Abstract
Since 2006, two new vaccines have been licensed to prevent rotavirus, the cause of 20% to 50% of severe acute gastroenteritis in young children worldwide. These vaccines have been implemented in national immunization programs in about 30 high- and middle-income countries, including the United States, and vaccine use has led to substantial decreases in diarrhea-related health care visits. In addition to reductions in diarrhea burden in vaccinated children, decreases have been observed in older, unvaccinated age groups in many settings, suggesting indirect benefits (i.e., herd immunity) from vaccination. Although the efficacy of these oral rotavirus vaccines is expectedly lower in developing countries in Asia and Africa, the public health benefits of vaccination in these settings, where more than 90% of the estimated 453,000 annual deaths from rotavirus occur, are likely to be substantial. Efforts continue to develop alternative rotavirus vaccines that could have a better efficacy and safety profile and may be less expensive.
INTRODUCTION
Acute gastroenteritis is one of the most common illnesses of infants and children worldwide and a major cause of childhood deaths in the developing world (1). As recently as 1970, the etiology of this condition was poorly understood. A few bacterial and parasitic pathogens (e.g., salmonella, cholera, shigella, and ameba) had been identified, but these could be found in fewer than 15% of fecal specimens from children with diarrhea. The remaining cases were assigned “descriptive” diagnoses, e.g., the diarrheas of weaning or malnutrition, “winter vomiting disease,” food allergy, or ”idiopathic diarrhea,‘ which disguised our complete lack of insight into their cause. Viruses were suspected, but no agent had been definitively linked to disease.
This scene changed in 1972 when electron microscopists turned their instruments to examine the stools and intestines of children with acute diarrhea. In 1972, the Norwalk virus was discovered by Kapikian and identified as the first virus to be specifically linked with a diarrheal illness, which was previously known as “winter vomiting disease,” in children (2). In 1973, Ruth Bishop identified an approximately 70-nm, round structured virus by thin section in the gut of Australian children with acute diarrhea (3). This new virus harbored a genome of dsRNA that placed it in the family Reoviridae. It was soon named rotavirus by Thomas Flewett because of its distinct wheel-like appearance (rota) by electron microscopy (EM) (4).
Early investigations demonstrated that rotavirus was common among children with winter diarrhea, but widespread studies were limited to research settings where EM was available. This problem was soon remedied when an enzyme-linked immunosorbent assay (ELISA) was developed that was sensitive, specific, simple, inexpensive, and could be used to detect rotavirus in fecal specimens of children even in the most elementary laboratories around the world (5). An explosion of studies ensued and, within a decade, rotavirus emerged to be the most common cause of severe diarrhea in children worldwide, responsible for 20% to 50% of patients hospitalized with diarrhea (1).
Because rotavirus was found as a pathogen in children in both industrialized and developing countries, it was hard to implicate poor sanitation or hygiene as critical factors in transmission. Rotavirus infected all children worldwide in their first few years of life. However, children in low-income settings were infected earlier in life and their illnesses were more often fatal, primarily because of suboptimal access to medical care. Surveillance of rotavirus among children younger than 5 years of age who were hospitalized with rotavirus diarrhea indicated that in India, approximately 70% to 80% of this severe disease occurred in the first year of life, whereas in the United States, only approximately 40% of disease was reported in infants, and older children up to 5 years of age were also being hospitalized for this infection (6).
In the United States, early surveillance of children hospitalized for diarrhea in Washington, DC, showed that rotavirus was the most common agent detected in more than a third of patients (7). Rotavirus was responsible for the huge annual winter peak of diarrhea, both mild and severe, that was long recognized by pediatricians. However, in 1985–1986, when the Institute of Medicine considered the possible need for a rotavirus vaccine for the world and for the United States, they estimated that rotavirus might be responsible for 873,000 deaths worldwide and that vaccine development should be a clear priority (8). However, they could not justify the need for a vaccine for the United States based on the perceived mild and treatable nature of this disease or evidence of the full burden of disease.
