Abstract
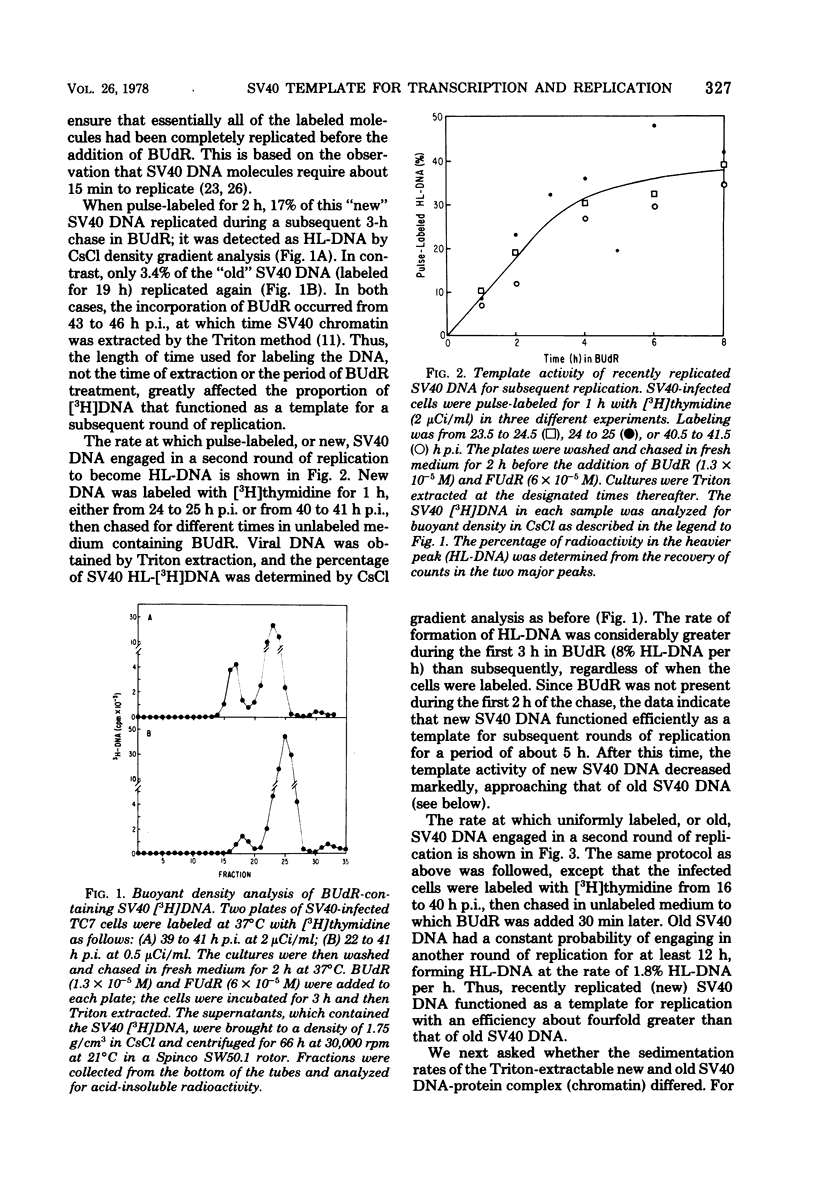
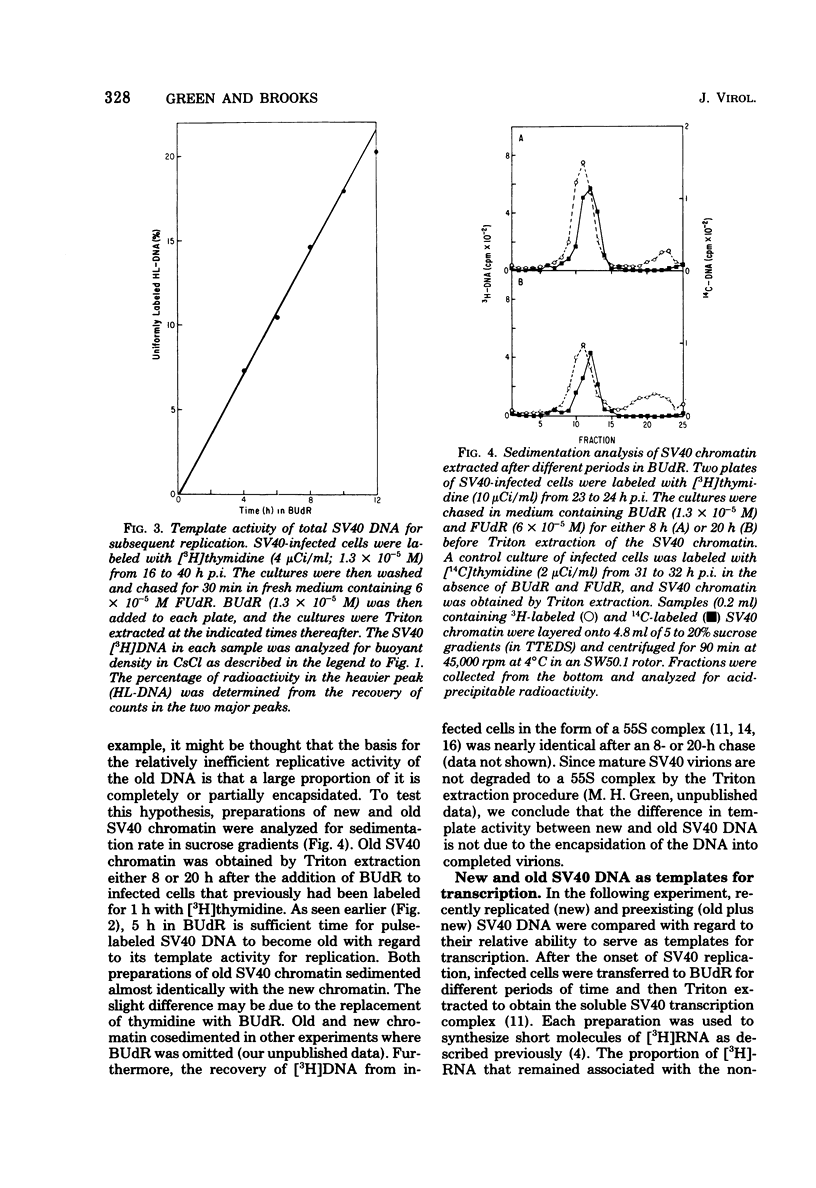
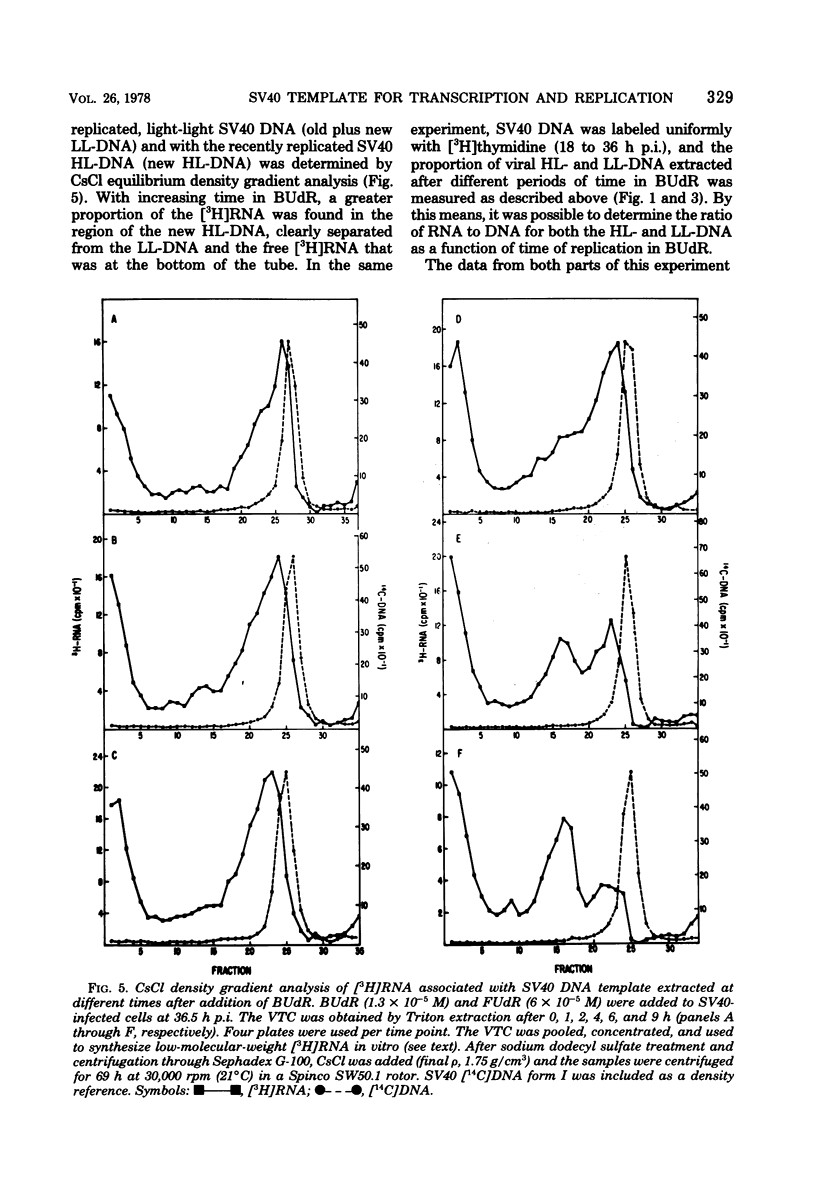
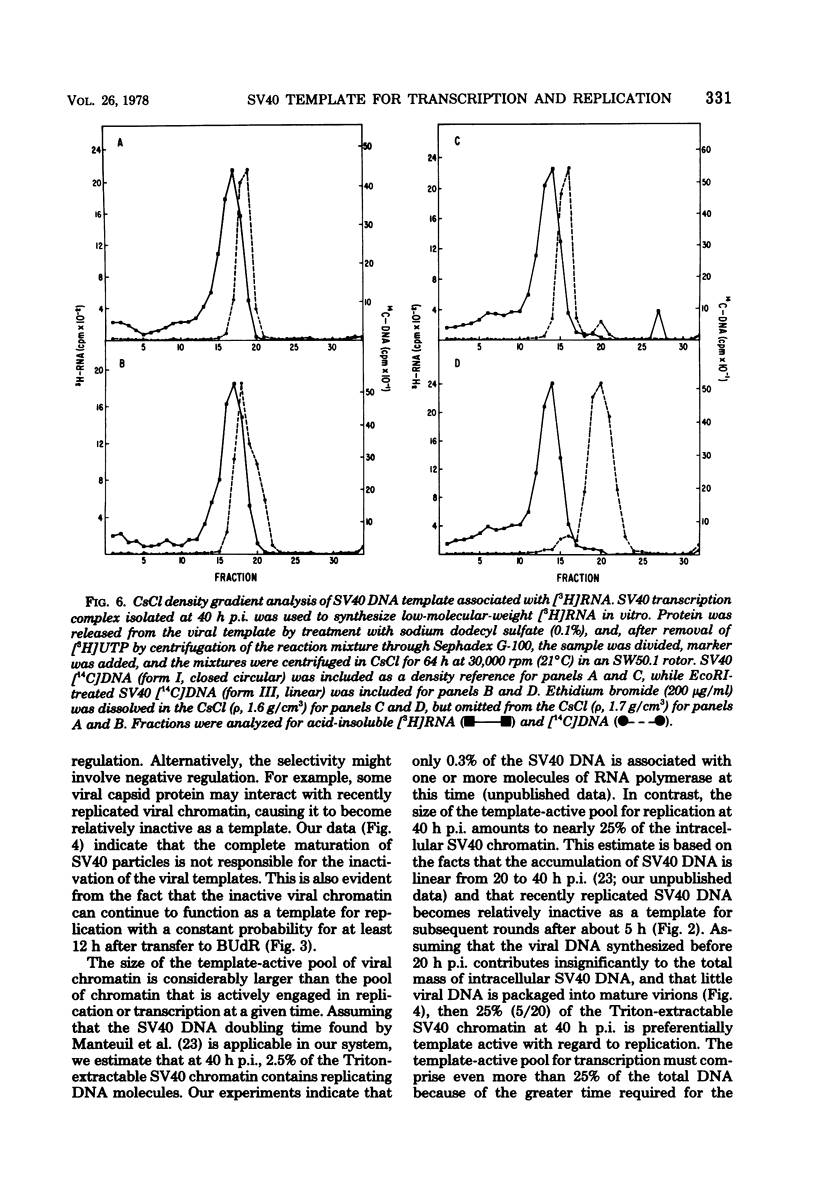
The template activities for the processes of replication and transcription were compared for recently replicated ("new") and uniformly labeled ("old") simian virus 40 (SV40) DNA in infected monkey cells (line TC7). New SV40 DNA (pulse-labeled for 1 h) served as a template for a second round of replication with a relatively high probability (8% of the DNA replicated per h) for a period of 5 h, after which time its template activity rapidly decreased by severalfold. Old SV40 DNA (labeled for 24 h) functioned as a template for replication with a constant probability (1.8% of the DNA replicated per h) for at least 12 h. The proportion of RNA polymerase with nonreplicated and with recently replicated (bromodeoxyuridine-substituted) viral DNA was determined by an assay that used the Triton-soluble SV40 transcription complex. The proportion of RNA polymerase associated with nonreplicated SV40 DNA decreased very slowly (to 50% in 6 h), strongly suggesting that replicating