Abstract
Introduction. We conducted a pilot project to test the hypothesis that decreasing insulin concentrations with diazoxide would affect parameters of vitamin D in obese women with and without polycystic ovary syndrome (PCOS).
Materials and Methods. Eight obese women with PCOS and nine matched controls participated in the study. Diazoxide was administered orally 100 mg three times daily for 10 days, and parameters of vitamin D were measured at baseline and end-of-study.
Results. At baseline, women with polycystic ovary syndrome had significantly lower serum 25-hydroxyvitamin D (25[OH]D) levels than controls. After treatment with diazoxide, there were no significant changes in vitamin D parameters when PCOS and control women were evaluated separately. Diazoxide exhibited differential effects on 25(OH)D concentrations in PCOS as compared with normal women (P for interaction =0.045), and serum 25(OH)D levels converged after diazoxide treatment.
Conclusions. Obese women with PCOS had significantly lower serum 25(OH)D levels at baseline than age- and body mass index–matched controls. Short-term administration with diazoxide seemed to have differential effects on 25(OH)D levels in PCOS as compared with control women. Further studies are necessary to confirm this finding.
INTRODUCTION
Recent interest in the non-skeletal actions of vitamin D has led to associations between vitamin D deficiency and a wide spectrum of disorders, including certain cancers (breast, prostate, colon), autoimmune diseases such as type 1 diabetes mellitus and multiple sclerosis, decreased T-cell mediated immune responses, and mortality from cardiovascular disease (1–3). Analysis of the National Health and Nutrition Examination Survey III (NHANES III) data also showed an inverse association between 25-hydroxyvitamin D (25[OH]D) and the risk of metabolic syndrome (4), and a large epidemiologic study showed prospectively that baseline 25(OH)D status was inversely associated with 10-year risk of hyperglycemia, insulin resistance, and metabolic syndrome (5).
Evidence suggests that vitamin D deficiency may also contribute to the insulin resistance observed in women with polycystic ovary syndrome (PCOS), a disorder affecting approximately 5% to 10% of women of childbearing age (6, 7). Insulin resistance and obesity are common features in women who have PCOS (8–10), and the prevalence of hypovitaminosis D (serum 25[OH]D <30 ng/mL) in women with PCOS has been found to be as high as 72.8% (7). A recent study reported that women with PCOS and metabolic syndrome had significantly lower vitamin D levels than women with PCOS who did not meet criteria for metabolic syndrome, and that serum 25(OH)D was an independent predictor of insulin resistance in women with PCOS (7). Furthermore, polymorphisms in the vitamin D receptor gene have been associated with insulin resistance, hyperinsulinemia, and PCOS (11). Interventional studies to determine if these relationships are causal or merely associational are scarce.
The converse relationship — whether hyperinsulinemia per se has an effect on vitamin D — has not been investigated. We performed a pilot study to test the hypothesis that hyperinsulinemia affects vitamin D parameters and reduces serum 25(OH)D levels in obese women with PCOS. To accomplish this, we examined the effects of reducing insulin secretion with diazoxide in obese women with PCOS and in age- and body mass index (BMI)–matched controls. Frozen serum samples from a previously conducted but not yet published study were thawed, and fasting parameters of vitamin D (serum 25-hydroxyvitamin D [25(OH)D], vitamin D binding protein [VDBP], and 1,25-dihydroxyvitamin D [1,25(OH)2D]) were measured at the beginning of the study and again after 10 days of treatment with diazoxide. Measures of glucose, insulin, and androgens were also assessed.
MATERIALS AND METHODS
Study Subjects
The study included eight obese (BMI 30 kg/m2) women with PCOS and nine obese normal women matched on average for age (between 18 and 40 years old) and BMI. PCOS was defined using the 1990 National Institute of Child Health and Human Development (NICHD) conference criteria, i.e., all women had chronic oligomenorrhea (eight or fewer menstrual periods annually) and biochemical hyperandrogenemia (elevated serum total or free testosterone concentration), and secondary causes of androgen excess or ovulatory dysfunction were excluded. Women with PCOS were recruited from Virginia Commonwealth University Health System subspecialty and primary care clinics. All women underwent a standard 75-g dextrose oral glucose tolerance test (OGTT), and those who met the Worth Health Organization criteria for diabetes mellitus (plasma glucose ≥ 200 mg/dL at 2 hours) were excluded from participating. Because of the high prevalence of impaired glucose tolerance in women with PCOS, this was not an exclusion criterion. Women with PCOS who had disorders associated with insulin resistance, such as hypertension or dyslipidemia, were also not excluded as long as they had been on a stable dose of medication for at least 6 months. Women who had used contraceptives within 3 months were excluded from the study.