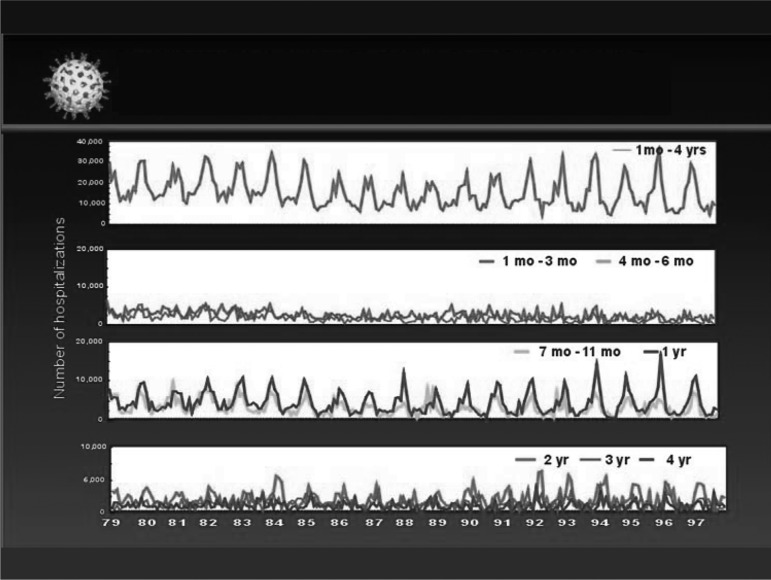
This conclusion led our group at the Centers for Disease Control and Prevention (CDC) to examine the burden of diarrhea hospitalizations and deaths among children younger than 5 years of age in the United States as a first step to assess the full burden of rotavirus disease. The problem was daunting because rotavirus was rarely diagnosed with laboratory testing by physicians because this would incur cost but not alter treatment with oral rehydration therapy. Furthermore, the clinical presentation, i.e., vomiting and diarrhea, was indistinguishable from other forms of childhood diarrhea. To assess the burden of rotavirus disease in the United States, we turned to national data on hospital discharges and mortality freely available from the National Center for Health Statistics, and plotted diarrhea events with a variety of ICD codes among children younger than 5 years of age by month of the year and age over a 20-year period (Figure 1) (9). The graphs indicated that approximately 200,000 children were hospitalized each year for diarrhea, and these admissions showed winter peaks every year in children from 6 months to 3 to 4 years of age. Nationally, these peaks each year were first seen in California and the Southwest in November, and moved across the country reaching New England in March and April. This unusual temporal and geographic pattern was soon matched with data on the detection of rotavirus in hospital laboratories across the country, and thus provided a “fingerprint” to ascertain the contribution of rotavirus to severe diarrhea in US children (10).
Fig. 1.
Diarrhea-associated hospitalizations by month and age among US children younger than 5 years of age from 1979 to 1997.
We soon understood that most of the winter seasonal peak was due to rotavirus. From these and other data, we estimated that in the United States, 40% to 50% of all diarrhea hospitalizations of children younger than 5 years of age was due to rotavirus, approximately 60,000 to 70,000 hospitalizations per year, and these were responsible for 4% to 6% of all hospitalizations of children younger than 5 years of age in American hospitals (11). Moreover, subsequent studies estimated that approximately 500,000 children would visit a clinic, emergency department, or doctor's office for treatment, more than 3 million children would become ill each year, and a small number (fewer than 100) would die of their infections (12). The cost of care approximated $300 million; and the total cost of the disease, including parents' time lost from work, was approximately $1 billion (13). Clearly, the United States had a lot to gain if a vaccine were made available as part of routine childhood immunizations.
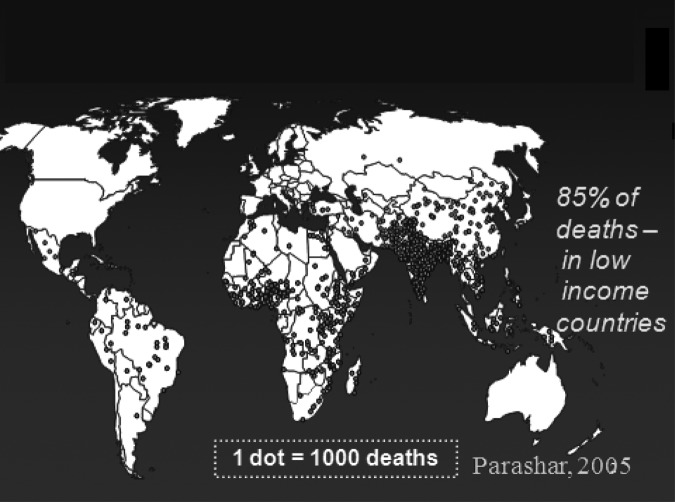
The global burden of disease has since been estimated from many surveys of childhood diarrhea from around the world where rotavirus has been specifically sought as the etiologic agent (Figure 2). With the World Health Organization (WHO), we established sentinel hospital surveillance of children hospitalized with severe diarrhea using a common protocol and a single diagnostic test, EIA, which is now ongoing in six regional networks and includes more than 60 countries (14). From these studies, rotavirus is generally detected in 30% to 55% of all children hospitalized with diarrhea. By extrapolation of these results to data on the global burden of diarrhea deaths in children, we have estimated that approximately 453,000 children die from rotavirus each year (15), making this one of the most common and potentially preventable approaches to improve child survival…if a vaccine were available.
Fig. 2.
Estimated global distribution of the 600,000 annual deaths caused by rotavirus.
DEVELOPMENT OF ROTAVIRUS VACCINES
The search for a rotavirus vaccine began in the early 1980s, when it was clear that rotavirus was the major cause of childhood diarrhea in every setting where laboratory surveillance had been set up. Japanese investigators learned to cultivate the virus, a key step in considering vaccine development (16). Research on the virus determined that there were only a few key serotypes that a vaccine would need to target (17). Epidemiologic evidence indicated that the virus was “democratic,” infecting all children, rich and poor; therefore, interventions to improve water quality, sanitation, or health behavior would be unlikely to change the incidence of the disease. At the same time, good evidence was emerging demonstrating that children developed protective immunity so that a vaccine approach to control might be feasible (18, 19).
