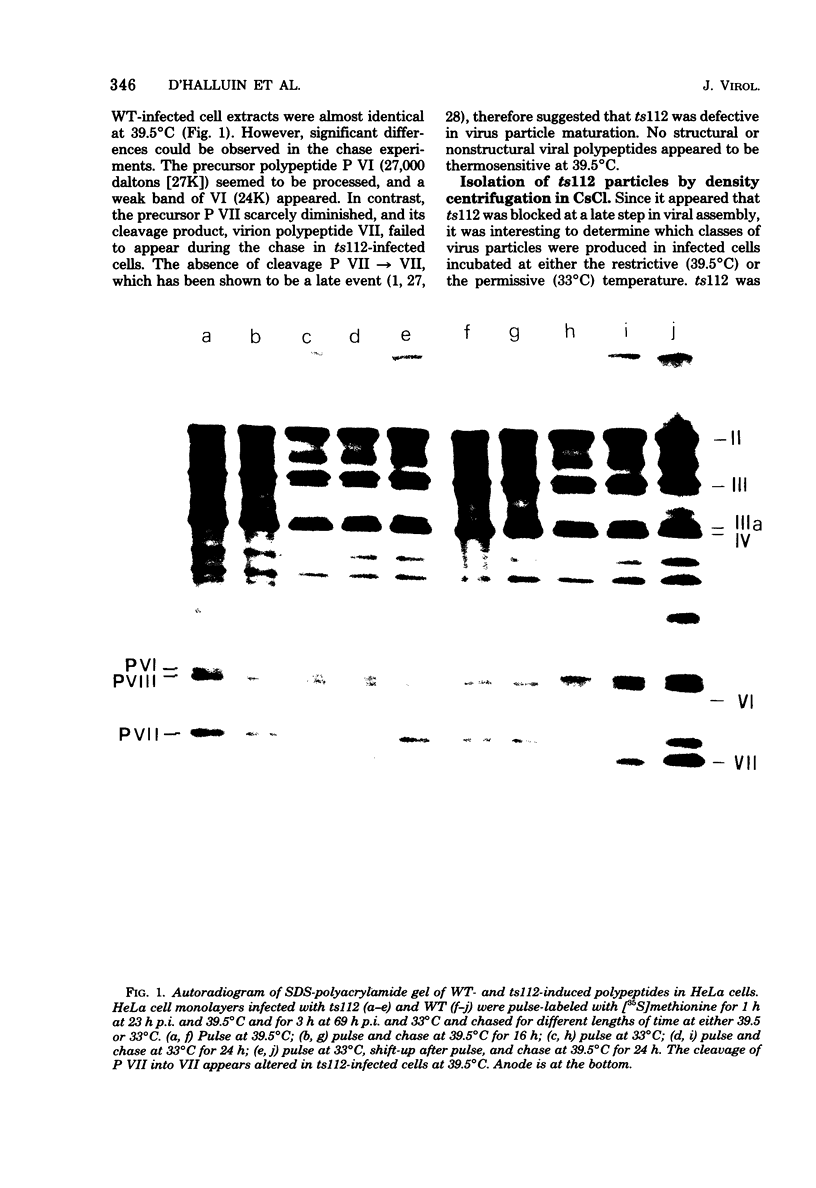
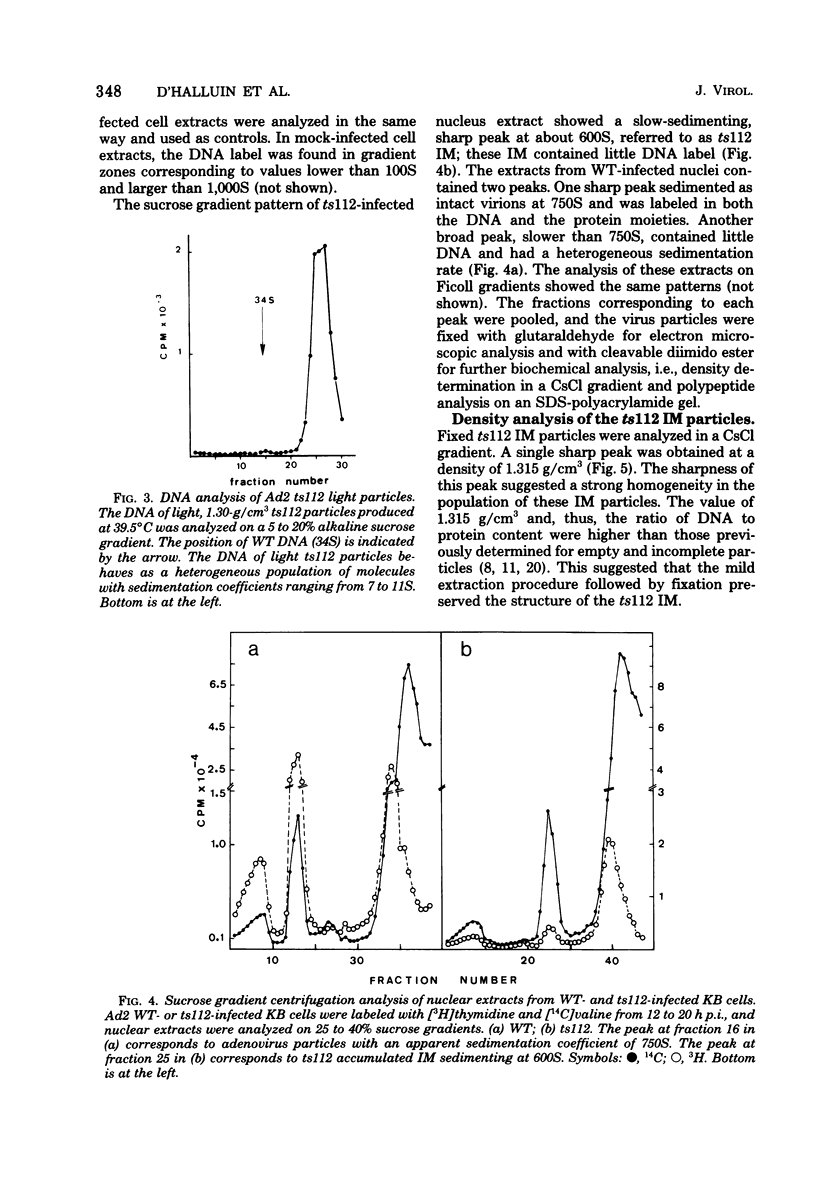
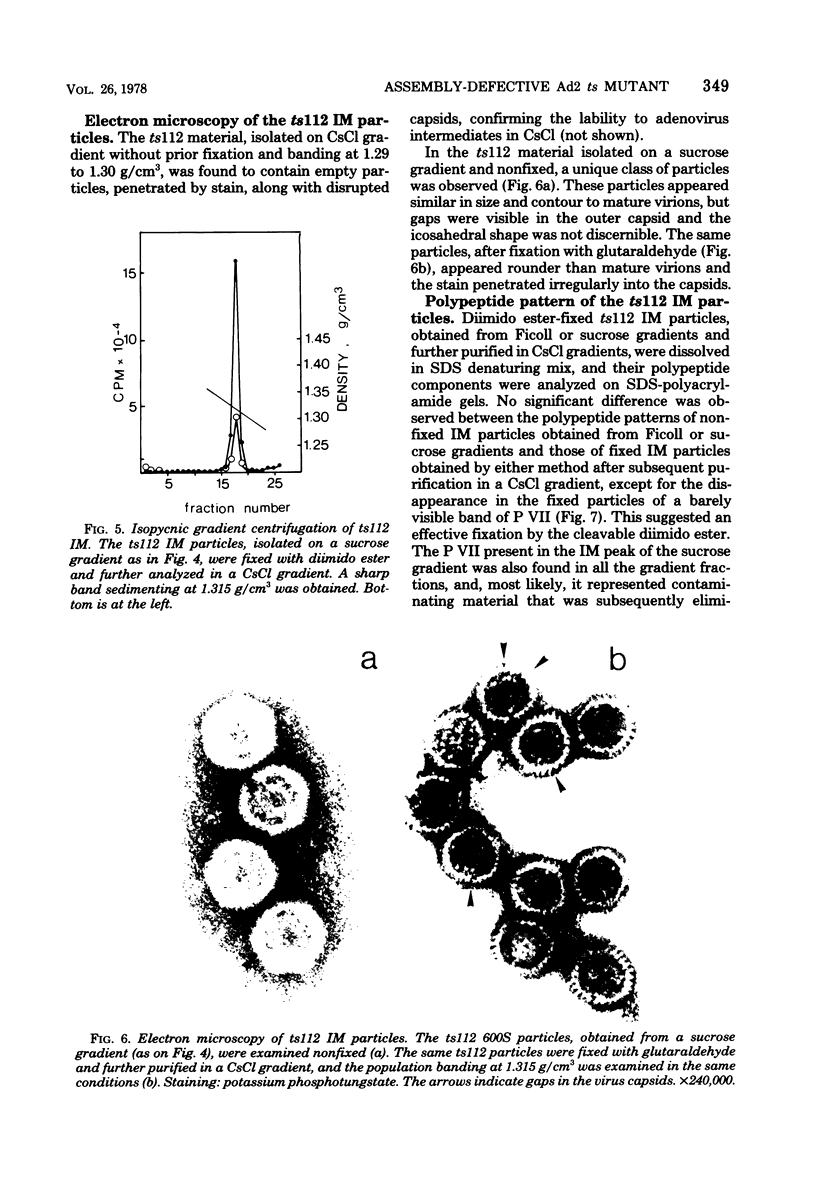
Abstract
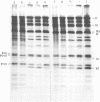
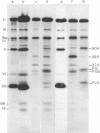
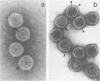
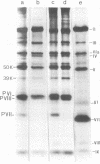
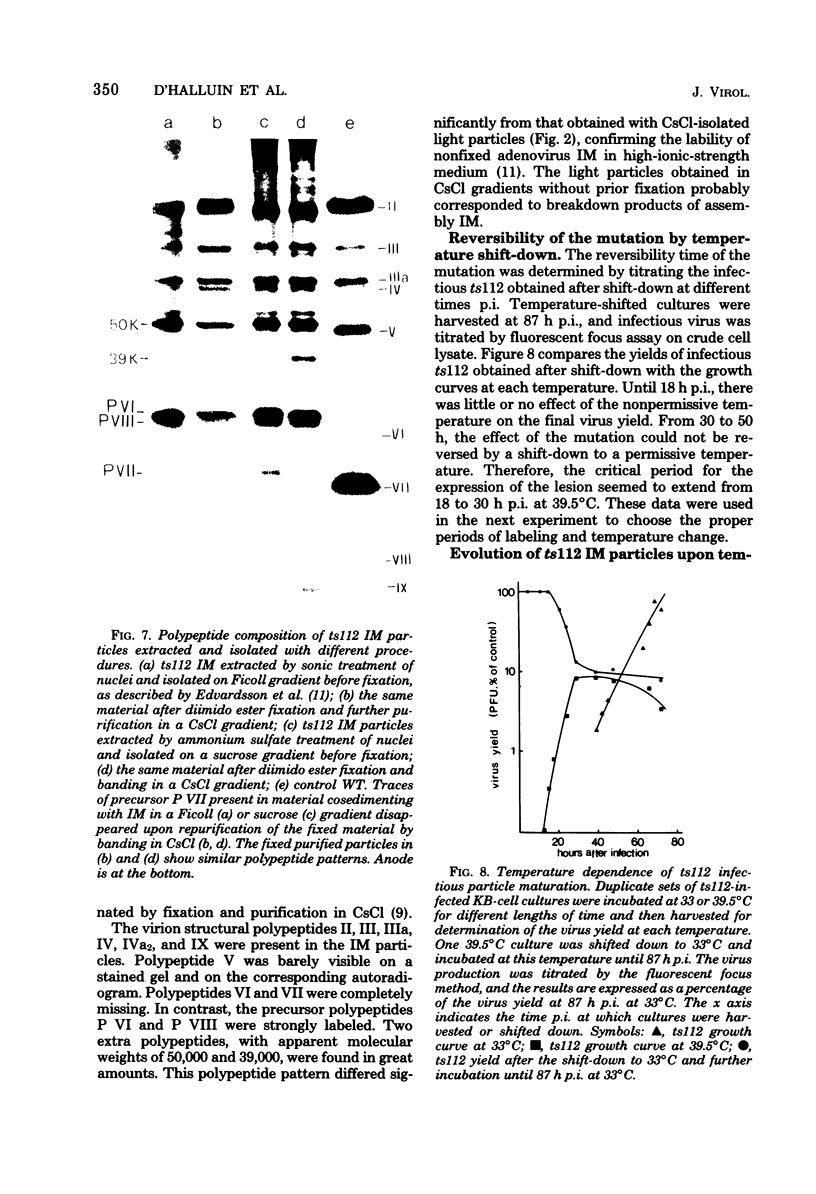
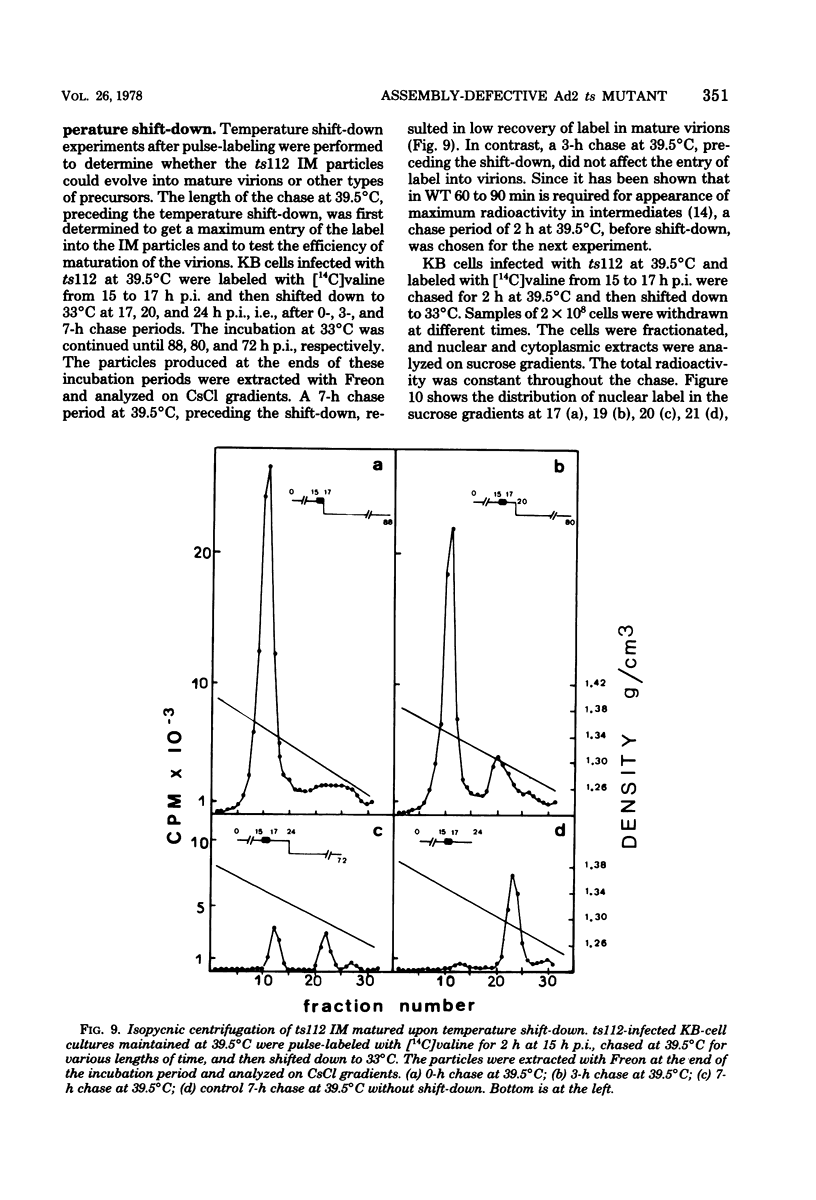
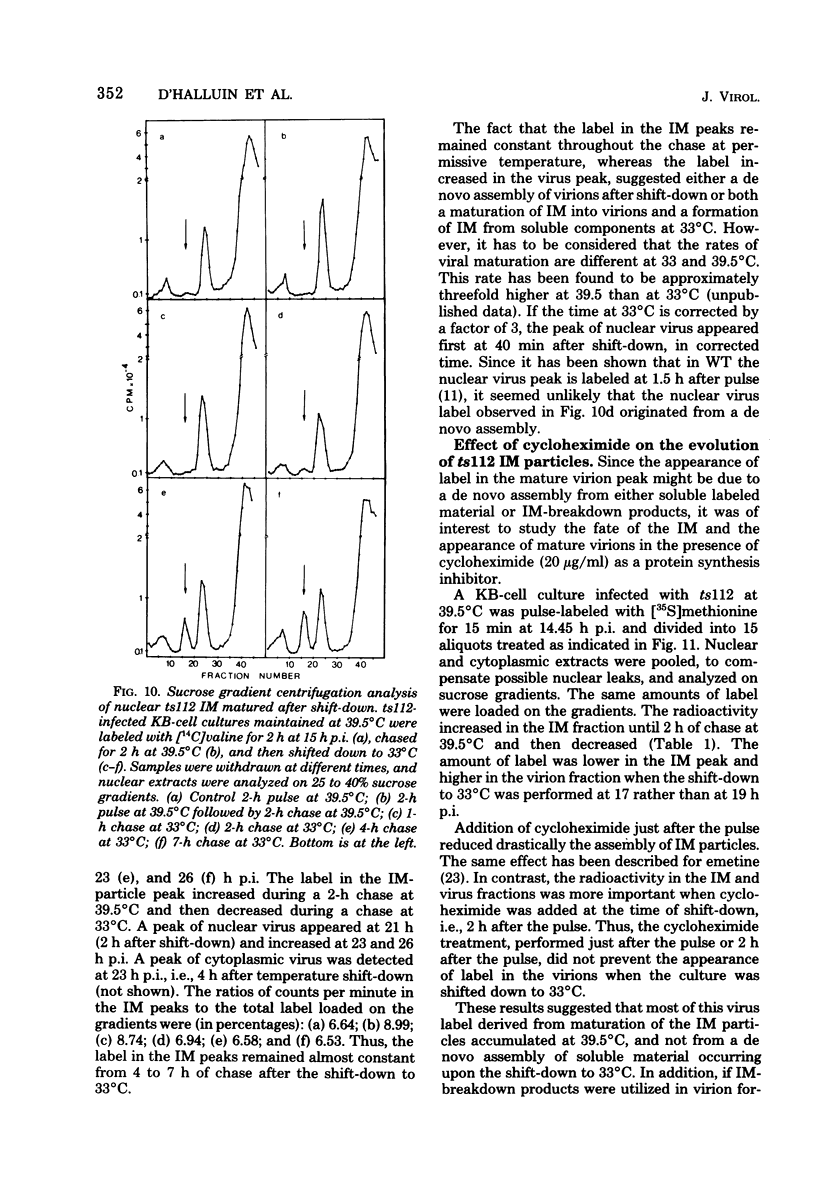
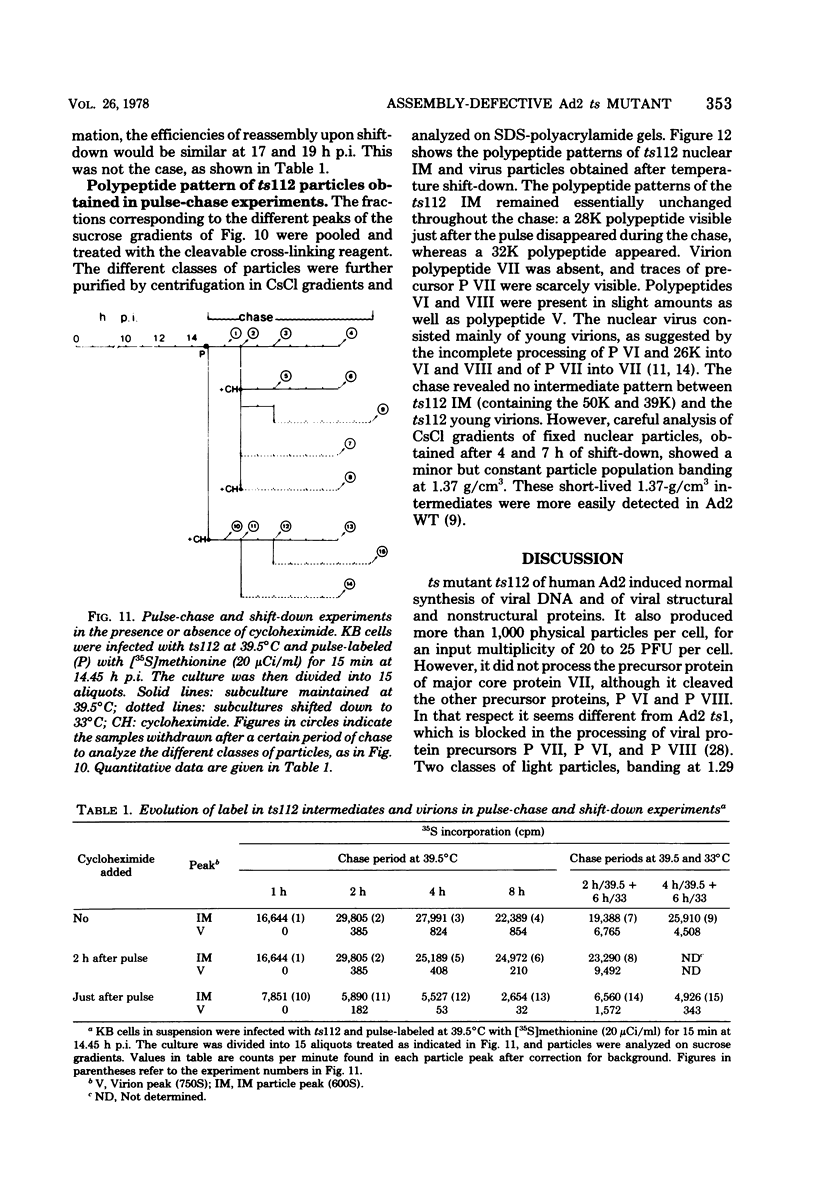
A temperature-sensitive mutant of human adenovirus type 2, ts112, was isolated and characterized, ts112 was blocked in a late function required for virus maturation. At restrictive temperature, it accumulated light precursor particles that were able to mature into infectious virions