Abstract
It has been proposed that painful and non-painful referred sensations (RSs) are associated with reorganization of sensory pathways in patients with complete spinal cord injury (SCI). In order to investigate the referred sensation (RS) phenomenon and its correlation with neuropathic pain (NP) 48 patients with complete SCI, 24 with chronic NP and 24 without pain or paraesthesias were studied using clinical examination and neurophysiological tests. Patients reporting RSs were re-examined at 2 and 10 weeks after the first examination. We defined the presence of RS as sensations perceived below the injury level in response to touch and pinprick stimuli in various body points above the injury level. The examination was carried out by one researcher applying the stimuli to the patient under two visual conditions (open and closed eyes), and then asking the patient to make tactile self-stimulation. Seven patients with SCI and NP (29%) reported RS below the injury level. RS were well located and consistently evoked at repeated examinations. Touch and pinprick stimulation elicited similar RS that were non-painful in six patients and painful in one. Visual feedback did not change RS perception and characteristics. None of the patients in the SCI group without NP presented RS. In conclusion, our results indicate that RS is relatively frequent in patients with complete SCI and NP. The common occurrence of RS in patients with NP and the location of the sensations in the same area as NP suggest that pain and RS share common pathophysiological mechanisms.
Keywords: Referred sensation, Spinal cord injury, Neuropathic pain
1. Introduction
Referred sensations (RSs) are sensations perceived as emanating from a body site other than the one stimulated. The topography of RSs can be stable [24] or can vary with time [15,16,31]. RSs can be elicited with different sensory modalities, and may be modality-specific [15,16,25] or of a different modality from the stimulation [9,12,16]. RSs have been described following limb amputation [1,2,6,8–10,12,15,20,22,23], brachial plexus avulsion [14], and stroke [26,31], and have also been reported in a few patients with spinal cord injury (SCI). Bors [3] described RSs in four patients with complete SCI and in two with incomplete SCI, and Moore et al. [21] reported RSs by contact stimulus in two patients with complete SCI.
Several authors have suggested that RSs are caused by reorganization of sensory processing in the brain. Indeed, RSs have been described in patients with chronic pain [14] and complex regional pain syndrome [19], presumably due to induced maladaptive plastic cortical changes. The relationship between RSs and pain has been reported in a few patients following limb amputation [10,14]. Recent studies in unilateral arm amputees using magnetoencephalography [6,9,15] showed the existence of different types of RSs related to cortical and subcortical reorganization. Non-painful RSs seem to be related to a widely distributed cross-modal cortico-subcortical network [6,21]. On the other hand, painful RSs appear to be related to reorganization of the primary somatosensory cortex [9], and a strong correlation has been demonstrated between the number of sites from where stimuli evoked RSs, the phantom limb pain experienced and the amount of cortical reorganization [15]. In this context, a correlation between RSs and chronic pain would be expected in situations in which there is significant reorganization of somatosensory pathways, such as in SCI. Considering that approximately one-third of all patients with complete SCI develop neuropathic pain (NP), located below the level of the injury [27,29], we hypothesized that RS is more common in patients with SCI and NP compared to patients with SCI without pain. To address this issue, we studied the presence and characteristics of RSs in patients with complete SCI with or without NP. The results of this study may offer additional insight into the changes in the somato-sensory system after SCI, and provide clinical and research markers for RSs in SCI.
