Abstract
The gut contains the bulk of the body's serotonin (5-hydroxytryptamine, 5-HT); nevertheless, the physiological role that enteric 5-HT plays has not been determined. 5-HT is linked to gastrointestinal (GI) motility; increased intraluminal pressure causes enterochromaffin (EC) cells to secrete 5-HT, which stimulates intrinsic primary afferent neurons that initiate peristaltic reflexes. 5-HT is also an enteric neurotransmitter. Surprisingly, deletion of tryptophan hydroxylase-1 (TPH1), upon which 5-HT biosynthesis in EC cells depends, does not alter constitutive GI motility, whereas deletion of TPH2, upon which biosynthesis of neuronal 5-HT depends, slows intestinal transit and accelerates gastric emptying. TPH1 deletion, however, protects mice from experimental inflammation; 5-HT potentiation and TPH2 deletion each make inflammation more severe. Neuronal 5-HT is neuroprotective and recruits stem cells to give rise to new enteric neurons in adult mice. Mucosal 5-HT, therefore, may mobilize inflammatory effectors, which protect the gut from invasion, whereas neuronal 5-HT shields enteric neurons from inflammatory damage.
INTRODUCTION
The gut contains far more serotonin (5-hydroxytryptamine; 5-HT) than does any other organ of the body (1). Although brain 5-HT has received much more attention than that in the bowel because of its seeming involvement in everything that makes life worthwhile, it is reasonable to assume that the massive enteric pool of 5-HT evolved for a purpose. Exactly what that purpose is, however, is still not entirely clear. This uncertainty does not reflect a lack of actions of 5-HT on the gut. In fact, it can be said that no pharmacologist ever went broke throwing 5-HT at the bowel. 5-HT does many things to the gut when applied in vitro or in vivo because almost all of the many subtypes of 5-HT receptor are expressed in the gut (1–3). The problem with determining which of the many actions of applied 5-HT are physiologically significant is that the application of exogenous 5-HT, or even the experimental evocation of the release of endogenous 5-HT, reveals what 5-HT can or might do in the gut, not what 5-HT actually does under physiological circumstances. Recent observations allowing identification of some of the functions that actually are 5-HT–dependent have been surprising.
5-HT BIOSYNTHESIS IN THE GUT
To comprehend recent discoveries concerning the roles that 5-HT plays in the drama of enteric behavior, it is first necessary to understand that there are two quite different 5-HT depots (1). 5-HT biosynthesis depends on an initial conversion of the amino acid L-tryptophan to 5-hydroxytryptophan, which the rate-limiting enzyme, tryptophan hydroxylase (TPH) mediates. A second, highly ubiquitous enzyme, aromatic L-amino acid decarboxylase, then rapidly converts 5-hydroxytryptophan to 5-HT. There are, however, two TPH molecules that are separate gene products. In one enteric 5-HT depot, 5-HT biosynthesis depends on tryptophan hydroxylase 1 (TPH1), whereas in the other, 5-HT biosynthesis depends on tryptophan hydroxylase 2 (TPH2). TPH1 is present in the enterochromaffin (EC) cells of the mucosal epithelium (Figure 1) and in mast cells (of mice and rats) (4–6), whereas TPH2 is located in neurons of the central (CNS) (7, 8) and enteric (ENS) nervous systems (Figure 1) (4, 9). As a result, TPH1 deletion (TPH1KO) does not alter the content of 5-HT in the brain, but reduces that in the gut to ∼2% of control. In contrast, deletion of TPH2 (TPH2KO) virtually eliminates brain 5-HT, but hardly alters the total 5-HT content of the gut at all. This is because the EC cell pool of 5-HT dwarfs that in enteric neurons (1). Mucosal 5-HT, moreover, does not reach or act directly on myenteric neurons (Figure 2) (10, 11). The two 5-HT compartments are kept apart (12). Most myenteric neurons, nevertheless, are 5-HT–responsive (13–15) and 5-HT is an enteric neurotransmitter (of myenteric interneurons) (1, 9, 16). The disparity in the sizes of the two 5-HT compartments creates a temptation to ignore enteric neuronal 5-HT; however, the size of a package does not necessarily determine its importance. Both 5-HT depots, moreover, are endowed with the critical molecule that inactivates free 5-HT after it has stimulated its receptors. The plasmalemmal serotonin transporter (SERT) is expressed both in the ENS (17) and in enterocytes of the intestinal mucosa (18–21); inhibition of SERT or its genetic deletion (SERTKO) thus potentiates mucosal and neural actions of 5-HT (22–26).
