Abstract
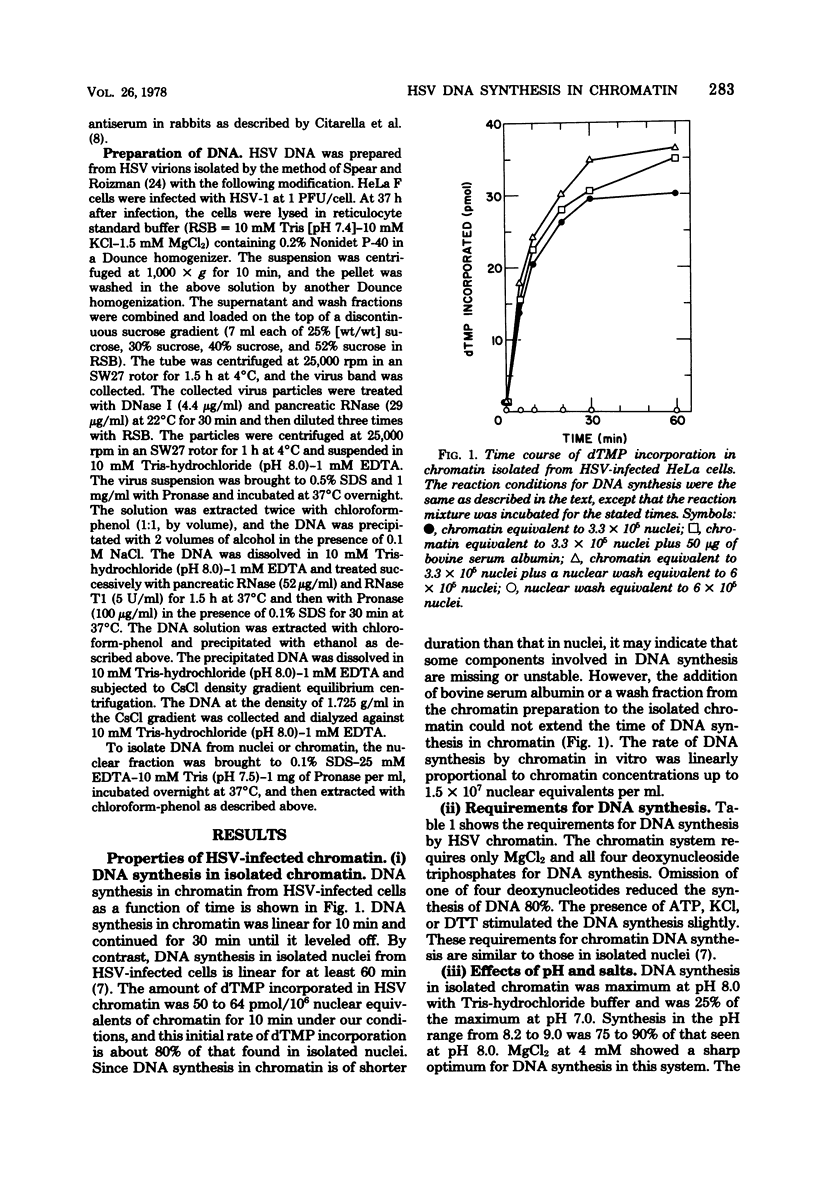
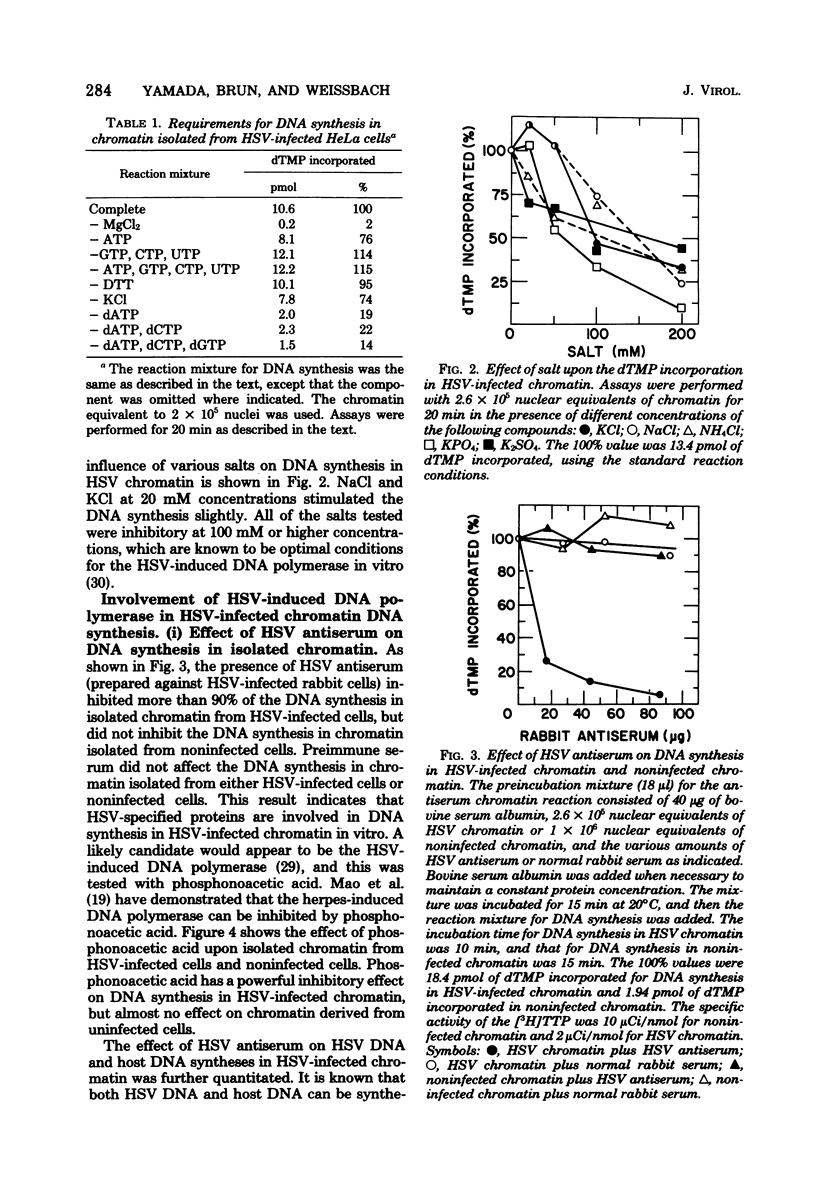
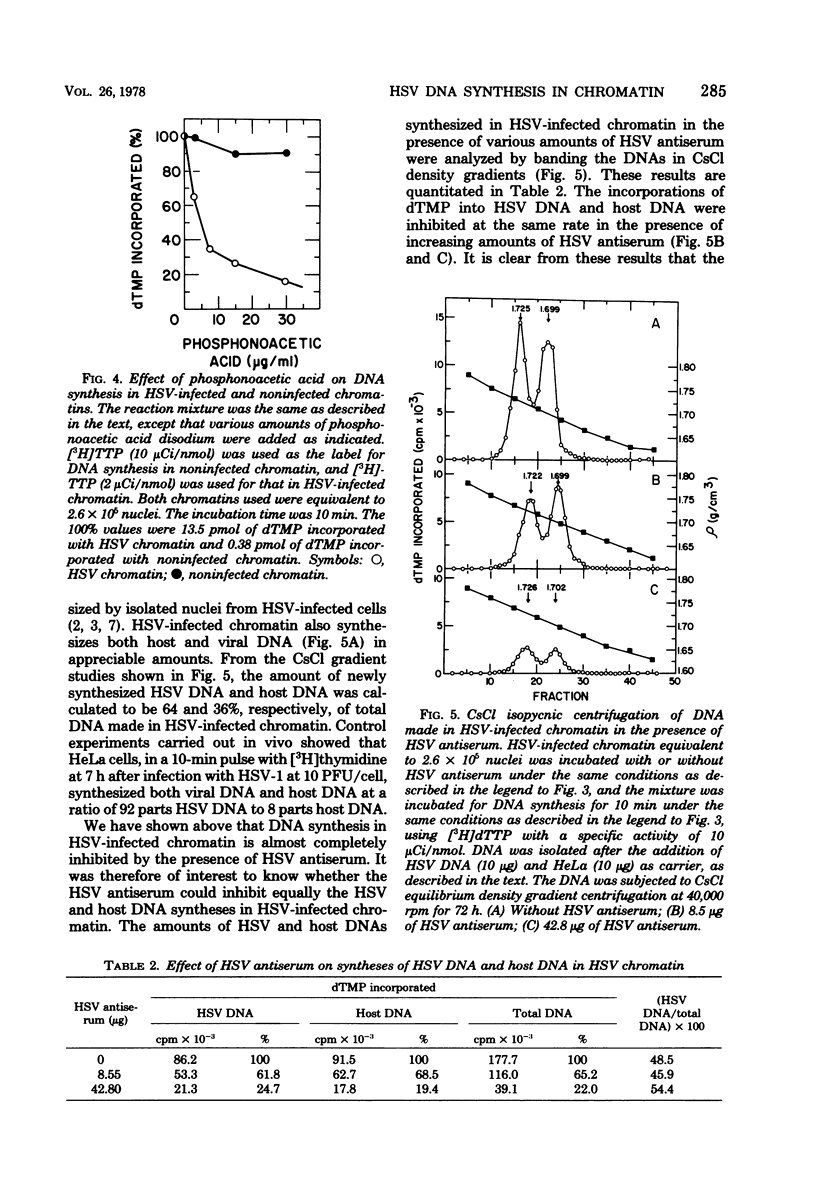
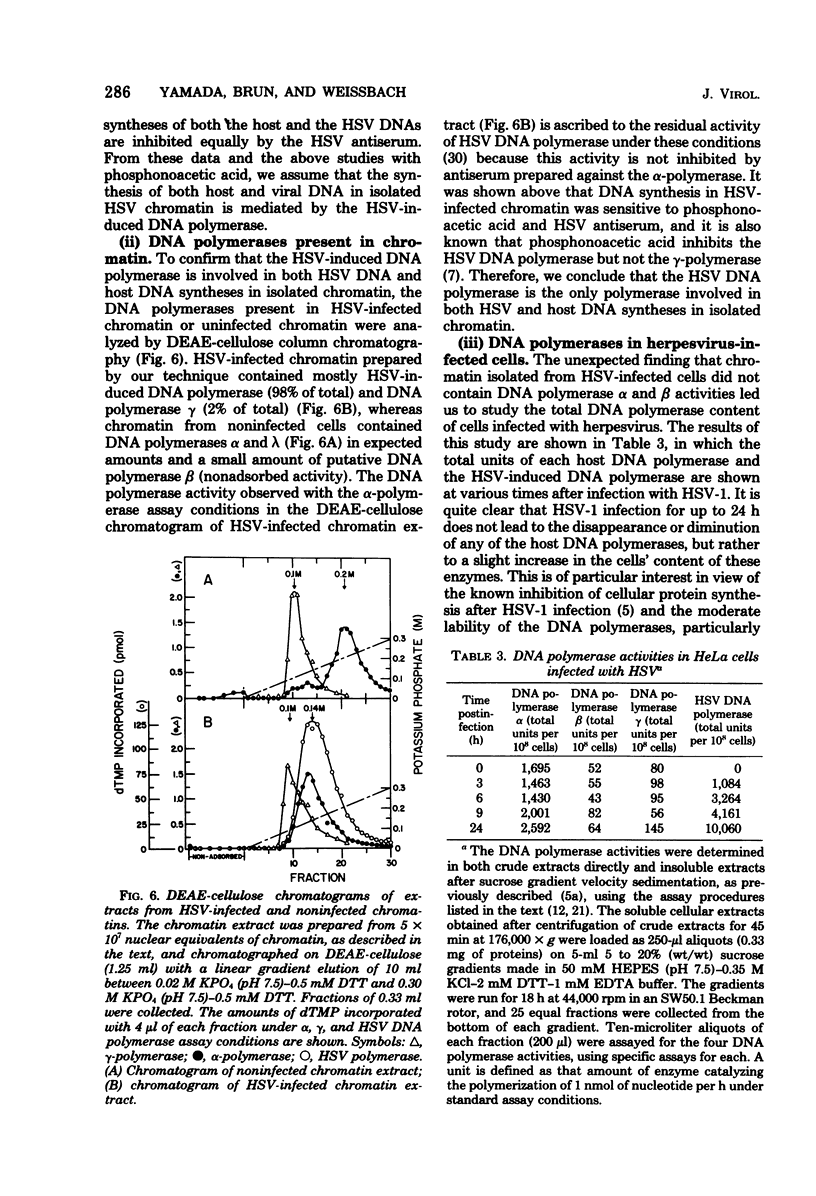
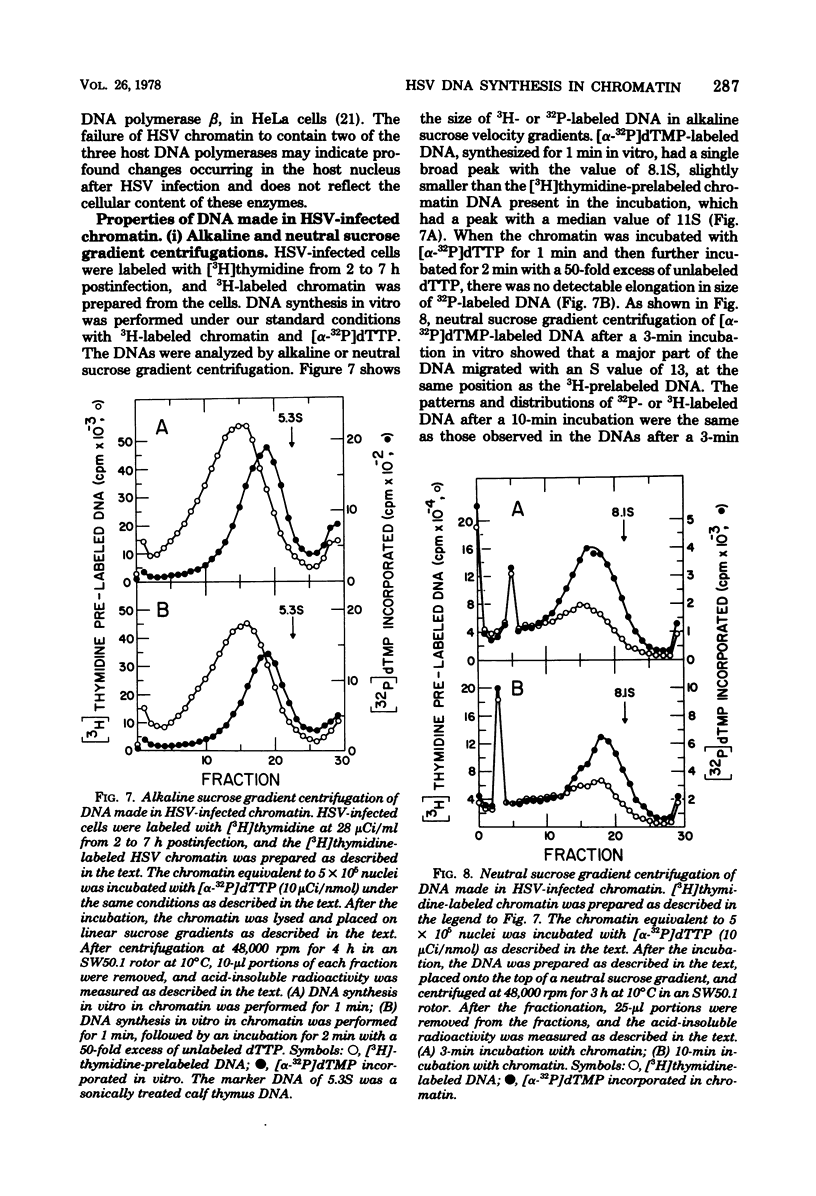
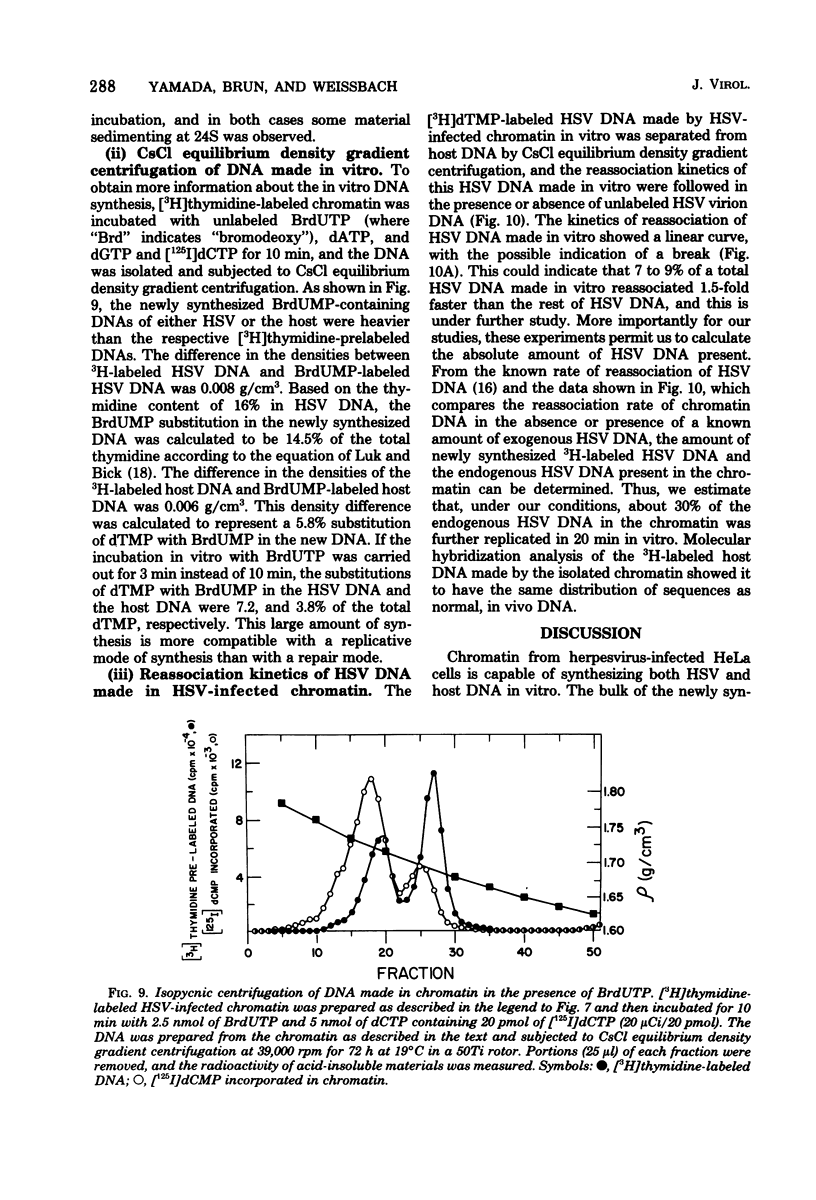
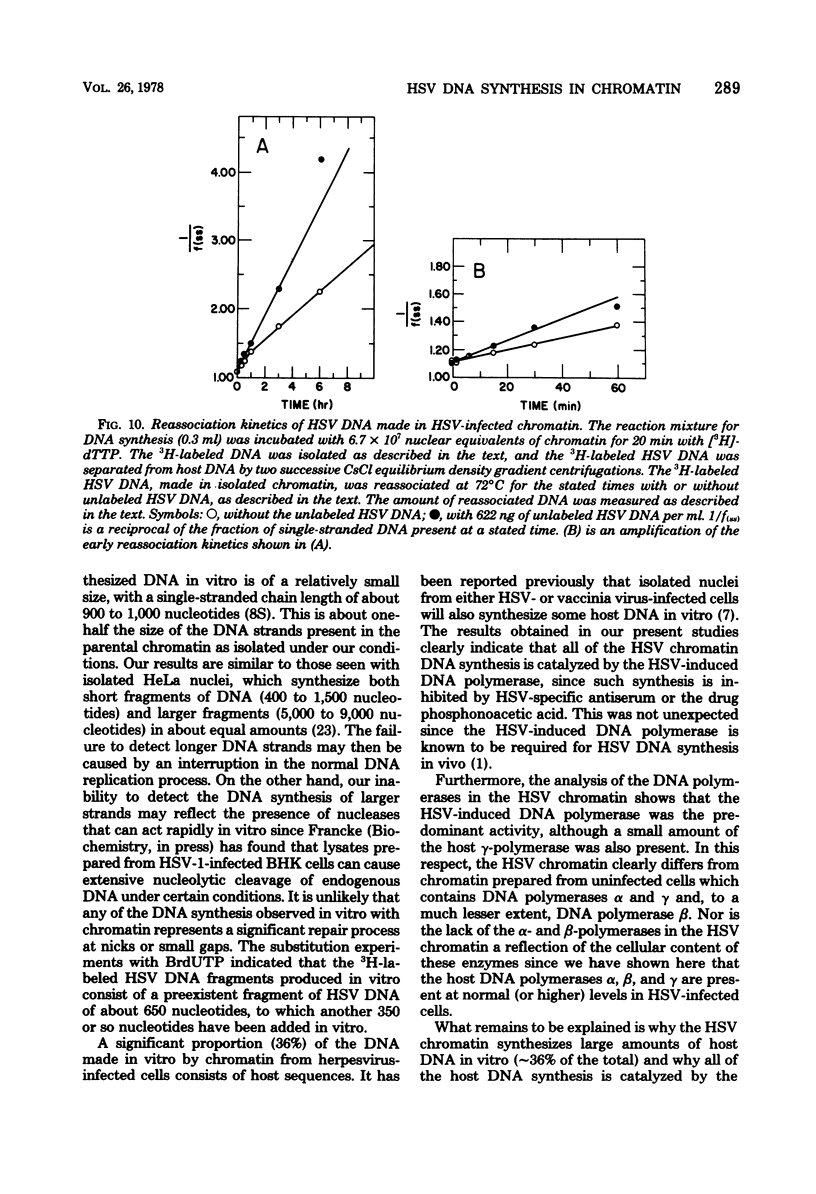
DNA synthesis in chromatin isolated from herpes simplex virus type 1-infected HeLa cells (HSV chromatin) was examined in vitro. The HSV chromatin was found to carry out an initial limited synthesis of DNA in vitro, 50 to 64 pmol of dTMP incorporated in 10(6) nuclei per 10 min, which is comparable to that found in