viral genomes are not required as templates for the initiation of late transcription. This hypothesis was supported by the finding that the RNA synthesized in vitro was associated with covalently closed circular SV40 DNA. Furthermore, after 9 h in bromodeoxyuridine, the recently replicated viral DNA had nearly three times more RNA polymerase per unit of DNA than did the nonreplicated DNA. We thus conclude that recently replicated SV40 DNA is utilized preferentially as a template for transcription and for replication.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Biogenesis and characterization of SV40 and polyoma RNAs in productively infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):165–178. doi: 10.1101/sqb.1974.039.01.023. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Priming of superhelical SV40 DNA by Escherichia coli RNA polymerase for in vitro DNA synthesis. Biochemistry. 1975 Jan 28;14(2):307–316. doi: 10.1021/bi00673a017. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Mousset S. Isolation and partial characterization of a nuclear RNA polymerase - SV40 DNA complex. FEBS Lett. 1975 Aug 1;56(1):149–155. doi: 10.1016/0014-5793(75)80130-x. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Brooks T. L. Isolation of two forms of SV40 nucleoprotein containing RNA polymerase from infected monkey cells. Virology. 1976 Jul 1;72(1):110–120. doi: 10.1016/0042-6822(76)90316-0. [DOI] [PubMed] [Google Scholar]
- Green M. H., Brooks T. L. The sv40 transcription complex. II. Non-dissociation of protein from SV40 chromatin during transcription. Nucleic Acids Res. 1977 Dec;4(12):4279–4289. doi: 10.1093/nar/4.12.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hall M. R., Meinke W., Goldstein D. A. Nucleoprotein complexes containing replicating Simian virus 40 DNA: comparison with polyoma nucleoprotein complexes. J Virol. 1973 Oct;12(4):901–908. doi: 10.1128/jvi.12.4.901-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Evidence for semiconservative replication of circular polyoma DNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):997–1004. doi: 10.1073/pnas.55.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Singer M. F. Localization of replicating DNA of simian virus 40 in monkey kidney cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2236–2240. doi: 10.1073/pnas.71.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuil S., Pages J., Stehelin D., Girard M. Replication of simian virus 40 deoxyribonucleic acid: analysis of the one-step growth cycle. J Virol. 1973 Jan;11(1):98–106. doi: 10.1128/jvi.11.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Attachment of nascent RNA molecules to superhelical DNA. J Mol Biol. 1975 Nov 5;98(3):565–579. doi: 10.1016/s0022-2836(75)80087-8. [DOI] [PubMed] [Google Scholar]
- Roman A., Dulbecco R. Fate of polyoma form IDNA during replication. J Virol. 1975 Jul;16(1):70–74. doi: 10.1128/jvi.16.1.70-74.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M., Birkenmeier E., May E., Salzman N. P. Properties of simian virus 40 transcriptional intermediates isolated from nuclei of permissive cells. J Virol. 1977 Jul;23(1):20–28. doi: 10.1128/jvi.23.1.20-28.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmookler R. J., Buss J., Green M. H. Properties of the polyoma virus transcription complex obtained from mouse nuclei. Virology. 1974 Jan;57(1):122–127. doi: 10.1016/0042-6822(74)90113-5. [DOI] [PubMed] [Google Scholar]