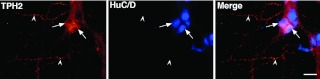
Fig. 1.
TPH2 immunoreactivity (red) is present in neurons of the myenteric plexus of the mouse intestine. Antibodies to HuC/D (blue) are used as a neuronal marker and reveal the cell bodies of all neurons. Double label immunoreactivity demonstrates both antigens in the same sections. Left panel: TPH2 immunoreactivity. Center panel: HuC/D immunoreactivity. Right panel: Merged image. Note that TPH2 is found in 2 neurons (arrows) but not in all and that it also extends into varicose axons running in interganglionic connectives (arrowheads). The marker = 25 μm.
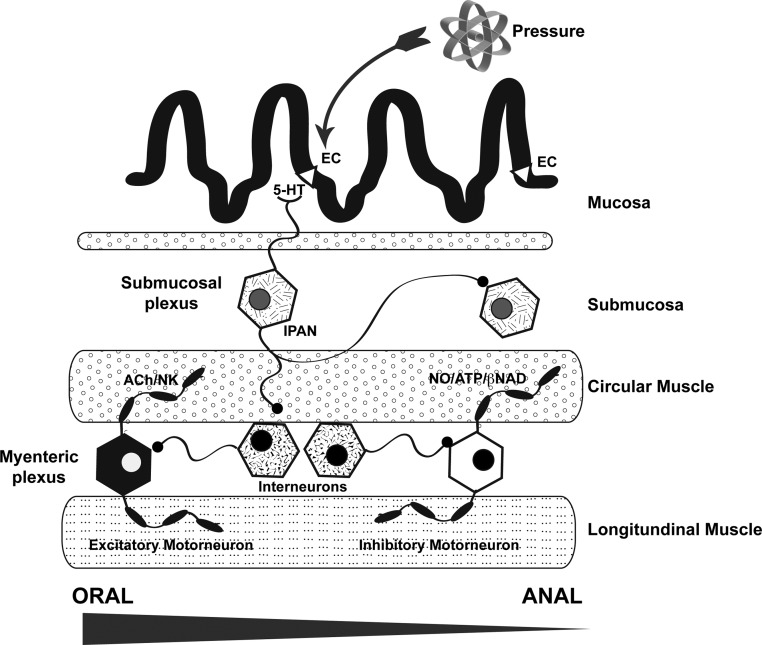
Fig. 2.
A cartoon showing the layers of the bowel wall and the cells thought to be active in mediating the peristaltic reflex. Increased intraluminal pressure initiates the secretion of 5-HT from EC cells in the epithelium of the intestinal mucosa. 5-HT stimulates the mucosal projections of intrinsic primary afferent neurons (IPANs). Although these cells are found in both the submucosal and myenteric plexuses, only a submucosal IPAN is shown. IPANs activate ascending and descending interneurons, which in turn stimulate orad excitatory and distal inhibitory motor neurons, respectively. The interneurons enable the peristaltic reflex to manifest ascending excitation and descending inhibition of smooth muscle. Excitatory motor neurons use acetylcholine (ACh) and/or a tachykin to trigger contraction of the circular muscle, whereas inhibitory motorneurons use NO and/or a purine (β-NAD or ATP) to evoke muscle relaxation. Most interneurons are cholinergic and activate nicotinic receptors; however, 5-HT is also a transmitter of descending myenteric interneurons.