The normal women were matched on average to the PCOS group for age and BMI. They had regular monthly menses, no clinical evidence of hyperandrogenism, demonstrated normal glucose tolerance on OGTT, and had no history of gestational diabetes or family history of a first-degree relative with diabetes. None of them had disorders linked to insulin resistance (i.e., hypertension or dyslipidemia), nor were they taking any medications known to affect insulin sensitivity.
PCOS women were studied during the equivalent of the follicular phase of the menstrual cycle, as documented by a serum progesterone < 2 ng/mL. Normal women were studied during the mid-follicular phase of the menstrual cycle (days 5–9).
Study Design
All women were interviewed by a dietician, and were given instructions for a balanced mixed diet which was started at least three days before the start of the study and maintained during the study.
Subjects came to the General Clinical Research Center at 0800 h on day 1 after a 12-hour overnight fast. Weight, height, waist-to-hip ratio, and supine blood pressure were measured. Fasting blood samples were drawn at 0800, 0815, and 0830 h and pooled for determination of plasma insulin, glucose, sex steroids (testosterone, androstenedione, dehydroepiandrosterone sulfate [DHEAS], estradiol, progesterone), and sex hormone-binding globulin (SHBG). Vitamin D parameters were later measured from these stored frozen samples. At 0900 h, an OGTT was performed by administering 75 g dextrose orally and collecting blood samples for determination of serum glucose and insulin every 15 minutes for 2 hours; responses were analyzed by calculating the areas under the response curves (AUC) by the trapezoidal rule.
After completing the OGTT, the women were started on diazoxide 100 mg orally three times daily (300 mg/d) for 10 days, a dose that had previously been shown to suppress endogenous insulin release in women with PCOS both in the fasting state and during an oral glucose challenge (1–3), decrease total testosterone, and increase SHBG concentrations (1, 2).
The last dose of diazoxide was taken in the morning on day 12. Afterward, all testing performed at baseline (day 1) was repeated.
Samples were stored at -80° Celsius in the dark until the vitamin D measurements were performed. Vitamin D parameters were analyzed in batch assays using the Virginia Commonwealth University Health System pathology laboratory tandem mass spectrometer (Perkin Elmer, Waltham, MA) for 25(OH)D and enzyme-linked immunosorbent assay from ALPCO (Salem, NH) for VDBP; radioimmunoassay for 1,25(OH)2D was performed by LabCorp (Richmond, VA).
Statistical Analysis
For continuous variables, normality of distribution was visualized by normal quantile plots. All continuous variables were presented as mean values and standard deviations. Baseline comparisons for parametric variables were performed by student's t test. We evaluated whether diazoxide treatment resulted in changes in glucose, insulin, and vitamin D parameters as compared with baseline using the two-sided paired t test. We performed these analyses in all women, and in women with and without PCOS as separate groups. Because we were interested in whether PCOS women had different trends of vitamin D parameters after diazoxide treatment as compared with normal women, we also tested for the interaction between time-trends and PCOS status in a repeated measures analysis. Analyses were performed using JMP 8.0.2 (SAS Institute, Cary, NC), and P values <0.05 were considered significant.
RESULTS
Baseline Characteristics
The control group did not differ from the PCOS group at baseline with regard to BMI, age, or blood pressure (Table 1). Women with PCOS at baseline had higher serum total testosterone levels (P =0.002), and SHBG tended to be lower (P =0.14) and androstenedione higher (P =0.09) than in controls (Table 1). Fasting plasma glucose did not differ significantly between the two groups; however, women with PCOS had significantly greater AUC for glucose (P =0.001; Table 1).
TABLE 1.