nuclei isolated from HSV-infected cells. DNA synthesis in vitro proceeded for only 30 min, and both HSV DNA and host DNA were synthesized in significant amounts. The HSV and host DNA synthesis in isolated chromatin were inhibited to the same extent by anti-HSV antiserum or by phosphonoacetic acid. The results indicate that the HSV-induced DNA polymerase is most likely involved in the synthesis of host and HSV DNA in isolated chromatin, even though this chromatin contains small amounts of the host gamma-polymerase in addition to the HSV-induced DNA polymerase. The HSV chromatin contains no detectable levels of DNA polymerases alpha and beta, even though infected cells have normal, or increased, levels of these enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Rakusanova T., Kaplan A. S. Early functions of the genome of herpesvirus. II. Inhibition of the formation of cell-specific polysomes. Virology. 1971 Dec;46(3):890–899. doi: 10.1016/0042-6822(71)90089-4. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Waqar M. A., Huberman J. A. Subnuclear systems for synthesis of simian virus 40 DNA in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4392–4396. doi: 10.1073/pnas.73.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Wang J. L., Edelman G. M. Initiation of replication in chromosomal DNA induced by extracts from proliferating cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2231–2235. doi: 10.1073/pnas.73.7.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- Krokan H., Bjorklid E., Prydz H. DNA synthesis in isolated HeLa cell nuclei. Optimalization of the system and characterization of the product. Biochemistry. 1975 Sep 23;14(19):4227–4232. doi: 10.1021/bi00690a012. [DOI] [PubMed] [Google Scholar]