2. Patients and methods
2.1. Subjects
Forty-eight patients who had SCI above the T12 level were studied at the Institut Guttmann (Barcelona, Spain) between January 2007 and August 2009 (Table 1). All patients had a complete SCI (Grade A, no sensory or motor function preserved in sacral segments S4–S5) according to the American Spinal Injury Association’s standards for classification of SCI (ASIA) [18]. Twenty-four patients had NP and the other 24 were without NP or spontaneous paraesthesias. Criteria for NP diagnosis were chronic pain in an area of sensory abnormality corresponding to the spinal cord lesion, and without primary relation to movement, inflammation or other local tissue damage [28]. Below-level pain was diagnosed if the NP was present more than three dermatomes below the neurological level of injury; at-level pain was diagnosed if the pain was located within the dermatome at and three dermatomes below the neurological level of injury. Patients completed the neuropathic pain symptom inventory (NPSI) [4]. NP intensity was scored using the numerical rating scale (NRS). Patients were informed that this study would not result in therapeutic benefit; however, the particular aims of the experiment were not disclosed to avoid bias. The study was approved by the Research Ethics Board of the Institute Guttmann, and all patients gave their written informed consent.
Table 1.
Characteristics of the SCI patients included in the study.
| Patients without NP | Patients with NP | p* | NP patients without RS | NP patients with RS | p* | |
|---|---|---|---|---|---|---|
| Number | 24 | 24 | 17 | 7 | ||
| Age (years), median (range) | 43.5 (21–77) | 43.5 (29–66) | 0.90β | 43.7 (29–69) | 43.2 (30–59) | 1.00β |
| Gender, male/female | 19/5 | 19/5 | 0.64α | 12/5 | 7/0 | 0.15α |
| Aetiology trauma/medical | 20/4 | 17/7 | 0.35α | 11/6 | 6/1 | 0.31α |
| Years postinjury median (range) | 14 (1–36) | 7 (1–31) | 0.44β | 8 (1–31) | 6 (3–19) | 0.90β |
| Level of lesion thoracic/cervical | 16/8 | 17/7 | 0.50α | 12/5 | 5/2 | 0.70α |
No significant differences were found either between groups of SCI patients without NP and with NP or between the subgroups of SCI patients with NP with and without RS.
Statistical comparisons for categorical variables by Chi-square test (α) and for continuous variables by Mann–Whitney U test (β).
2.2. Examination of referred sensations
The presence of RSs was evaluated through a detailed examination of perceptions experienced by the patient in response to light touch and pinprick stimulation. The examinations were performed by the same clinician (MDS) on all patients at entry into the study; patients reporting RSs were then re-examined at 2 and 8–10 weeks after the first examination to assess possible changes in the characteristics and location of RSs. A RS was defined as a sensation noted below the spinal cord lesion level coincident in time with stimulation applied at a body site above the lesion level (reference zone). Between 10 and 80 standardized key sensory points [18] on dermatomes located above the level of the lesion were stimulated in a random order for the two modalities tested (light touch and pin-prick) and body side (right–left) and segment (head–trunk–upper limbs). During the examination, patients who reported a RS were asked to define the characteristics of their RS in terms of quality and location. If the patient reported a RS when a given point was stimulated, we then systematically examined an area up to 2 cm around the point that upon being touched evoked the RS until the patient stopped feeling it. Stimulation was applied by two modalities: light touch with a soft cotton swab to the skin [24] and pinprick applied with a needle [16]. The duration of each stimulus was approximately 2 s for both modalities. If the patient experienced a RS, the location of the stimulated point and the area of referral were drawn on a body figure. The examination lasted between 30 and 150 min depending on the presence of RSs, and also on the neurological level of the lesion. If the subject reported a RS, the same stimulation was repeated twice immediately. At the end of the examination, the points that elicited RSs were stimulated again to ensure consistency.
The same stimulation protocol was carried out twice: first, with the patient’s eyes closed and second, with the patient’s eyes open, while the patient was in front of a full-length mirror and was asked to observe his/her body. Patients who felt a RS were asked to try to evoke the RS by tactile self-stimulation if able to do so (lesion level at or below C7).
2.3. Neurophysiological studies
Patients underwent a neurophysiological evaluation. A Medelec synergy electromyograph (Oxford Instruments, England) was used for all the tests.