FUNCTIONS OF THE TWO ENTERIC 5-HT DEPOTS
The availability of TPH1KO, TPH2KO, and double knockout (TPH1/2dKO) mice makes it possible to investigate the functions of the two enteric 5-HT depots (3). TPH1KO animals lack mucosal 5-HT but retain neuronal, TPH2KO mice lack neuronal 5-HT but retain mucosal, whereas 5-HT1/2dKO animals, which are very irritable, have no 5-HT at all. We have studied parameters of GI motility, inflammation, and ENS development in each of these mice. We expected that the peristaltic reflex and propulsive motility would be deficient in TPH1KO mice. Those expectations were based on the evidence that pressure and chemical stimuli release 5-HT from EC cells, which then activates peristaltic reflexes (23, 27–31). The idea, illustrated in Figure 2, is that stimulated EC cells secrete 5-HT that acts on the mucosal projections of intrinsic primary afferent neurons (IPANs) (27). These cells, in turn, synapse with ascending and descending interneurons, which finally activate, respectively, excitatory (cholinergic/neurokinin-containing) and inhibitory (nitrergic/purinergic) motor neurons that are responsible for the oral contraction and anal relaxation of the peristaltic reflex (1, 32). The peristaltic reflex increases propulsion of luminal contents. The reflex has been known since Bayliss and Starling, at the end of the nineteenth century, first described that behavior of the bowel, termed it the “law of the intestine,” and attributed the activity to the “local nervous mechanism” of the gut (33–35). Bayliss and Starling made this attribution because they found that elevated intraluminal pressure would evoke a reproducible oral contraction and anal relaxation of a dog's intestine in vivo, even when they cut all of the intestine's extrinsic innervation. Trendelenburg, in 1917, changed the name of the phenomenon from Bayliss and Starling's awe-inspiring “law of the intestine” to the more prosaic “peristaltic reflex” and demonstrated that it can be evoked in vitro (a condition in which the brain, spinal cord, and sensory ganglia are all absent) (36). That demonstration leaves little doubt that Bayliss and Starling had been correct in their attribution of the peristaltic reflex to the “local nervous mechanism” of the gut, which is now called the ENS.
The early work on the peristaltic reflex was popularly linked to 5-HT as a result of a pioneering series of experiments that Bülbing et al. performed with isolated preparations of guinea pig ileum (10, 30, 37–39). These experiments linked 5-HT, seemingly inextricably, to the peristaltic reflex and the stimulation of intestinal propulsion (Figure 2). Carcinoid tumors, moreover, which are derived from EC cells and secrete copious amounts of 5-HT, are also associated with severe diarrhea and enhanced peristaltic activity (40). Some of the same stimuli that cause EC cells to secrete 5-HT activate carcinoid tumor cells to do the same (41). 5-HT3 receptor antagonists oppose carcinoid diarrhea (42), supporting the idea that mucosal 5-HT secretion promotes motility. 5-HT4 agonists, furthermore, are prokinetic (43) and were useful in the treatment of chronic constipation and non-diarrhea–predominant irritable bowel syndrome (IBS) before they were removed from the market for safety reasons that were unrelated to mimicry of responses of the gut to 5-HT (44, 45). It therefore came as a surprise when we discovered that total gastrointestinal transit time (GIT), gastric emptying, small bowel transit, and colonic motility were unchanged in TPH1KO mice (Figure 3) (3). Those observations suggest that if mucosal and mast cell stores of 5-HT are empty, the constitutive motility of the bowel is not different from that in mice in which these stores are full.
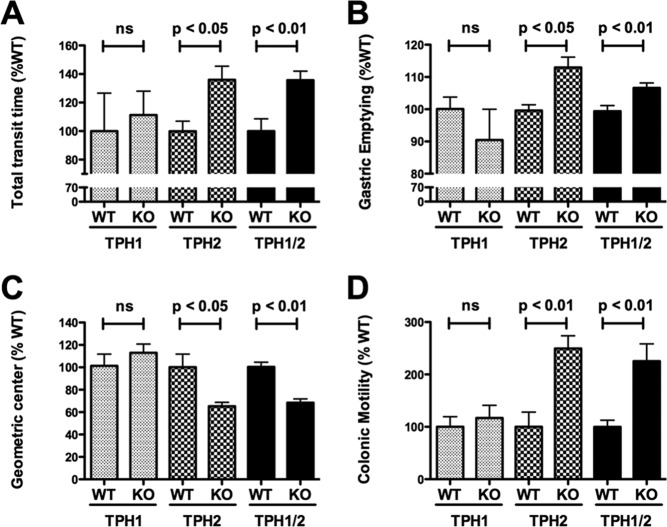
Fig. 3.