- Luk D. C., Bick M. D. Determination of 5'-bromodeoxyuridine in DNA by buoyant density. Anal Biochem. 1977 Feb;77(2):346–349. doi: 10.1016/0003-2697(77)90247-0. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. 3. The nucleotide sequence at the RNA-DNA junction of nascent strands. J Mol Biol. 1974 Mar 25;84(1):197–216. doi: 10.1016/0022-2836(74)90222-8. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. R., Mueller G. C. Dissociation of the DNA replicase system of bovine lymphocyte nuclei. Biochim Biophys Acta. 1975 Dec 19;414(3):231–241. doi: 10.1016/0005-2787(75)90162-8. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. Evidence for covalent association of RNA with nascent DNA in human lymphocytes. J Mol Biol. 1975 Dec 5;99(2):339–346. doi: 10.1016/s0022-2836(75)80150-1. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]
- Yamada M., Weissbach A. Abnormal sequence distribution in DNA synthesized by isolated nuclei from normal or mitomycin C-treated HeLa cells. Biochem Biophys Res Commun. 1977 Jul 25;77(2):642–649. doi: 10.1016/s0006-291x(77)80027-2. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. II. Synthesis of viral deoxyribonucleic acid in vitro by a nuclear membrane fraction from infected KB cells. J Biol Chem. 1975 May 10;250(9):3273–3279. [PubMed] [Google Scholar]