Baseline Anthropomorphic and Laboratory Data
| Variable | PCOS | Control | P Value |
|---|---|---|---|
| BMI (kg/m2) | 40.9 +/− 9.9 | 38.4 +/− 6.6 | 0.53 |
| Age (years) | 27.1 +/− 6.7 | 28.0 +/− 8.63 | 0.80 |
| Systolic BP (mm Hg) | 132 +/− 13 | 121 +/− 13 | 0.10 |
| Diastolic BP (mm Hg) | 72 +/− 4 | 70 +/− 5 | 0.34 |
| Testosterone (ng/dL) | 50 +/− 15 | 26 +/− 9 | 0.002 |
| SHBG (nmol/L) | 33.5 +/− 10.8 | 51.7 +/− 31.6 | 0.14 |
| DHEAS (μg/mL) | 1.49 +/− 0.63 | 1.73 +/− 1.18 | 0.62 |
| Androstenedione (ng/mL) | 2.7 +/− 1.5 | 1.7 +/− 0.6 | 0.09 |
| Fasting glucose (mg/dL) | 85 +/− 5.5 | 84 +/− 8.2 | 0.76 |
| Fasting insulin (μIU/mL) | 10.7 +/− 6.0 | 7.2 +/− 2.5 | 0.1331 |
| AUC glucose (mg/dL×min) | 16,447 +/− 1264 | 13,168 +/− 1247 | 0.001 |
| AUC insulin (μIU/mL×min) | 12,466 +/− 13,239 | 6,208 +/− 4347 | 0.22 |
| 25(OH)Vitamin D (ng/dL) | 7.6 +/− 2.02 | 15.6 +/− 7.62 | 0.012 |
| 1,25(OH)2 Vitamin D (pg/mL) | 21.5 +/− 5.29 | 22.9 +/− 5.22 | 0.57 |
| Vitamin D binding protein (mg/dL) | 51.1 +/− 48.18 | 72.9 +/− 67.47 | 0.46 |
Abbreviations: PCOS, polycystic ovary syndrome; BMI, body mass index; BP, blood pressure; SHBG, sex hormone-binding globulin; DHEAS, dehydroepiandrosterone sulfate; AUC, area under the curve.
In women with PCOS, baseline serum 25(OH)D levels were significantly lower than in the control group (P =0.012). The prevalence of vitamin D deficiency, defined as a serum 25(OH)D level < 20 ng/mL, was 100% (8/8 subjects) in the PCOS group versus 55.6% (5/9 subjects) in the control group. Moreover, the prevalence of severe vitamin D deficiency, defined as a serum 25(OH)D level < 10ng/mL, was 87.5% (7/8 subjects) in the PCOS group versus 22.2% (2/9 subjects) in the control group.
Serum VDBP and 1,25(OH)2D levels did not differ significantly between the two groups at baseline (Table 1).
Effects of Diazoxide Administration on Weight, Fasting Serum Insulin and Glucose, and Serum Insulin and Glucose Responses During the OGTT
After treatment with diazoxide, the BMI in the PCOS and placebo groups were 40.8 ± 10.4 kg/m2 and 39.0 ± 6.6 kg/m2, respectively, and had not changed from baseline in either group.
In both groups, fasting serum glucose levels increased significantly after diazoxide (PCOS, P =0.03; control, P =0.05; Table 2). AUC for glucose increased significantly in the control group (<0.04) and the group as a whole (P =0.016; Table 2); in the PCOS group there was a trend toward an increase (P =0.10; Table 2).
TABLE 2.
Change in Glucose, Insulin, and Androgen Levels After Treatment With Diazoxide*
| PCOS |
Control |
All Women |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | P Value | Start | End | P Value | Start | End | P Value | |
| Fasting glucose (mg/dL) | 85 +/− 5.5 | 99 +/− 14 | 0.03 | 84 +/− 8.2 | 92 +/− 11.4 | 0.05 | 85 +/− 6.9 | 95 +/− 13 | 0.003 |
| Fasting insulin (μIU/mL) | 10.7 +/− 6.0 | 9.7 +/− 7.00 | 0.30 | 7.2 +/−2.5 | 7.5 +/ 3.7 | 0.96 | 8.8 +/− 4.7 | 8.4 +/− 5.5 | 0.50 |
| AUC glucose (mg/dL/min) | 16,447 +/− 1,264 | 18,377 +/− 3787 | 0.10 | 13,168+/−1,247 | 14,117+/− 1,753 | <0.044 | 14,711+/− 2,079 | 16,122+/− 3,552 | 0.016 |
| AUC insulin (μIU/mL/min) | 12,466 +/− 13,239 | 5,099 +/− 3813 | 0.08 | 6,208+/−4,347 | 4,158+/− 2,979 | 0.09 | 9,153+/− 9823 | 4,601+/− 3321 | <0.025 |
| SHBG (nmol/L) | 33.5+/−10.8 | 33.4+/−10.7 | 0.9776 | 51.7+/− 31.6 | 59.4+/−35.9 | 0.2274 | 43.1+/−25.2 | 47.2+/−29.5 | 0.2950 |
| Total testosterone (ng/dL) | 49.9+/− 14.8 | 39.1+/−8.3 | 0.0369 | 26.4 +/− 8.6 | 34.6+/−14.3 | 0.0672 | 37.3+/−16.7 | 36.7+/−11.7 | 0.8725 |
Abbreviation: PCOS, polycystic ovary syndrome.