Total GI transit time, small intestine transit, and colonic motility are decreased in TPH2KO and TPH1/2dKO mice; however, gastric emptying is accelerated in the same animals. (A) Carmine red was administered orally and transit time was considered as the time required for the red color to appear in feces. GI transit time in TPH1KO, TPH2KO, and TPH1/2dKO mice was compared, respectively, to that of the wild-type littermates of each of these types of mouse. Total GI transit time was increased (slower than littermates) in TPH2KO and TPH1/2dKO animals but not in TPH1KO mice. Total GI transit time in TPH1/2dKO animals was not significantly different from that in TPH2KO mice. (B) Gastric emptying was measured after rhodamine dextran gavage. The proportion of gavaged rhodamine dextran emptying from the stomach in 15 minutes was compared in TPH1KO, TPH2KO, and TPH1/2dKO mice and their respective wild-type littermates. Gastric contents emptied to a greater extent in TPH2KO and TPH1/2dKO, but not in TPH1KO, animals than in their littermates. Gastric emptying in TPH1/2dKO animals was not significantly different from that in TPH2KO mice. (C) Small intestinal transit was evaluated as the geometric center of rhodamine dextran in the small bowel. Geometric centers of 1 to 10 represent slow to fast small intestine transit. Small intestinal transit was significantly slower in TPH2KO and TPH1/2dKO but not in TPH1KO animals than in their respective wild-type littermates. Small intestinal transit in TPH1/2dKO animals was not significantly different from that in TPH2KO mice. D. Colonic motility was estimated as the time for expulsion of a glass bead pushed into the rectum a distance of 2 cm from the anal verge. This time was significantly greater (slower motility) in TPH2KO and TPH1/2dKO but not in TPH1KO animals than in their respective wild-type littermates. Expulsion time in TPH1/2dKO mice was not significantly different from that in TPH2KO mice. (ns = not significant.) (From Li et al. [3].)
DEPENDENCE OF GI MOTILITY ON NEURONAL 5-HT
In contrast to GI motility in TPH1KO mice, motility in TPH2KO animals is strikingly abnormal (Figure 3) (3). Gastric emptying accelerates significantly in TPH2KO mice, but small bowel and colonic motility slows so substantially that the net effect is that GIT is significantly longer in TPH2KO mice than in their wild-type (WT) littermates. Motility in TPH1/2dKO mice is not significantly different from that of TPH2KO animals. Our observations that GIT, small bowel transit, and colonic motility are abnormally slow in TPH2KO mice are consistent with prior suggestions that 5-HT mediates propagating contractile complexes (9), as well as slow (46–48) and fast excitatory synaptic transmission (13). The seemingly paradoxical acceleration of gastric emptying in TPH2KO and TPH1/2dKO mice is consistent with prior reports that 5-HT participates in vagal inhibition of the stomach (49) and that gastric emptying accelerates when 5-HT1P receptors are antagonized (50). These data support the hypothesis that 5-HT functions in vagal gastric accommodation reflexes (51); furthermore, 5-HT3 agonists (52) and fenfluramine, which releases endogenous 5-HT (53, 54), each retard gastric emptying. These observations suggest that neuronal 5-HT excites gastric inhibitory neurons, which promote accommodation and delay gastric emptying.