Motor evoked potentials (MEPs) were elicited by single trans-cranial magnetic stimulus (TMS) using a Magstim Super Rapid (Magstim Company, Spring Gardens, Whitland, UK) and delivered through a double-cone coil centered over the vertex. MEPs were recorded by means of surface electrodes placed bilaterally over tibialis anterior (TA) and soleus (SOL) muscles, with the active electrode over the motor point and the reference electrode 2 cm distally. If there was no response at rest following TMS with 100% maximum stimulator output intensity, the patient was asked to imagine bilateral foot dorsiflexion during stimulus delivery. Single sweeps of 100 ms were recorded, filtered at 10 Hz–2 kHz and amplified with a gain of 0.1 mV/div.
For sensory evoked potentials (SEPs) the tibial nerve on both sides was stimulated dorsally to the medial malleolus, and cortical potentials were recorded at Cz′ against the reference Fz. Stimulus intensity was adjusted to produce a clear muscular response (max 40 mA) in order to assess all sensory fibres. Two sets of responses were averaged with sweeps of 100 ms, filters set at 3 and 3000 Hz, and amplified at a gain of 2 μV/div [30].
2.4. Data analysis
Comparisons were made between the groups of patients with and without NP. Patients with NP were also divided into two subgroups: those without RSs and those with RSs. Demographic data and characteristics of patient groups were compared using the Mann–Whitney U test for continuous variables and the Chi-square test for categorical data. Significance level was set at p < 0.05.
3. Results
No significant differences were observed between SCI patients, with and without NP, in age and time since injury, aetiology, sex and neurological level of injury (Table 1).
Seven of the 24 patients with NP experienced RSs (29%) (Table 1). No significant differences were found in age, aetiology, disease duration, type of pain, descriptors of pain, neurological level of the lesion and medication between the subgroups of patients with or without RSs (p > 0.1 for all these variables). Detailed information on each patient with NP regarding SCI level, aetiology and NP characteristics is shown in table 2. The seven patients with RSs had below-level NP as defined by the International Association for the Study of Pain SCI Pain Taxonomy [28]. These patients described their NP as burning (4/7), pressing (3/7), paroxysmal (1/7) and dysaesthesia (2/7). These descriptors were similar to those chosen by patients without RS. Only one of the patients with RSs had been aware of them prior to the study (Table 2). The characteristics of these seven patients are described briefly in the following paragraphs:
Table 2.
Characteristics of the SCI patients with neuropathic pain.
| Subject | Sex | Age | Years since SCI | Neurological level of injury | Type of paina | NRS for pain | Analgesic medications |
|---|---|---|---|---|---|---|---|
| SCI patients with neuropathic pain and referred sensations | |||||||
| 1 | M | 50 | 18 | T9 | Below | 9 | CNZ, TMD |
| 2 | M | 41 | 6 | T5 | Below | 5 | PGB, CNZ, FE |
| 3 | M | 43 | 19 | T10 | Below | 8 | PGB, ANT |
| 4 | M | 59 | 5 | T12 | Below | 10 | PGB, OXT, CNZ, ANT |
| 5 | M | 35 | 3 | C6 | Below | 10 | PGB, CNZ |
| 6 | M | 45 | 5 | C6 | Below | 8 | PGB, CNZ, OXT |
| 7 | M | 30 | 12 | T4 | Below | 5 | – |
| SCI patients with neuropathic pain without referred sensation | |||||||
| 8 | F | 55 | 21 | C7 | At + below | 7 | – |
| 9 | M | 57 | 29 | T9 | At + below | 8 | PGB |
| 10 | M | 32 | 8 | T10 | At + below | 8 | PGB, ANT |
| 11 | F | 36 | 2 | C4 | At | 9 | GBP |
| 12 | M | 29 | 5 | T6 | At + below | 6 | CNZ, PGB, TMD |
| 13 | M | 53 | 9 | T6 | At + below | 7 | GBP, PGB |
| 14 | M | 44 | 3 | T12 | At | 6 | TMD, CNZ, GBP |
| 15 | M | 42 | 25 | T7 | Below | 6 | – |
| 16 | F | 44 | 5 | T3 | Below | 6 | ANT |
| 17 | F | 50 | 2 | C5 | Below | 6 | GBP, ANT |
| 18 | M | 29 | 8 | L1 | Below | 7 | ANT |
| 19 | M | 37 | 1 | T12 | At | 9 | GBP |
| 20 | F | 49 | 2 | C7 | Below | 5 | CNZ, PGB |
| 21 | M | 39 | 5 | C7 | Below | 4 | – |
| 22 | M | 62 | 19 | T11 | Below | 5 | GBP, CNZ |
| 23 | M | 35 | 9 | T4 | Below | 7 | GBP, PGB, ANT |
| 24 | M | 66 | 31 | T11 | Below | 5 | CNZ, GBP, ANT |
Abbreviations: NRS, numerical rating scale from 0 (no pain) to 10 (unbearable pain).
