potentials for G1/S phase progression. Exp Hematol. 2002;30:792–800. doi: 10.1016/s0301-472x(02)00814-7. [DOI] [PubMed] [Google Scholar]
- 41.Berrios VM, Dooner GJ, Nowakowski G, et al. The molecular basis for the cytokine-induced defect in homing and engraftment of hematopoietic stem cells. Exp Hematol. 2001;29:1326–35. doi: 10.1016/s0301-472x(01)00734-2. [DOI] [PubMed] [Google Scholar]
- 42.Becker PS, Nilsson SK, Li Z, et al. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Exp Hematol. 1999;27:533–41. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 43.Reddy GP, Tiarks CY, Pang L, et al. Cell cycle analysis and synchronization of pluripotent hematopoietic progenitor stem cells. Blood. 1997;90:2293–9. [PubMed] [Google Scholar]
- 44.Colvin GA, Lambert JF, Moore BE, et al. Intrinsic hematopoietic stem cell/progenitor plasticity:inversions. J Cell Physiol. 2004;199:20–31. doi: 10.1002/jcp.10436. [DOI] [PubMed] [Google Scholar]
- 45.Colvin GA, Lambert JF, Carlson JE, et al. Rhythmicity of engraftment and altered cell cycle kinetics of cytokine-cultured murine marrow in simulated microgravity compared with static cultures. In Vitro Cell Dev Biol Anim. 2002;38:343–51. doi: 10.1290/1071-2690(2002)038<0343:ROEAAC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Colvin GA, Dooner MS, Dooner GJ, et al. Stem cell continuum: directed differentiation hotspots. Exp Hematol. 2007;35:96–107. doi: 10.1016/j.exphem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Cerny J, Dooner M, McAuliffe C, et al. Homing of purified murine lymphohematopoietic stem cells: a cytokine-induced defect. J Hematother Stem Cell Res. 2002;11:913–22. doi: 10.1089/152581602321080574. [DOI] [PubMed] [Google Scholar]
- 48.Habibian HK, Peters SO, Hsieh CC, et al. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med. 1998;188:393–8. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters SO, Kittler EL, Ramshaw HS, et al. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–7. [PubMed] [Google Scholar]
- 50.Peters SO, Kittler EL, Ramshaw HS, et al. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23:461–9. [PubMed] [Google Scholar]
- 51.Nilsson SK, Dooner MS, Tiarks CY, et al. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89:4013–20. [PubMed] [Google Scholar]
- 52.Quesenberry PJ, Dooner G, Dooner M, et al. Developmental biology: ignoratio elenchi: red herrings in stem cell research. Science. 2005;308:1121–2. doi: 10.1126/science.1104432. [DOI] [PubMed] [Google Scholar]
- 53.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25:2245–56. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aliotta JM, Pereira M, Johnson KW, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38:233–45. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]