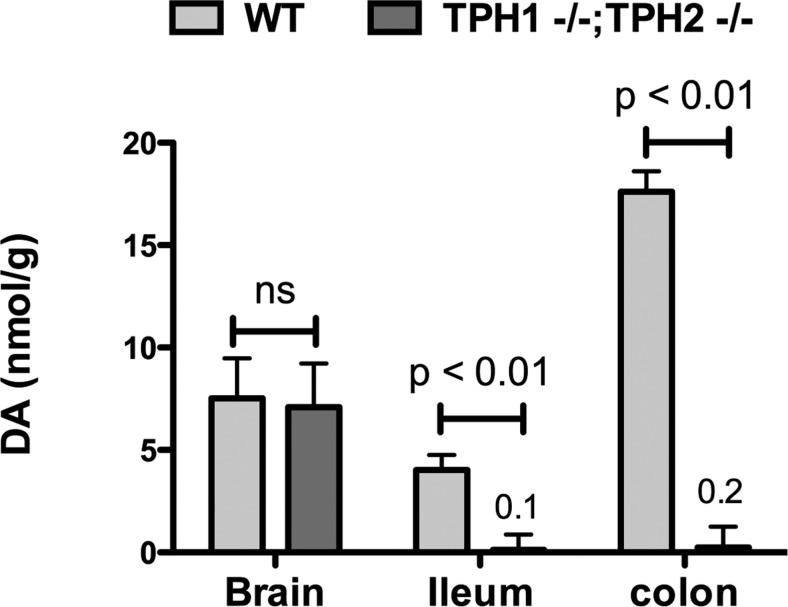
The most parsimonious explanations of data from TPH1KO and TPH2KO mice are that the large amount of 5-HT in EC cells does not affect constitutive motility of the bowel whereas the small amount of neuronal 5-HT not only does so but is indispensable (3). It is, of course, possible, even likely, that situations arise that are not applicable to the unstressed gut of mice living in Institutional Animal Care and Use Committee–approved animal quarters, in which 5-HT from EC cells is mobilized to alter transit and other functions of the bowel. It is also possible that the very large amount of 5-HT in EC cells serves purposes that are not related to GI motility. A caveat about the effects of an absence of neuronal 5-HT is that we have discovered that neuronal 5-HT is a growth factor for neurons of the ENS as well as a neurotransmitter (55). The total numbers of enteric neurons, as well as specific subsets expressing gamma-aminobutyric acid, NO, and calcitonin gene-related peptide (CGRP), are significantly reduced in the ENS of TPH2KO mice; moreover, dopaminergic neurons are virtually absent in the TPH2KO bowel (Figure 4) (3). 5-HT also promotes development of dopaminergic neurons when added to cultures of isolated enteric neural crest-derived precursors. Specific metabolites of dopamine, including dihydroxyphenylactic acid (DOPAC) and homovanillic acid (HVA), furthermore, are increased in SERTKO mice, suggesting that dopaminergic neurons receive a serotonergic innervation and depend on it for development and/or survival. The ENS of TPH2KO mice is therefore deficient in more properties than just its content of 5-HT, which complicates explanations of the abnormal motility of the TPH2KO intestine. Slow motility could as easily be due to an inadequate number of enteric neurons as to the absence of serotonergic neurotransmission.
Fig. 4.
Dopamine is depleted in the intestines but not in the brains of mice lacking TPH2. Levels are shown for TPH1/2dKO animals and their wild-type littermates; however, the level of dopamine in the gut of TPH1KO mice is not significantly different from that of wild-type animals. The levels of dopamine in the bowel of TPH2KO mice are not significantly different from those of the TPH1/2dKO mice that are shown.
5-HT AND INTESTINAL INFLAMMATION
One purpose, other than triggering peristaltic reflexes, that is served by mucosal 5-HT appears to be to promote intestinal inflammation. Experimentally induced colitis is less severe in TPH1KO mice than in their WT littermates (6). Amplifying the action of 5-HT exerts the opposite effect and makes colitis more severe than in the respective WT littermates (56). Amplification of the effects of 5-HT was studied in SERTKO mice, which lack a high affinity mechanism to take up extracellular 5-HT. In SERTKO animals, the inactivation of released 5-HT is compromised. Enterocytes express SERT (19, 20), which removes 5-HT from extracellular fluid and thus helps to terminate the actions of mucosal 5-HT. Serotonergic neurons also express SERT; however, neuronal 5-HT is not pro-inflammatory, but anti-inflammatory. The severity of colitis is more severe in TPH2KO mice than in WT controls. We found, for example, that the mortality in TPH2KO mice due to dextran sodium sulfate–induced (DSS; 5% in drinking water for 7 days) colitis, was ∼ 80%, whereas that in WT littermates was only ∼20%; P <0.05; Fisher's exact test); moreover, the clinical scores, a quantitative evaluation of the severity of colitis, were approximately twice as great in TPH2KO mice subjected to DSS-induced colitis than in WT littermates (P <0.05) and the colonic levels of the pro-inflammatory cytokines, interleukin-1β, interleukin-6, and tumor necrosis factor-α were all significantly higher in TPH2KO than WT mice (P <0.05). Promotion of inflammation by the deletion of neuronal 5-HT in TPH2KO mice could be related to the slowing of intestinal motility in those animals; however, because we have found that 5-HT is neuroprotective, inflammation-induced neuronal damage (57) may exacerbate the effects of inflammation in TPH2KO mice. Inflammation of the bowel is certainly counterproductive when it occurs abnormally in individuals with inflammatory bowel disease (IBD) (58); however, it also might be beneficial in helping to prevent or counter microbial invasion (59). Inflammation, even when needed to fight infection, can be damaging to enteric neurons (57); thus, a means of protecting the ENS from the destructive force of inflammation would be useful. Neuronal 5-HT may do this.