Below: “below-level pain” was defined as neuropathic pain present more than three dermatomes below the neurological level of injury, At: “at level pain” was defined as pain localized to the same dermatome or within three dermatomes below the neurological level. Below-level pain extending to the at-level area is classified as below-level pain if the patient is unable to distinguish two separate pain types. At + below: when the patient presents both types of pain and is able to distinguish two separate pain types.
Medication GBP = gabapentin, CNZ = clonazepam, PGB = pregabalin, TMD = tramadol, FE = fentanyl, KTM = ketamine, OXT = oxycontin, ANT = antidepressants.
3.1. Patient 1
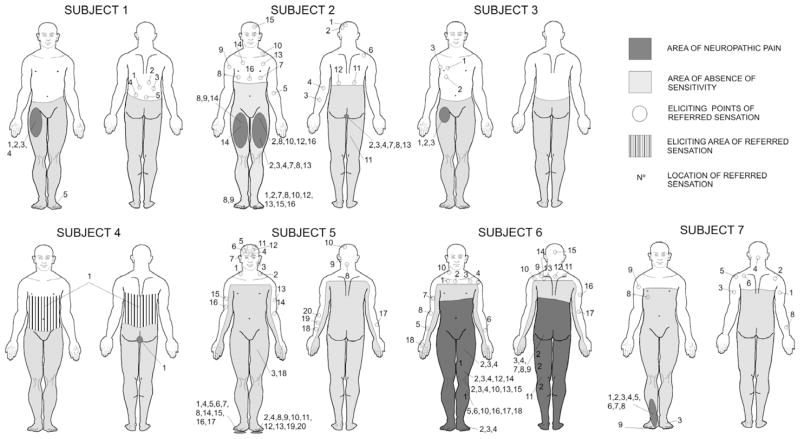
A 50-year-old man who began to complain of NP 5 months after the onset of a thoracic SCI (Table 3), following a car accident, 20 years prior to our study. He described NP as stabbing pain affecting his right leg. He reported around 20 episodes of pain per day, unrelated to position or movement but worsened by urinary tract infections. RSs were generated when five points on the back between the T5 and T10 levels were stimulated by (Fig. 1, subject 1), using both light touch and pinprick. He described his RSs as non-painful electric currents evoked by stimulation and located in the ipsilateral thigh, where the NP was located, and in the contralateral toes, where he had no pain. The subject could evoke the same RSs by light touch self-stimulation. Visual feedback did not change RS perception and characteristics.
Table 3.