The first effort to develop a vaccine was jump-started in the early 1980s by pioneering studies in Belgium and Finland by SmithKline RIT (20). This group developed a first candidate live oral vaccine derived from a single bovine strain of rotavirus that shared no antigens responsible for serotypes with human strains. This candidate vaccine, RIT 4237, was tested in rapid succession in adults for safety and in infants for safety and immunogenicity, and the investigators proceeded directly to a small field trial in Finnish children led by Timo Vesikari. The surprising results of this trial laid down the key features of rotavirus vaccines that have stood the test of time. This simple live oral monovalent vaccine protected infants against rotavirus diarrhea and was more protective against severe disease than against milder disease. Of note, this bovine strain that contained no common neutralizing antigens with human strains protected children against disease caused by a range of human serotypes. Development of this vaccine seemed promising, but as more trial results became available, the efficacy ranged widely when tested at sites in the United States and in several low income countries for reasons that were not fully understood (21). Further development was halted before the reasons for this variability could be assessed. Another animal rotavirus vaccine candidate, based on rhesus monkey rotavirus strain (RRV), gave significant protection only against human strains with the same G serotype, suggesting that vaccines might need to include common human rotavirus serotypes to achieve maximal effectiveness and prompting efforts to develop reassortant vaccines containing human and animal rotavirus genes.
It took another 15 years to develop and bring to licensure the first rotavirus vaccine, Rotashield (Wyeth Lederle), a product derived from strain of rotavirus from a rhesus monkey, RRV, that was naturally attenuated for humans and had been reassorted to include single outer capsid genes from the four most common G serotypes of rotavirus (22). The four strains in this vaccine (the parent rhesus strain and three rhesus-human reassortant strains) were combined as a single tetravalent oral vaccine administered in three doses at the time of routine childhood immunizations, i.e., at 2, 4, and 6 months. Trials showed the vaccine to be safe and effective, protecting American, Finnish, and Venezuelan children against severe rotavirus diarrhea caused by a diversity of serotypes (22–24).
In 1998, Rotashield was licensed in the United States and recommended by CDC for the routine immunization of American children (25). Uptake was rapid, and more than 600,000 infants received the vaccine in the first 9 months after its introduction. Then, a rare and unanticipated adverse event, intussusception, was noted in infants after the first dose of the vaccine (26, 27). Intense epidemiologic investigation showed that this excess was indeed a rare complication of the vaccine that occurred in approximately 1 of 10,000 vaccine recipients. The manufacturer removed the vaccine from production, and use and vaccine development went back to the drawing board. Future live oral vaccines in development all had to deal with this difficult legacy that their vaccines might also be associated with the same risk of intussusception (28).
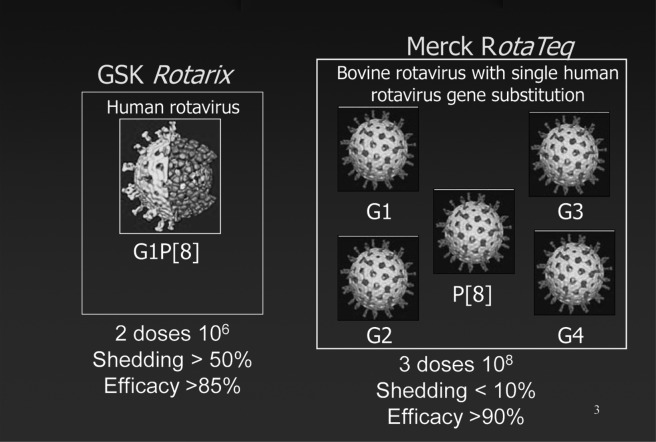
In 2006, after much investigation of the cause of intussusception from Rotashield, two manufacturers, Merck and GSK, each completed development and testing of their distinct live oral rotavirus vaccines (29–31) (Figure 3). The monovalent GSK vaccine, Rotarix, was derived from the single most common serotype of rotavirus infecting humans, serotype G1, that had been attenuated by multiple passage in tissue culture and was delivered as two oral doses (30). The pentavalent Merck vaccine was derived from a single bovine strain that was naturally attenuated for humans and then reassorted with the five most common serotypes of human rotavirus (29). Through reassortment, the novel strains carried the attenuation properties of the bovine strain, but a single outer capsid antigen from each of the five most common human serotypes, four G serotypes and one P serotype. This vaccine was administered in a three-dose oral regimen. To assess a possible risk of intussusception of the level seen with Rotashield from these new live oral vaccines, the United States Food and Drug Administration (FDA) insisted that they be tested in trials involving more than 60,000 infants, an expensive hurdle to consider for vaccine developers (31). Fortunately, in large clinical trials conducted in the United States, Europe, and Latin America, a level of risk similar to that after Rotashield was excluded. Furthermore, these vaccines each proved to be both safe and highly effective in preventing rotavirus diarrhea in infants and could be administered as part of the routine program for childhood immunizations (29–31).
Fig. 3.
New rotavirus vaccines.
VACCINE INTRODUCTION IN THE UNITED STATES
Shortly after completion of the pivotal studies of these two new vaccines, vaccine introduction began in a variety of countries. In the United States, the Merck vaccine was licensed by the FDA in 2006 and was immediately recommended by the Advisory Committee on Immunization Practices of the CDC and the American Academy of Pediatrics for the routine immunization of all American children (12). The vaccine also became part of the Vaccines for Children program of CDC that permitted the government to purchase vaccine. Some feared that uptake might be slow because of the unfortunate legacy left from the withdrawal of Rotashield 7 years before, an event that had energized the anti-vaccine community and might have tainted introduction of these new vaccines. However, within 2 years, approximately 60% of infants were receiving the vaccine on schedule at 2, 4, and 6 months of age (32).