5-HT AND NEUROGENESIS IN INTESTINES OF ADULT MICE
The post-natal growth of the ENS from infancy to maturity depends on the addition of new neurons from a retained population of neural crest-derived stem cells (60). This accretion of neurons does not occur when 5-HT4 receptors are deleted (5-HT4KO). Stimulation of 5-HT4 receptors also protects enteric neurons from apoptotic death, which occurs when enteric neurons are isolated, and inhibits inflammation-induced axon terminal degeneration and autophagy (60). 5-HT4 stimulation, moreover, mobilizes adult stem cells to form new neurons that might replace neurons damaged or killed by inflammation. One source of such cells is an extraganglionic niche that contains inactive stem cells. 5-HT4 stimulation induces these cells to proliferate, then withdraw from the cell cycle and ultimately turn into new neurons that migrate to and become integrated into intestinal ganglia. More recent studies have shown that new enteric neurons can also arise from glial precursors in the adult ENS, at least in vitro (61, 62); however, the experiments that suggest that glia can give rise to neurons have used culture conditions that include the use of enriched media, which contain 5-HT. It is thus conceivable that 5-HT stimulates glia, as well as extraganglionic stem cells, to undergo neurogenesis (63).
CONCLUSIONS
Observations made in TPH1KO, TPH2KO, SERTKO, and 5-HT4KO mice are consistent with the ideas that 5-HT is both a sword and a shield of the gut. Mucosal 5-HT wields the sword. EC cells secrete 5-HT when the bowel is threatened. This release of 5-HT promotes an inflammatory offensive that protects against invasion but would cause collateral damage if the ENS were not to be protected. Neuronal 5-HT wields the shield because it is neuroprotective and it can activate 5-HT4 receptors to induce neurogenesis. Thus, the two sources of 5-HT exert effects that are seemingly antagonistic, but in reality they are synergistic and allow the gut to have its inflammation and survive it too.
ACKNOWLEDGMENTS
This work was supported by grants NS12969 and NS14447 from the National Institutes of Health.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Zeidel, Boston: Wonderful talk. I want to ask a question in a somewhat related organ, at least topologically, and that's the bladder because there we have an epithelium which is highly specialized. We have a muscle layer underneath and we have lots of innervation. What's interesting is that the motility stimuli are coming from here and not from stretch of the muscle wall from what you are saying and we have recently developed evidence that if you do an epithelial-specific knockout of an integrin in the urothelium that you, in fact, disrupt normal accommodation reflexes in the bladder, and so there seem to be a lot of similarities. It also appears that if you block serotonin activities of the neurons, you can reduce inflammation. The question I have is: when the bowel wall gets infected, is the stimulus coming from the wall itself to bring in the inflammatory mediators because in the bladder the cells get infected and they get full of the E. coli and then they release various factors? We don't know which exactly then cause inflammatory response, so what is the process of that in the bowel? I think we need to do a lot more analogizing between the two tissues.
Gershon, New York: Well, there is a difference between the nervous system of the bladder and the enteric nervous system. That is that in the bladder the critical factors are reflexes that go through the spinal cord, whereas in the enteric nervous system, the critical reflexes are intrinsic. Also, the way it seems to be affecting our postulate is that in the gut, particularly the EC cells have toll-like receptor 4 and toll-like receptor 5 on their surfaces; therefore, the part of the cell that actually projects into the lumen may be sensitive to bacteria. EC cells may thus be able to sense early threats and serotonin, which promotes inflammation, probably by activating dendritic cells. So, that's a mucosal effect. Now, there are other effects as well. I mean, if bacteria get beyond the epithelium, then all bets are off in terms of the sequelae.