Characteristics of neuropathic pain, localization of referred sensations in the complete SCI patients with pain.
| Subject | Neuropathic pain
|
Referred sensation
|
|||||
|---|---|---|---|---|---|---|---|
| Localization | Description NPSI | Light touch* | Pin-prick* | Self stimulation | Localization** | Description | |
| 1 | Right thigh | Paroxysmal | 5 | 5 | + | Right thigh (4) great toes (1) | Non-painful soft electrical currents |
| 2 | Great toes perianal area upper legs | Pressing, burning | 16 | 16 | + | Great toes (11) perianal area (6) upper legs (15) | Non-painful, tingling. More vivid awareness of the body |
| 3 | Right thigh | Pressing | 3 | 3 | + | Right thigh (3) | Non-painful, vivid, soft and electrical current |
| 4 | Perianal area | Burning, pressing provoked pain | Any area between T4–T12 | Any area between T4–T12 | + | Perianal area | Painful. Vivid burning and oppressive |
| 5 | Soles | Burning, dysesthesia | 20 | 20 | n.a. | Left sole/foot (10), right sole/foot (10) and groin (2) | Non-painful, tingling |
| 6 | Thoracic area both legs | Burning | 18 | 16 | n.a. | Left leg (15), left foot (3) and bottom (5) | Non-painful, tingling |
| 7 | Right foot | Dysesthesia | 9 | 3 | + | Chest (1), right (9) and left foot (1) | Non-painful, tingling |
Abbreviations: NPSI, neuropathic pain symptom inventory; n.a. = not available, because physical limitation of patient.
Number of stimulated areas above the SCI level from where RSs could be elicited, which was consistent at the two examinations.
Number of RSs points at each location.
Fig. 1.
Representation on the body figure of the area of sensory deficit, area of neuropathic pain, area of referred sensations, and location of points on the skin that elicited referred sensations with light touch in each of the seven patients with referred sensations.
3.2. Patient 2
A 41-year-old man with traumatic thoracic SCI following a motorcycle accident 6 years earlier had begun to experience chronic NP some 3 months after the injury. He described NP mainly as pressure and a burning sensation, located in legs, toes and the perineal region. The NP was continuous and unrelated to position or movement, but exacerbated by fatigue. Most of the points eliciting RSs were located above but close to the lesion level and others in the head and upper limbs. Stimulation of some of these points generated RSs in multiple areas below the lesion, but predominantly in areas where the patient also reported NP (Fig. 1, subject 2). In general, the RSs were ipsilateral to the stimulated side, but a few stimulated points triggered RSs in the contralateral side or bilaterally. The subject described the RSs as non-painful sensations induced by light touch and pinprick stimulation, and reported that when stimulated he also had awareness of the body areas where he experienced RSs. Sites eliciting RSs were stable on the three different exams performed. Two points near the transition lesion area (points #7 and #8 in Fig. 1, subject 2) always elicited the most intense RSs. The patient liked to self-produce the RSs because he perceived sensations from an otherwise anaesthetic part of his body.
3.3. Patient 3
A 43-year-old man who had been injured 19 years earlier in a motorcycle accident (Table 3). He began to complain of NP 1 year after the thoracic SCI. He described NP as a constant pressure located in the right upper leg and groin that was affected by mood, attention and weather changes. RSs were elicited ipsilaterally by stimulation on the right chest (Fig. 1, subject 3) and described as vivid, non-painful electric current sensations, following light touch and pinprick stimulation, located in the same area. The region eliciting RSs was very well defined and consistent on the repeat examinations. RSs could be evoked by light touch self-stimulation. Visual feedback did not change the perception and characteristics of the RS.
3.4. Patient 4
A 59-year-old man with thoracic SCI following a work accident 5 years before enrolment in our study. He reported severe chronic NP dating back to six months after the injury. His NP had a burning and oppressive nature, located in the genital area, together with a sensation of a constant desire to defecate (Fig. 1, subject 4). He experienced RSs when light touch or pinprick stimuli were applied anywhere on the chest and back between T5 and T12. The quality and intensity of the RS were similar to those of the NP that the patient usually experienced. The patient was able to self-evoke the RSs by touching himself in the same areas. In addition, he experienced worsening of the NP when he moved his trunk or moved fast in his wheelchair.