*Comparison between baseline and post-diazoxide levels.
AUC for insulin decreased significantly in the group as a whole (P <0.025), and also trended down in the control and PCOS groups after diazoxide (Table 2). There were no significant changes in fasting insulin levels in either group or in the group as a whole (Table 2).
Effects of Diazoxide Administration on Serum Steroid and SHBG Concentrations
In the PCOS group, testosterone levels decreased significantly after diazoxide (from 49.9 ± 14.8 ng/dL to 39.1 ± 8.3 ng/dL; P =0.036), whereas in the control group testosterone levels did not change (Table 2). There were no significant changes in SHBG levels in either group (Table 2).
Effects of Diazoxide Administration on Vitamin D Parameters and Correlation With Changes in Insulin
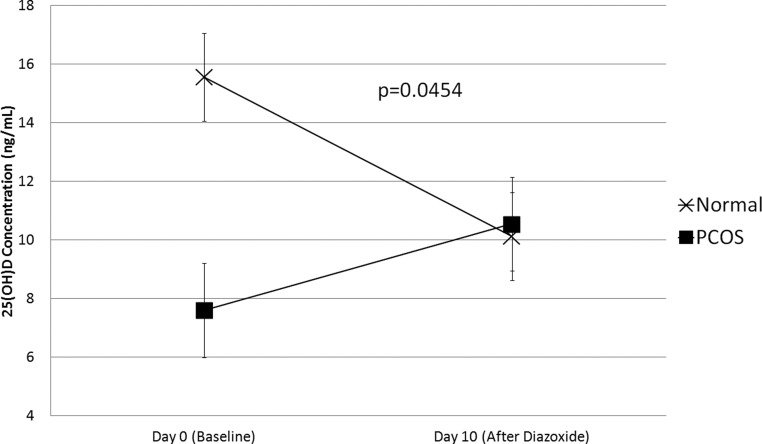
There were no significant changes in serum 25(OH)D, VDBP, or 1,25(OH)2D after diazoxide administration in either group (Table 3). However, we observed a differential effect of diazoxide on 25(OH)D levels in PCOS as compared with control women (Figure 1). Specifically, there was a significant interaction between time trends of 25(OH)D concentrations and PCOS status upon diazoxide administration (P =0.0454; Figure 1).
TABLE 3.
Change in Vitamin D Parameters After Treatment With Diazoxide*
| PCOS |
Control |
|||||
|---|---|---|---|---|---|---|
| Start | End | P Value | Start | End | P Value | |
| VDBP (mg/dL) | 51.05 +/− 48.17 | 46.16 +/− 41.81 | 0.62 | 72.92 +/− 67.47 | 78.49 +/− 83.61 | 0.58 |
| 1,25(OH)2D (pg/mL) | 21.46 +/− 5.29 | 20.44 +/− 9.56 | 0.72 | 22.92 +/− 5.22 | 20.53 +/− 7.53 | 0.32 |
| 25(OH)D (ng/mL) | 7.7 +/− 2.0 | 10.6 +/− 5.4 | 0.21 | 15.6 +/− 7.6 | 10.1 +/− 4.6 | 0.12 |
Abbreviations: PCOS, polycystic ovary syndrome; VDBF, vitamin D binding protein.
*Comparison between baseline and post-diazoxide levels.
Fig. 1.
Serum 25(OH)D levels in PCOS and control women before and after treatment with diazoxide for 10 days. There was a significant interaction between time trends of 25(OH)D concentrations and PCOS status upon diazoxide administration (P =0.0454).
Baseline serum 25(OH)D levels had a near significant negative correlation with AUC for glucose in the group as a whole (P =0.051). VDBP also had a significant positive correlation at baseline with fasting glucose levels in the group as a whole (P =0.0062). Changes in any of the three vitamin D parameters did not correlate with AUC for insulin, BMI, or testosterone in either group (data not shown).