Zeidel, Boston: And just how do the EC cells sense pressure or stretch? Do we know?
Gershon, New York: How does it?
Zeidel, Boston: Yeah.
Gershon, New York: We don't know for sure. It is known that if you put pressure on the cells, they depolarize, but other than that I don't know.
REFERENCES
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–G63. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JJ, Wan S, Gershon MD. Expression of two isoforms of tryptophan hydroxylase (TPH1 and TPH2) in human and mouse gut. Gastroenterology. 2004;126(Suppl 2):A411. [Google Scholar]
- 5.Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–30. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–60. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 8.Gutknecht L, Kriegebaum C, Waider J, Schmitt A, Lesch KP. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol. 2009;19:266–82. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–86. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bülbring E, Lin RCY. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis, the local production of 5-hydroxytryptamine and its release in relation to intraluminal pressure and propulsive activity. J Physiol (Lond) 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon MD, Tamir H. Release of endogenous 5-hydroxytryptamine from resting and stimulated enteric neurons. Neuroscience. 1981;6:2277–86. doi: 10.1016/0306-4522(81)90017-8. [DOI] [PubMed] [Google Scholar]
- 12.Gershon MD, Bursztajn S. Properties of the enteric nervous system: limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J Comp Neurol. 1978;180:467–88. doi: 10.1002/cne.901800305. [DOI] [PubMed] [Google Scholar]
- 13.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 14.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–9. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 15.Galligan JJ, Parkman H. Recent advances in understanding the role of serotonin in gastrointestinal motility and functional bowel disorders. Neurogastroenterol Motil. 2007;19(Suppl 2):1–4. doi: 10.1111/j.1365-2982.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 16.Gershon MD. Enteric serotonergic neurones … finally! J Physiol. 2009;587:507. doi: 10.1113/jphysiol.2008.167676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon MD, Altman RF. An analysis of the uptake of 5-hdyroxytryptamine by the myenteric plexus of the small intestine of the guinea pig. J Pharmacol Exp Ther. 1971;179:29–41. [PubMed] [Google Scholar]
- 18.Linden DR, White SL, Brooks EM, Mawe GM. Novel promoter and alternate transcription start site of the human serotonin reuptake transporter in intestinal mucosa. Neurogastroenterol Motil. 2009;21:534–41. e10–1. doi: 10.1111/j.1365-2982.2008.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J-X, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–G48. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 20.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–64. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and IBS. Gastroentrology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Bian X, Patel B, Dai X, Galligan JJ, Swain G. High mucosal serotonin availability in neonatal guinea pig ileum is associated with low serotonin transporter expression. Gastroenterology. 2007;132:2438–47. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel BA, Bian X, Quaiserova-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2007;132:41–7. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand PP, Hu X, Mach J, Bertrand RL. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1228–36. doi: 10.1152/ajpgi.90375.2008. [DOI] [PubMed] [Google Scholar]
- 25.Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil. 2006;18:464–71. doi: 10.1111/j.1365-2982.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 26.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–70. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 27.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil. 2004;16:511–4. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 29.Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007;19(Suppl 2):25–31. doi: 10.1111/j.1365-2982.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 30.Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol (Lond) 1959;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke HJ, Sidhu M, Wang YZ. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol Motil. 1997;9:181–6. doi: 10.1046/j.1365-2982.1997.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 32.Wood JD. Enteric nervous system: sensory physiology, diarrhea and constipation. Curr Opin Gastroenterol. 2010;26:102–8. doi: 10.1097/MOG.0b013e328334df4f. [DOI] [PubMed] [Google Scholar]