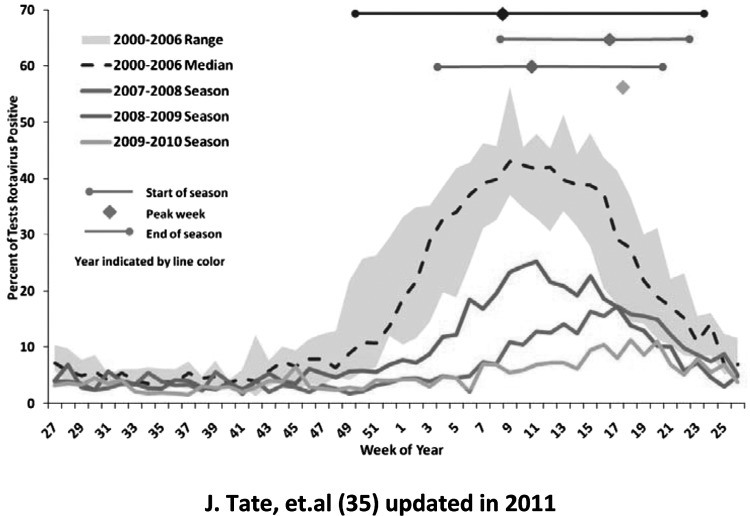
Surveillance activities established at the CDC were most helpful in assessing the early impact of vaccine introduction in the United States. In the 1990s, rotavirus surveillance had been established in hospital laboratories that routinely tested fecal specimens of children admitted for diarrhea and reported their results to the CDC on a weekly or monthly basis (10). By 2008, merely 2 years after vaccine introduction, these laboratories noted the first evidence of the impact of vaccine to reduce hospitalizations of American children with diarrhea (Figure 4) (33). The marked winter seasonal peak of hospitalizations for diarrhea, evidenced as the number of fecal specimens submitted to these laboratories, decreased dramatically, and the proportion of these specimens that were positive for rotavirus decreased markedly as well. Moreover, the annual winter peak of childhood diarrhea occurred later, moving from February-March to April and May, an observation predicted by mathematical models and attributed to removal of susceptible infants from the population, delaying the speed of spread of disease, and increasing the mean age of illness (34, 35).
Fig. 4.
Percentage of rotavirus tests with positive results from NREVSS laboratories by week of year in the United States from June–July 2000–2006, 2007–2008, 2008–2009, 2009–2010. From Tate et al (34), updated in 2011.
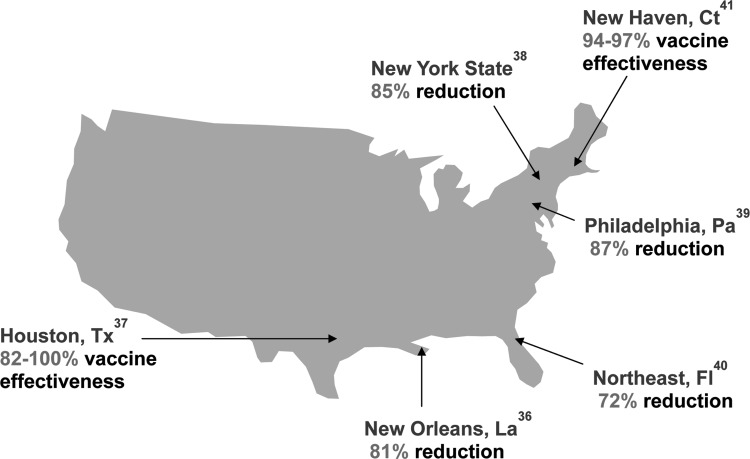
Then, a series of studies from individual hospitals around the country documented a marked reduction in diarrhea and rotavirus hospitalizations and emergency room visits among children younger than 2 to 3 years of age in the same 2008 rotavirus season (Figure 5) (36–41). Examination of data from a large insurance claims database showed a decrease of approximately 40,000 hospitalizations annually in 2008 and 2009, resulting in an annual reduction of about $140 million in treatment costs (42). The same evaluation confirmed that the vaccine was 89% effective in preventing rotavirus-coded hospitalizations in routine use in US children, which compared favorably with the efficacy of 96% in the ideal conditions of the pre-licensure clinical trial. The contribution of rotavirus to severe diarrhea in US children was further shown by the 44% to 59% decrease in hospitalizations and 37% to 48% decrease in emergency room visits for diarrhea from all causes during winter months in vaccinated compared with unvaccinated children.
Fig. 5.
A review of published studies regarding the impact of rotavirus vaccination against rotavirus gastroenteritis in the United States. Studies assessed impact against rotavirus hospitalizations and emergency department visits or all rotavirus healthcare visits among children 2 to 5 years of age (35–40).