DISCUSSION
It has been reported that the majority of women with PCOS have vitamin D deficiency (7, 12), and that women with PCOS have lower 25(OH)D levels than normal age- and BMI-matched women (13). Others have reported that vitamin D levels were inversely correlated with BMI and insulin resistance, but did not independently correlate with PCOS status (6). In our study, we confirmed that women with PCOS had lower baseline serum 25(OH)D levels than their matched controls. Moreover, we found that the prevalences of vitamin D deficiency and severe deficiency were much higher in the PCOS versus the control group (100% and 87.5%, versus 55.6% and 22.2%, respectively). In our cohort, baseline serum 25(OH)D levels also had a near significant negative correlation with AUC glucose levels (P =0.051), providing further support for the association of insulin resistance and low vitamin D.
Recent investigations (6, 7) have examined a possible role for vitamin D deficiency contributing to the insulin resistance commonly observed in women with PCOS (14). It has been shown that serum 25(OH)D levels are inversely correlated with — and may be an independent predictor of — insulin resistance in women with PCOS (6, 7). Additionally, polymorphisms in the gene for the vitamin D receptor were associated with insulin resistance in women with PCOS (11, 15).
Interventional studies assessing the effects of vitamin D in women with PCOS are scarce. A pilot study by Thys-Jacobs et al. reported that, in a small cohort of women with PCOS and hypovitaminosis D, treatment with calcium and ergocalciferol (to a serum 25[OH]D concentration of 30 to 40 ng/dL) improved menstrual regularity within 2 months of treatment (16). Another group reported improvements in insulin resistance in vitamin D–deficient women with PCOS just 3 weeks after a single large (300,000 units) dose of vitamin D3 (15). Treatment with activated vitamin D (calcitriol; 1,25(OH)2D) was also studied, and was found to increase first phase insulin secretion and improve the lipid profile in obese, vitamin D–deficient women with PCOS (17).
Previously, a study performed by our group found that decreasing insulin levels in obese women with PCOS decreased androgen levels (18). Based on this finding, we speculated that hyperinsulinemia might influence circulating concentrations of vitamin D. Potential mechanisms by which hyperinsulinemia could decrease vitamin D levels include increased turnover of 25(OH)D to 24,25(OH)2D due to upregulation of 24-hydroxylase; increased sequestration of vitamin D in fat stores; shifts in plasma volume; and/or changes in VDBP levels. The results of the present pilot study indicate that short-term suppression of insulin levels by diazoxide in obese women with PCOS and obese normal controls did not significantly change vitamin D parameters in either group. However, it is notable that the difference in serum 25(OH)D levels between the PCOS and control groups (i.e., significantly lower at baseline in the PCOS group) disappeared after treatment with diazoxide due to an apparent differential effect of diaxozide on serum 25(OH)D levels in PCOS versus control women. Whether this represented a true biological change, indicative of an effect of insulin on vitamin D metabolism, or simply a regression to the mean, is unknown at this time.
One limitation of our study is the relatively small number of subjects in this pilot study, which might have limited our power to detect changes in vitamin D levels; although the cohort size was large enough to show a statistically significant change in testosterone levels. Another possible shortfall of the study is that we did not calculate “free” serum 1,25(OH)2D levels (19), but we did measure serum 1,25(OH)2D and VDBP levels, and found no change in these levels when hyperinsulinemia was reduced. Finally, considering that vitamin D is a fat soluble vitamin, and thus may take weeks to months to equilibrate, results could potentially have been different had the study been performed for a longer duration; however, feasible duration of treatment with diazoxide is limited due to side effects.
This study has several strengths. To our knowledge, it was the first study to assess the effects of decreasing insulin levels on vitamin D parameters in women with PCOS. We confirmed that diazoxide: 1) lowers insulin levels; 2) increases glucose levels; and 3) decreases testosterone levels in women with PCOS. We also found, consistent with several recent studies, that baseline serum 25(OH)D levels were significantly lower in women with PCOS than in age- and BMI-matched controls. In addition, we compared the PCOS group with an age- and BMI-matched control group and characterized the metabolic requirements of all subjects with OGTTs.
CONCLUSIONS
In this pilot study, short-term reduction of circulating insulin with diazoxide did not alter vitamin D parameters in women with PCOS or control subjects. However, it is notable that the difference in serum 25(OH)D levels between the two groups at baseline was abolished by diazoxide treatment, suggesting that insulin may influence vitamin D metabolism, perhaps in divergent fashion in PCOS women versus normal women. These results suggest that larger and more long-term studies of an effect of insulin on vitamin D metabolism may be warranted.