3.5. Patient 5
A 35-year-old man with a traumatic cervical SCI caused by a work accident (Table 3). The patient started to suffer NP a few months after the injury. The NP quality was mainly burning, “pins and needles” and tingling sensations on the soles of both feet. The trigger points that evoked RSs were located on the head, shoulders and arms. RSs were described as tingling, non-painful sensations following light touch and pinprick stimulation, and perceived in the same area as NP (Fig. 1, subject 5). In addition, he reported RSs in the left thigh, ipsilateral to stimulation to the head and forearm points. The patient was not able to self-provoke RSs owing to the high neurological level of the lesion.
3.6. Patient 6
A 45-year-old man with cervical SCI following an intra-medullary haemorrhage (5 years ago) who began to suffer chronic NP 6 months after the lesion (Table 3). He described pain in the thoracic area and in both legs as a constant burning sensation that worsened with bowel function problems. During the examination, most points eliciting RSs were located within the transition area of the lesion (i.e. approximately two dermatomes above the neurological level of the injury). Stimulation of some of these points generated RSs in multiple areas, but predominantly in the left leg where the patient also had NP (Fig. 1, subject 6). The RSs were described as tingling, non-painful sensations, after both light touch and pinprick stimulation. Three points (#1,3,10 in Fig. 1, subject 6) always elicited the most intense RS in the leg. The patient had not been aware of such RSs before the study. The stimulated points inducing RSs and the location of RSs below the lesion were stable on the three repeated examinations. Visual feedback did not change RSs characteristics. The patient was not able to self-provoke RSs owing to the high neurological level of the lesion.
3.7. Patient 7
A 30-year-old man with traumatic thoracic SCI who began to complain of NP after the injury (Table 3) following a motorcycle accident, 12 years ago. The NP was described as constant and moderately intense pins and needles (dysaesthetic pain) [4] in the right foot. Pharmacological treatment failed to alleviate his pain and for this reason he had stopped the treatment. Light touch and pinprick stimulation above the lesion was reported to produce a tingling, non-painful sensation in the right foot (Fig. 1, subject 7), coincident with the area of dysaesthetic pain. In addition, he reported RSs in the right chest (non-painful area), ipsilateral to the stimulation. The patient had not been aware of RS before the first examination. Similar RSs were elicited when the patient had his eyes closed, when the examination was performed in front of a mirror, and with self-stimulation.
In summary, 29% of our patients (7 of 24) with NP after complete SCI presented RSs below the level of the lesion, whereas none of the patients without NP after complete SCI presented RSs. RSs location coincided in most instances with body regions of maximal NP. Different stimulation modalities (light touch and pinprick) elicited similar RSs that were non-painful in six patients and painful in only one. The stimulated areas that evoked RSs and location of the RS below the lesion were stable on the repeated examinations in all patients with NP. Only the patient with painful RSs had been aware of them prior to the present study. RS could be evoked by self-stimulation in five patients (two were unable to perform the self-stimulation), and visual feedback did not change RSs perception in any of the patients.
3.8. Neurophysiological results
Six of the 24 patients with NP declined to come to the hospital for the neurophysiological studies because of transportation (3 subjects) or health (3 subjects) issues. At TMS intensity of 100% of maximum stimulator output, MEPs were absent in TA and SOL muscles of all studied patients. Mean stimulus intensity used in the SEPs study was 34.4 ± 3.8 mA in the right leg and 29.9 ± 5.7 mA in the left leg. SEPs were absent in all studied patients following stimulation of the tibial nerve on both sides.
4. Discussion
4.1. Frequency of RS in patients with SCI with and without NP
Our results show that RSs are relatively common in patients with complete SCI and NP, (appearing in about a third of patients), but not in those without NP. The exact incidence of RSs following SCI is difficult to ascertain since there are few reports in the literature. Moore et al. [21] studied 12 patients with complete SCI at thoracic level, evaluating the RSs evoked by light touch and moving stimuli, and found that 16% of their patients had ipsilateral, non-painful RSs. Bors et al. [3] reported RSs in 6 of 50 patients (12%) with complete or incomplete SCI. In both these studies it was unclear whether the patients had NP. The higher percentage of RSs in our patients with NP in comparison with patients without NP supports the hypothesis that pain and RS share some common mechanisms.