In addition to the direct benefits of vaccination that reaffirmed the high efficacy of vaccine seen in clinical trials, unanticipated indirect effects were also noted (43, 44). The data provided several surprises: the marked reduction in hospitalizations for rotavirus diarrhea was greater than that which could be explained by the actual coverage of the vaccine in the population. Furthermore, the decrease in hospitalizations occurred not only in those children who had been vaccinated but also among older age groups that had not received the vaccine. These findings, repeated in larger numbers in the national data, showed that the vaccine was having a distinct herd effect, reducing illness mostly in those children vaccinated, but also in those who remained unvaccinated. Further evidence of this indirect effect came from a clever analysis of hospital discharge data for older children and adults, groups already thought to be immune to rotavirus for which rotavirus would not represent a significant disease risk (45). When winter seasonal hospitalizations for diarrhea of any cause were plotted by age group for older children (e.g., 5 to 14 years old, 14 to 25 years old) and adults, there was also a distinct decrease in events during exactly the same peak rotavirus season months of the year as the decrease noted in those children vaccinated. Because the only intervention had been introduction of rotavirus vaccine in infants, these data suggested that rotavirus remained a significant cause of diarrhea in these older populations as well, and that vaccinating infants prevented spread of infection from the vaccinated infants to their older siblings, contacts, parents, and grandparents. Rotavirus in children, such as influenza virus, was a reservoir of infection for infections in older children and adults. The vaccine was clearly having an additional impact to reduce hospitalizations for diarrhea in these older age groups, representing an additional 15% to 20% benefit to the program in terms of hospitalizations averted in these age groups or costs saved for medical care (46).
IMPACT OF ROTAVIRUS VACCINES IN OTHER COUNTRIES
To date, rotavirus vaccines have been licensed in more than 100 countries and introduced into the routine childhood immunization schedules in more than 30 countries, mostly high- and middle-income countries in the Americas, few European countries, and in Australia. The rate of uptake has been influenced by two key factors, demonstration of the efficacy of the vaccine in low-income settings, and the cost of the vaccine versus the perceived benefits. Live oral vaccines, such as polio, cholera, and typhoid, have performed less well in low-income settings compared with richer countries for reasons that are not fully understood (47). Some data suggests that in low- and middle-income countries, higher titers of transplacental antibodies in the infant, breast feeding at the very time of immunization, an altered microbiome, or micronutrient nutritional (e.g., Zinc or vitamin A) and other host factors (e.g., other diseases such as diarrhea, HIV, and malaria) might be responsible (47). Consequently, the WHO required the vaccine manufacturers to conduct trials in a poor African and Asian setting before they could make a universal recommendation for the use of the vaccines.
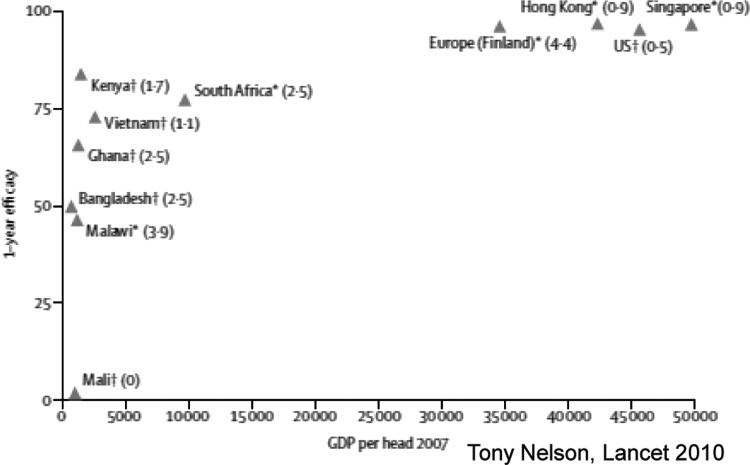
These studies in low-income countries have now been concluded, and both products do work in these populations, but the efficacy varies greatly (Figure 7) (48). For the GSK Rotarix vaccine, the efficacy from trials in South Africa and Malawi ranged from 76% to 49%, respectively, consistent with the observation that the infants in low-income settings have lower immune responses to the same vaccine product (49). For the Merck vaccine, RotaTeq, studies from five countries in Africa and Asia had an overall efficacy of 64%, with values that ranged from 1% in Mali to 83% in Kenya (50, 51). This variability calls for a robust research agenda to understand how to improve the “take” of the vaccine. That being said, the use of these vaccines, even if they are only 50% effective, was seen as a great benefit to protecting the lives of children from severe diarrhea. Consequently, the WHO has recommended rotavirus immunization for all children worldwide (52).
Fig. 7.
Relationship between RV vaccine efficacy and per capita gross income.