upon temperature shift-down. Use of a mild extraction procedure and a reversible fixation by a cleavable diimido ester permitted the isolation and analysis of these labile intermediates in the adenovirus assembly. These accumulated particles had a sedimentation coefficient of about 600S and a buoyant density of 1.315 g/cm3 in CsCl. They contained a DNA fragment of 7--11S and two nonvirion proteins having molecular weights of 50,000 (50K) and 39.000 (39K), respectively. They resembled in composition and morphology the light intermediate particles found in wild-type adenovirus 2, which were identified as precursors of heavy intermediates, preceding the young virions. The ts112 lesion was apparently located at the exit of either the 50K and/or 39K proteins and at the entry of viral DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Puvion F. Adenovirus assembly. Cross-linking of adenovirus type-2 hexons in vitro. Eur J Biochem. 1974 Apr 16;43(3):465–470. doi: 10.1111/j.1432-1033.1974.tb03433.x. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Puvion F. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur J Biochem. 1973 Nov 1;39(1):37–42. doi: 10.1111/j.1432-1033.1973.tb03100.x. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Brown D. T., Doerfler W. Incomplete particles of adenovirus. I. Characteristics of the DNA associated with incomplete adenovirions of types 2 and 12. Virology. 1974 Aug;60(2):419–430. doi: 10.1016/0042-6822(74)90336-5. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Martin G. R., Torpier G., Boulanger P. A. Adenovirus type 2 assembly analyzed by reversible cross-linking of labile intermediates. J Virol. 1978 May;26(2):357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976 Aug;19(2):685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Dickson R. C. Assembly of viruses. Annu Rev Biochem. 1972;41:467–502. doi: 10.1146/annurev.bi.41.070172.002343. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Khittoo G., Weber J. Genetic analysis of adenovirus type 2. VI. A temperature-sensitive mutant defective for DNA encapsidation. Virology. 1977 Aug;81(1):126–137. doi: 10.1016/0042-6822(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwirth B., Tjia S., Westphal M., Doerfler W. Incomplete particles of adenovirus. II. Kinetics of formation and polypeptide composition of adenovirus type 2. Virology. 1974 Aug;60(2):431–437. doi: 10.1016/0042-6822(74)90337-7. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Oberg B. In vivo and in vitro synthesis of adenovirus type 2 early proteins. J Virol. 1976 Mar;17(3):865–875. doi: 10.1128/jvi.17.3.865-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist B., Everitt E., Philipson L., Hoglund S. Assembly of adenoviruses. J Virol. 1973 Mar;11(3):449–459. doi: 10.1128/jvi.11.3.449-459.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjia S., Fanning E., Schick J., Doerfler W. Incomplete particles of adenovirus type 2. III. Viral and cellular DNA sequences in incomplete particles. Virology. 1977 Jan;76(1):365–379. doi: 10.1016/0042-6822(77)90309-9. [DOI] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warocquier R., Boulanger P. A. Adenovirus protein maturation at 42 degrees C. J Gen Virol. 1976 Feb;30(2):233–242. doi: 10.1099/0022-1317-30-2-233. [DOI] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Wadell G. Structural polypeptides of adenovirus type 16 incomplete particles. J Virol. 1977 May;22(2):389–401. doi: 10.1128/jvi.22.2.389-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters W. D., Russell W. C. Studies on the assembly of adenovirus in vitro. J Gen Virol. 1971 Feb;10(2):181–194. doi: 10.1099/0022-1317-10-2-181. [DOI] [PubMed] [Google Scholar]