ACKNOWLEDGMENTS
Supported in part by NIH grants R01HD35629 and U54HD034449 (J.E.N), and K23HD049454 (K.I.C.), and by award number UL1RR031990 from the National Center for Research Resources.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Lippman, Miami: I really enjoyed that very much. The one thing that I think is interesting, and I think even national guidelines get wrong, is: what are appropriate vitamin D levels? I think, frankly, all of your patients' vitamin D levels are deficient, and therefore, it may well be that you're looking at an extreme end of this variability and you rejected the first question. You wanted the second question to be more interesting, but I'd like it if you would opine about the first question: whether or not substantial vitamin D supplementation would have had a beneficial effect on PCOS or testosterone levels, since lowering insulin is kind of a loser's corner for people who are already diabetic.
Nestler, Richmond: I wasn't trying to suggest that lowering insulin is a way to treat vitamin D deficiency. It was more just an intervention to perturb the system and to find out if lowering insulin has an effect on vitamin D metabolism. I think there is a controversy right now about what constitutes vitamin D deficiency. Some people argue vitamin D levels should be up in the 40s, and some would say that levels in the low 20s are perfectly fine. There are a couple of studies that people have been doing with patients, exploring the effect of vitamin D supplementation on insulin sensitivity or glucose tolerance, and the results have been variable. Some studies have shown an improvement in glucose tolerance with vitamin D supplementation, for example, while others have not. We are actually beginning a clinical study now where we will assess the effects of vitamin D administration on insulin sensitivity, and we will perform hyperinsulinemic-euglycemic studies to more accurately measure insulin sensitivity.
Hochberg, Baltimore: So, you mentioned that you pulled these sera out of the freezer, and obviously your study was not designed to look at vitamin D as the endpoint. So, that raises a number of issues in terms of interpreting the data with regard to dietary intake: whether you collected data on calcium intake, supplement utilization; whether you had measures of renal function; whether you had measures of serum calcium; whether the groups were racially and ethnically balanced (because there's also lower mean 25-hydroxy-D levels in African-American women even after adjusting for body mass index compared to Caucasian women); so, a number of these other factors.
Nestler, Richmond: Correct. So, I can tell you that the groups were racially and ethnically balanced. I don't have information about a number of the other things you mentioned, although keep in mind that this was a 10-day study, so there shouldn't have been much change during the duration of the study in terms of diet or exercise; in fact, the women were specifically instructed not to change their diet or regular routine. So, I don't think that could account for the divergent results between groups. I do not have calcium levels. What we plan to do now, given these results from the pilot study, is to actually do the proper study and measure many of the variables you mentioned as well. Even though this is a freezer study, we did check that the 25-hydroxy-D level would be stable and everything else we measured is supposed to be stable as well.
Hochberg, Baltimore: We've recently measured several thousand 25-hydroxy-D levels on sera that have been in the freezer for 15 years plus. Very stable.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Ajani UA, McGuire LC, et al. Concentration of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–30. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 5.Forouhi NG, Luan J, Cooper A, et al. Baseline serum 25-hydroxyvitamin D is predictive of future glycemic status and insulin resistance. Diabetes. 2008;57:2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn S, Haselhorst U, Tan S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 7.Wehr E, Pilz S, Schweighofer N, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–82. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 8.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 9.Asuncion M, Calvo RM, San Millan JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 10.Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoudi T. Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril. 2009;92:1381–3. doi: 10.1016/j.fertnstert.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Thys-Jacobs S, Donovan D, Papadopoulos A, et al. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroid. 1999;64:430–5. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 13.Wehr E, Trummer O, Guiliani A, et al. Vitamin D associated polymorphisms are related to insulin resistance and vitamin D deficiency in PCOS. Eur J Endocrinol. 2011;164:741–9. doi: 10.1530/EJE-11-0134. [DOI] [PubMed] [Google Scholar]
- 14.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocrine Rev. 1999;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 15.Selimoglu H, Duran C, Kiyici S, et al. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33:234–8. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- 16.Thys-Jacobs S, Donovan D, Papadopoulos A, et al. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64:430–5. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 17.Kalliopi K, Yavropoulou M, Anastasiou O, et al. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Feril Steril. 2009;92:1053–8. doi: 10.1016/j.fertnstert.2008.07.1757. [DOI] [PubMed] [Google Scholar]
- 18.Nestler JE, Barlascini CO, Matt DW, et al. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. JCEM. 1989;68:1027–32. doi: 10.1210/jcem-68-6-1027. [DOI] [PubMed] [Google Scholar]
- 19.Vieth R. Simple method for determining specific binding capacity of vitamin D-binding protein and its use to calculate the concentration of “free” 1,25-dihydroxyvitamin D. Clin Chem. 1994;40:435–41. [PubMed] [Google Scholar]