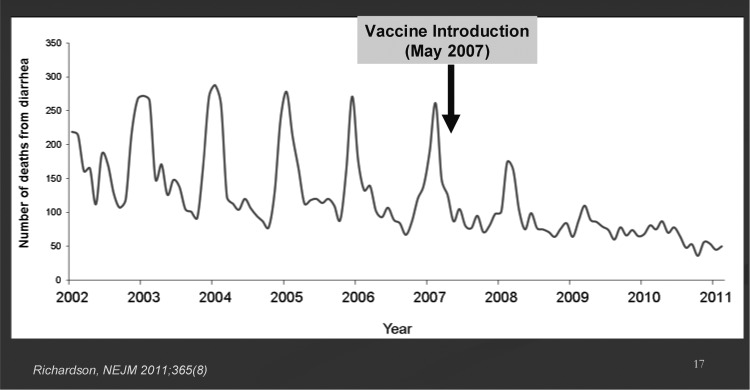
Some impressive data on the impact of these vaccines in Mexico and Brazil have shown what can be achieved in these middle-income settings (Figure 6) (53–55). In Mexico, 3 years after the national introduction of the vaccine, diarrhea mortality has decreased by 39%; and in Brazil, a 22% to 28% decrease has been reported. Additional data is amassing from impact studies in many other settings, most of which show major decreases in hospitalizations for diarrhea due to rotavirus. As yet, rotavirus vaccine is just being introduced into a number of low-income countries in sub-Saharan Africa; therefore, full evaluation of impact on diarrhea mortality will require several years to assess.
Fig. 6.
Sustained decreases in deaths due to diarrhea in Mexico from 2008 to 2010 in children younger than 5 years of age.
The price of rotavirus vaccines has been severely tiered by the companies and has had a major impact on the rate of uptake of the vaccine. In the United States, the price of the vaccines remains at approximately $60 to $100 per dose, or approximately $200 per child vaccinated (56). In Europe, as of 2011, and despite similar assessments of the burden of disease, the vaccine has only been introduced in four countries, in part due to cost-benefit analyses that indicate that the high price of the vaccine does not match the perceived value of medical costs alone for a disease that can be treated and is rarely fatal in these settings. For middle-income countries in Latin America, the Revolving Fund for Vaccine Procurement of the Pan American Health Organization has established a purchase price for vaccine at approximately $15 per child, a level that has permitted the vaccine to be introduced in 14 countries (57). Finally, for low-income countries where 85% of the fatal cases of rotavirus occur, vaccine introduction has just begun. The Global Alliance for Vaccines and Immunizations, GAVI, will provide vaccines for a subsidized price of $0.15-$0.30 per dose for a scaled 5-year introduction, but ministers have feared that after this concessionary period, they will be burdened with paying a newly tiered price for the vaccine of between $5.00 and $15.00 per child (58). Nonetheless, vaccines have been approved for 16 GAVI-eligible countries, including 12 countries in Sub-Saharan Africa, with the hope that the price of vaccines will decrease over the next 5 years to a level that is affordable by even the poorest countries.
FUTURE OUTLOOK
We are at an interesting time in the history of rotavirus vaccine introduction. We have seen tremendous benefits from the vaccine in the United States and a number of middle-income countries where the vaccine has been introduced on a national basis. Clearly, the approximate 50% decrease in hospitalizations for diarrhea in the United States and the 4% to 6% decrease in hospitalizations of children younger than 5 years of age for all causes is having a positive effect on use of our health services, particularly in the winter season (32). The decrease in doctor, clinic, and emergency visits plus mild illness treated at home adds additional value to the program. We are still learning about the indirect and herd effects of vaccine introduction which can only be appreciated after widespread use and assessment. It remains to be seen whether these indirect herd effects will be observed when the vaccines is introduced in low-income countries. Herd effects over time could render vaccination programs more effective than the results of efficacy trials, perhaps by increasing the age of severe disease, thereby decreasing the risk of dying in infancy.
The lower efficacy of live oral vaccines in children in low-income settings remains a real target for research and has led to additional efforts to develop some alternative vaccine strategies (21). One strategy has been to develop a rotavirus vaccine derived from newborn strains of rotavirus that are highly immunogenic even in the presence of high titers of transplacental IgA antibody in the infant, a known inhibitor of vaccine take. Two vaccines based on newborn rotavirus strains, RV3 in Australia (59) and 116E in India (60), are both in clinical trials and could address at least one major impediment to better efficacy. Alternatively, a number of parenteral rotavirus vaccines are in early stage development with the thought that these injected products are independent of the problems posed by oral immunization and could provide a high level of efficacy regardless of the income setting of the child (61). Ultimately, these vaccines could be included in other combination vaccines that would be easier to deliver to all children.
Finally, concerns regarding a risk of intussusception with current rotavirus vaccines linger. Post-marketing safety data from some populations (Mexico, Brazil, and Australia) suggest a low-level increased risk of approximately 1 to 2 cases per 100,000 vaccinated infants, primarily in the first week after the first vaccine dose. An increased intussusception risk has not been documented in the United States to date, but available US data do not allow us to reliably exclude the possibility of a low-level risk seen in other settings. Benefit-risk analyses for several settings, including a US analyses based on a hypothetical risk, show that the well-documented health benefits of vaccination clearly exceed the potential risks and various regulatory and policy committees have made no changes to recommendations for vaccine use. Besides improved efficacy in low-income settings, a parenteral rotavirus vaccine, when available, is likely to also be free of a risk of intussusception.
The field of rotavirus vaccines is rapidly evolving with a robust agenda to introduce vaccines in many countries and monitor the outcome. Research will be imperative to make these vaccines more effective in low-income settings and to understand the indirect effects as these programs unfold. All activities and efforts are now directed towards the ultimate prevention and control of this most common cause of childhood diarrheal illness in all children worldwide.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
DuPont, Houston: Roger, thank you so much for a wonderful presentation, and thank you even more for what you've done to fight this disease of children which causes such important morbidity but very serious mortality in the developing world. You've shown, once more, that biology is complicated. It looked like there were vaccines on the way and that we would be able to eliminate this problem. You've worked with companies, you've gotten prices down, and so that looks good. In Houston, we are seeing as rotavirus goes down, norovirus rates are going up. My question is: are we going to get rid of this virus only to have other viruses such as noroviruses just come in and take its place? Is there some end to this story?