The prevalence of RSs in patients with complete SCI and NP is lower than in amputees, in whom the presence of RSs has been reported in 38–75% of the subjects [9,12,15]. These differences may be due to the fact that, in complete SCI, patients do not feel their legs but do see them, and in fact need to pay particular attention to them to prevent injuries from trauma. In amputees, the loss of visual feedback from the missing part of the body might play a role in plastic reorganization, leading to a more frequent experience of RSs. We hypothesize that visual differentiation (as in the case of amputations) is most conducive to the induction of plastic changes that may lead to RSs.
Although visual feedback plays a critical role in sensory reorganization in patients with amputation [12], in our study, visual feedback did not change the quality or frequency of RSs in patients with SCI. One of the reasons might be that visual feedback cannot influence the underlying mechanisms of RSs, or at least that its effects cannot be detected by our method of evaluation.
4.2. Location and characteristics of RS
The characteristics of RSs, experienced as mild electric current and tingling sensations, were different from the normal sensations elicited by the used stimuli (light touch and pinprick stimulation) and independent of the type of stimulus triggering the RSs in six of our seven patients with complete SCI and NP. Reports on RS in the literature are quite variable in this respect. In some patients, RSs matched the modality of the triggering stimulus [15,24], but lacked such specificity in others [6,12,16].
One of our patients experienced RSs as painful and similar to his spontaneous NP, and referred to increasing pain with more intense stimuli (touch and pinprick). The fact that in this patient all stimuli triggered painful RSs in the same body part suggests a striking, maladaptive central reorganization. Knecht et al. [15] demonstrated for the first time a direct relationship between the amount of phantom limb pain experienced and the number of locations from where painful stimuli could evoke painful RS in the phantom limb. Other studies have indicated a correlation between cortical and thalamic reorganization phenomena and the presence of pain in amputees [7,8,15].
In all our patients, the stimulated points evoking RSs, location and characteristics of the RSs remained highly stable and reproducible over 3 months. This appears to contrast with findings in amputees, whose RSs tend to change over hours, days or weeks [11,16,22,25]. Changes over time in RSs have been explained as the result of changing patterns of sensory inputs and spontaneous activity from the amputated stump [24]. The stability of RSs in our patients might be related to the existence of chronic and stable NP, possibly secondary to the persistence of residual spinothalamic tract pathways [32], and the maintained visual input that might reduce the amount of sensory reorganization and thus preserve awareness of the anaesthetic limbs.
Five patients in this study could self-produce the same type of RSs with touch. In the other two patients it could not be demonstrated due to physically limitations. This is a surprising finding that contrasts with the results in patients with amputations [12,13,25]. The absence of RSs in amputees with eyes open has been explained by the strong modulatory effect of vision on tactile RSs [12]. Seeing the actual stimulation typically serves as a “reality check” negating the percept of the stimulus applied to the missing body part in amputees. However, this does not appear to be the case in our patients with SCI. All our patients reported preserved whole-body awareness. Apparently, in patients with complete SCI, the presence of visual but absence of somatosensory awareness of the legs promotes different plastic processes from those encountered in amputees. Such differences might also be related to the differential impact of primarily central (SCI) versus peripheral nervous system damage (amputees).