- 33.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol (Lond) 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol (Lond) 1900;26:125–38. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol (Lond) 1900;26:107–18. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trendelenburg P. Physiologische und pharmakologische versuche über die dünndarm peristaltick. Naunyn-Schmiedebergs Archiv fur Experimentellan Pathologie Pharmakologie. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- 37.Bülbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol. 1958;13:444–57. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bülbring E, Crema A. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol (Lond) 1959;146:29–53. doi: 10.1113/jphysiol.1959.sp006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Q J Exp Physiol. 1958;43:26–37. doi: 10.1113/expphysiol.1958.sp001305. [DOI] [PubMed] [Google Scholar]
- 40.Ahlman H. Serotonin and carcinoid tumors. J Cardiovasc Pharmacol. 1985;7(Suppl 7):S79–S85. doi: 10.1097/00005344-198500077-00023. [DOI] [PubMed] [Google Scholar]
- 41.Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. 2008;295:G260–72. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 42.Saslow SB, Scolapio JS, Camilleri M, et al. Medium-term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut. 1998;42:628–34. doi: 10.1136/gut.42.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beattie DT, Armstrong SR, Shaw JP, et al. The in vivo gastrointestinal activity of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:139–47. doi: 10.1007/s00210-008-0281-z. [DOI] [PubMed] [Google Scholar]
- 44.Fried M, Beglinger C, Bobalj NG, Minor N, Coello N, Michetti P. Tegaserod is safe, well tolerated and effective in the treatment of patients with non-diarrhoea irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2005;17:421–7. doi: 10.1097/00042737-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Brandt LJ. The FDA's decision-making process: isn't it time to temper the principle of protective paternalism? Am J Gastroenterol. 2008;103:1226–7. doi: 10.1111/j.1572-0241.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 46.Erde SM, Sherman D, Gershon MD. Morphology and serotonergic innervation of physiologically identified cells of the guinea pig's myenteric plexus. J Neurosci. 1985;5:617–33. doi: 10.1523/JNEUROSCI.05-03-00617.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takaki M, Mawe GM, Barasch J, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neurosci. 1985;16:223–40. doi: 10.1016/0306-4522(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 48.Wade PR, Tamir H, Kirchgessner AL, Gershon MD. Analysis of the role of 5-HT in the enteric nervous system using anti-idiotypic antibodies to 5-HT receptors. Am J Physiol. 1994;266:G403–G16. doi: 10.1152/ajpgi.1994.266.3.G403. [DOI] [PubMed] [Google Scholar]
- 49.Bülbring E, Gershon MD. Serotonin participation in the vagal inhibitory pathway to the stomach. Symposium on the biological role of indolealkylamine derivatives. Adv Pharamcol. 1967;6A:323–34. doi: 10.1016/s1054-3589(08)61188-6. [DOI] [PubMed] [Google Scholar]
- 50.Mawe GM, Branchek T, Gershon MD. Blockade of 5-HT-mediated enteric slow EPSPs by BRL 24924: gastrokinetic effects. Am J Physiol. 1989;257:G386–96. doi: 10.1152/ajpgi.1989.257.3.G386. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504(Pt 2):479–88. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coleman NS, Marciani L, Blackshaw E, et al. Effect of a novel 5-HT3 receptor agonist MKC-733 on upper gastrointestinal motility in humans. Aliment Pharmacol Ther. 2003;18:1039–48. doi: 10.1046/j.1365-2036.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 53.Davies RF, Rossi J, 3rd, Panksepp J, Bean NJ, Zolovick AJ. Fenfluramine anorexia: a peripheral locus of action. Physiol Behav. 1983;30:723–30. doi: 10.1016/0031-9384(83)90169-5. [DOI] [PubMed] [Google Scholar]
- 54.Booth DA, Gibson EL, Baker BJ. Gastromotor mechanism of fenfluramine anorexia. Appetite. 1986;7(Suppl):57–69. doi: 10.1016/s0195-6663(86)80052-6. [DOI] [PubMed] [Google Scholar]
- 55.Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010;33:446–56. doi: 10.1016/j.tins.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–95. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 57.Linden DR, Couvrette JM, Ciolino A, et al. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil. 2005;17:751–60. doi: 10.1111/j.1365-2982.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 58.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 59.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–99. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–24. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joseph NM, He S, Quintana E, Kim YG, Nunez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121:3398–411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gershon MD. Behind an enteric neuron there may lie a glial cell. J Clin Invest. 2011;121:3386–9. doi: 10.1172/JCI59573. [DOI] [PMC free article] [PubMed] [Google Scholar]