Glass, Bethesda: I think that's a good question. Noroviruses are the next major target for us, especially as virologists, but the fact that hospitalizations have gone down by half in the United States and that diarrhea mortality in Mexico has gone down by 40% is an indication to me that we are removing a large part of the pie of diarrheal morbidity and mortality already. Once this is accomplished, we can address the remaining pathogens including norovirus and some of the other bacterial diseases, including cholera.
Barondess, New York: Dr. Glass, that was a powerful and clear presentation. Thank you. I'm curious about the mechanism of the intussusceptions caused by this vaccine.
Glass, Bethesda: So are we. We looked extensively at the pathologic specimens from children who were operated on for intussusception thinking that we might find hyperplastic lymph nodes at the leading edge of the intussusceptions that would provide a clear mechanism of action to explain these rare events. We did not find this, although our specimens were limited. Consequently, we don't have a clue as to why this happened. However, we did learn that intussusceptions are rare in the first 3 months of life and the incidence increases 10-fold between 3 and 7 months of age. We, therefore, recommended that first doses of the new oral vaccines be administered before an infant reached 90 days of age, and this may explain in part why the live oral vaccines tested subsequently did not have this problem.
REFERENCES
- 1.Kapikian AZ. Viral gastroenteritis. JAMA. 1993;269:627–30. [PubMed] [Google Scholar]
- 2.Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10:1075–81. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;2:1281–3. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- 4.Flewett TH, Bryden AS, Davies H, Woode GN, Bridger JC, Derrick JM. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet. 1974;2:61–3. doi: 10.1016/s0140-6736(74)91631-6. [DOI] [PubMed] [Google Scholar]
- 5.Yolken RH, Wyatt RG, Barbour BA, Kim HW, Kapikian AZ, Chanock RM. Measurement of rotavirus antibody by an enzyme-linked immunosorbent assay blocking assay. J Clin Microbiol. 1978;8:283–7. doi: 10.1128/jcm.8.3.283-287.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresee JS, Hummelman E, Nelson EA, Glass RI. Rotavirus in Asia: the value of surveillance for informing decisions about the introduction of new vaccines. J Infect Dis. 2005;192(Suppl 1):S1–5. doi: 10.1086/431515. [DOI] [PubMed] [Google Scholar]
- 7.Brandt CD, Kim HW, Rodriguez WJ, et al. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol. 1983;18:71–8. doi: 10.1128/jcm.18.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. New Vaccine Development: Diseases of Importance in Developing Countries. Washington: National Academy Press; 1986. The prospects of immunizing against rotavirus; pp. D13-1–D13-12. [Google Scholar]
- 9.Ho MS, Glass RI, Pinsky PF, Anderson LJ. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J Infect Dis. 1988;158:1112–6. doi: 10.1093/infdis/158.5.1112. [DOI] [PubMed] [Google Scholar]
- 10.LeBaron CW, Lew J, Glass RI, Weber JM, Ruiz-Palacios GM. Annual rotavirus epidemic patterns in North America. Results of a 5-year retrospective survey of 88 centers in Canada, Mexico, and the United States. Rotavirus Study Group. JAMA. 1990;264:983–8. doi: 10.1001/jama.264.8.983. [DOI] [PubMed] [Google Scholar]
- 11.Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887–92. doi: 10.1542/peds.2005-2351. [DOI] [PubMed] [Google Scholar]
- 12.Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-12):1–13. [PubMed] [Google Scholar]
- 13.Tucker AW, Haddix AC, Bresee JS, Holman RC, Parashar UD, Glass RI. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 1998;279:1371–6. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- 14.Rotavirus surveillance worldwide - 2009. Wkly Epidemiol Rec. 2011;86:174–6. [PubMed] [Google Scholar]
- 15.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Inaba Y, Shinozaki T, Fujii R, Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69:155–60. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- 17.Kapikian AZ, Hoshino Y, Chanock RM, Perez-Schael I. Jennerian and modified Jennerian approach to vaccination against rotavirus diarrhea using a quadrivalent rhesus rotavirus (RRV) and human-RRV reassortant vaccine. Arch Virol Suppl. 1996;12:163–75. doi: 10.1007/978-3-7091-6553-9_18. [DOI] [PubMed] [Google Scholar]
- 18.Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–7. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–8. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T, Isolauri E, D'Hondt E, Delem A, Andre FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–81. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- 21.Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010;6:532–42. doi: 10.4161/hv.6.7.11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein DI, Glass RI, Rodgers G, Davidson BL, Sack DA. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus Vaccine Efficacy Group. JAMA. 1995;273:1191–6. [PubMed] [Google Scholar]
- 23.Perez-Schael I, Guntinas MJ, Perez M, et al. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997;337:1181–7. doi: 10.1056/NEJM199710233371701. [DOI] [PubMed] [Google Scholar]
- 24.Vesikari T, Ruuska T, Green KY, Flores J, Kapikian AZ. Protective efficacy against serotype 1 rotavirus diarrhea by live oral rhesus-human reassortant rotavirus vaccines with human rotavirus VP7 serotype 1 or 2 specificity. Pediatr Infect Dis J. 1992;11:535–42. doi: 10.1097/00006454-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Glass RI, Bresee JS, Parashar UD, Holman RC, Gentsch JR. First rotavirus vaccine licensed: is there really a need? Acta Paediatr Suppl. 1999;88:2–8. doi: 10.1111/j.1651-2227.1999.tb14318.x. [DOI] [PubMed] [Google Scholar]
- 26.Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- 27.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 28.Glass RI, Bresee JS, Parashar UD, Jiang B, Gentsch J. The future of rotavirus vaccines: a major setback leads to new opportunities. Lancet. 2004;363:1547–50. doi: 10.1016/S0140-6736(04)16155-2. [DOI] [PubMed] [Google Scholar]
- 29.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 31.Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–32. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 32.Glass RI, Patel M, Parashar U. Lessons from the US rotavirus vaccination program. JAMA. 2011;306(15):1701–2. doi: 10.1001/jama.2011.1475. [DOI] [PubMed] [Google Scholar]
- 33.Delayed onset and diminished magnitude of rotavirus activity—United States, November 2007–May 2008. MMWR Morb Mortal Wkly Rep. 2008;57:697–700. [PubMed] [Google Scholar]
- 34.Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science. 2009;325:290–4. doi: 10.1126/science.1172330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate JE, Mutuc JD, Panozzo CA, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30(1 Suppl):S30–4. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 36.Begue RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics. 2010;126:e40–5. doi: 10.1542/peds.2009-2069. [DOI] [PubMed] [Google Scholar]
- 37.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125(2):e199–207. doi: 10.1542/peds.2009-1021. 2010. [DOI] [PubMed] [Google Scholar]
- 38.Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine. 2010;28:754–8. doi: 10.1016/j.vaccine.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 39.Clark HF, Lawley D, Matthijnssens J, DiNubile MJ, Hodinka RL. Sustained decline in cases of rotavirus gastroenteritis presenting to the Children's Hospital of Philadelphia in the new rotavirus vaccine era. Pediatr Infect Dis J. 2010;29:699–702. doi: 10.1097/INF.0b013e3181d73524. [DOI] [PubMed] [Google Scholar]
- 40.Custodio H, Masnita-Iusan C, Wludyka P, Rathore MH. Change in rotavirus epidemiology in northeast Florida after the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2010;29:766–7. doi: 10.1097/INF.0b013e3181dbf256. [DOI] [PubMed] [Google Scholar]
- 41.Desai SN, Esposito DB, Shapiro ED, Dennehy PH, Vazquez M. Effectiveness of rotavirus vaccine in preventing hospitalization due to rotavirus gastroenteritis in young children in Connecticut, USA. Vaccine. 2010;28:7501–6. doi: 10.1016/j.vaccine.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes JE, Curns AT, Tate JE, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med. 2011;365:1108–17. doi: 10.1056/NEJMoa1000446. [DOI] [PubMed] [Google Scholar]
- 43.Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis. 2011;53:245–53. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 44.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128(2):e267–e75. doi: 10.1542/peds.2010-3722. [DOI] [PubMed] [Google Scholar]
- 45.Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204:980–6. doi: 10.1093/infdis/jir492. [DOI] [PubMed] [Google Scholar]
- 46.Glass RI. Unexpected benefits of rotavirus vaccination in the United States. J Infect Dis. 2011;204:975–7. doi: 10.1093/infdis/jir477. [DOI] [PubMed] [Google Scholar]
- 47.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200(Suppl 1):S39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson EA, Glass RI. Rotavirus: realising the potential of a promising vaccine. Lancet. 2010;376:568–70. doi: 10.1016/S0140-6736(10)60896-3. [DOI] [PubMed] [Google Scholar]
- 49.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 50.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 51.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 52.WHO. Meeting of the Strategic Advisory Group of Experts on immunization, October 2009—conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:518. [PubMed] [Google Scholar]
- 53.do Carmo GM, Yen C, Cortes J, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011;8:e1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 55.Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med. 2010;365:772–3. doi: 10.1056/NEJMc1100062. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control. [Accessed June 22, 2011]. Available at: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm.
- 57.de Oliveira LH, danovaro-Holliday MC, Sanwogou JN, Ruiz-Matus C, Tambini G, Andrus J. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr Infect Dis. 2011;30(Suppl):s61–6. doi: 10.1097/INF.0b013e3181fefdd6. [DOI] [PubMed] [Google Scholar]
- 58.Gavi Alliance. [Accessed June 22, 2011]. Available at: http://www.gavialliance.org/media_centre/press_releases/vaccine_prices.php.
- 59.Barnes GL, Lund JS, Mitchell SV, et al. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine. 2002;20:2950–6. doi: 10.1016/s0264-410x(02)00235-9. [DOI] [PubMed] [Google Scholar]
- 60.Bhandari N, Sharma P, Taneja S, et al. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200:421–9. doi: 10.1086/600104. [DOI] [PubMed] [Google Scholar]
- 61.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–8. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]