4.3. The relationship between RS and NP
One important finding of our study was the co-location of RS and NP in SCI patients. NP below-level in SCI is generally located distally from the site of injury and characterized by an asymmetric presentation, without dermatomal organization [5,27,28]. The consequences of neural reorganization are not always adaptive for the patient. Injuries to the peripheral and central nervous system trigger a series of plastic events that result in final increases in the excitability of neurons in the sensory pathways, thereby contributing to the physiopathology of NP. Different studies have revealed a strong correlation between the magnitude of phantom limb pain in amputees and the amount of reorganization in the thalamus and in the primary somatosensory cortex [7,8,17]. Recently a relationship between the degree of cortical reorganization and the intensity of NP below the level of the lesion has been shown in subjects with SCI [34]. Similarly, RS has been related to plastic changes in the nervous system [6,8,21]. The common occurrence of RS in our SCI patients with NP, but not in those without NP, supports the notion that NP and RS might be caused by similar or related plastic changes in the central nervous system. Recent studies demonstrated that SCI triggers dysregulated Na+ channel expression at several levels within the neuroaxis, specifically in dorsal horn and thalamic neurons [33]. In patients with complete SCI, it has been suggested that intact spinothalamic tract afferents projecting through a damaged spinal cord region can contribute to central pain [32]. The partially preserved spinothalamic tract pathways might function as a ‘pain generator’. Changes in excitability and discharge patterns in thalamic neurons may be triggered by ongoing activity in these preserved spinothalamic afferents [32]. These continuing nociceptive inputs might lead to cortical reorganization which may contribute to induce RSs associated with NP. Further mechanistic studies investigating RSs might shed light on the mal-adaptive sensory reorganization mechanisms in SCI.
Acknowledgments
The authors thank Eloy Opisso and Raquel López for their valuable technical input as well as our patients with SCI who generously offered their time and collaboration. This work was supported in part by grants from the Cátedra BBVA (CAT06/023), the FIS (PI082004) and the Foundation La Marató TV3 (071931), as well as by the Harvard-Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center integrated in the Harvard Clinical and Translational Science Center (Grants M01-RR-01066 and UL1 RR025758 from the National Center for Research Resources, National Institutes of Health) and a grant from the National Institutes of Health (K24 RR018875).
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
References
- 1.Aglioti S, Cortese F, Franchini C. Rapid sensory remapping in the adults human brain as inferred from phantom breast perception. Neuroreport. 1994;5:473–6. doi: 10.1097/00001756-199401120-00026. [DOI] [PubMed] [Google Scholar]
- 2.Berlucchi G, Aglioti S. The body in the brain: neural bases of corporeal awareness. Trends Neurosci. 1997;20:560–4. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- 3.Bors E. Extinction and synesthesia in patients with spinal cord injuries. Paraplegia. 1979;17:21–31. doi: 10.1038/sc.1979.9. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–57. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Finnerup NB, Gyldensted C, Fuglsang-Frederiksen A, Bach FW, Jensen TS. Sensory perception in complete spinal cord injury. Acta Neurol Scand. 2004;109:194–9. doi: 10.1034/j.1600-0404.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- 6.Flor H, Mühlnickel W, Karl A, Denke C, Grüsser S, Kurth R, Taub E. A neural substrate for non-painful phantom limb phenomena. Neuroreport. 2000;11:1407–11. doi: 10.1097/00001756-200005150-00011. [DOI] [PubMed] [Google Scholar]
- 7.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–4. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 8.Florence SL, Hackett TA, Strata F. Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol. 2000;83:3154–9. doi: 10.1152/jn.2000.83.5.3154. [DOI] [PubMed] [Google Scholar]
- 9.Grüsser SM, Winter C, Mühlnickel W, Denke C, Karl A, Villringer K, Flor H. The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience. 2001;102:263–72. doi: 10.1016/s0306-4522(00)00491-7. [DOI] [PubMed] [Google Scholar]
- 10.Grüsser SM, Mühlnickel W, Schaefer M, Villringer K, Christmann C, Koeppe C, Flor H. Remote activation of referred phantom sensation and cortical reorganization in human upper extremity amputees. Exp Brain Res. 2004;154:97–102. doi: 10.1007/s00221-003-1649-4. [DOI] [PubMed] [Google Scholar]
- 11.Halligan PW, Marshall JC, Wade DT. Sensory disorganization and perceptual plasticity after limb amputation: a follow-up study. Neuroreport. 1994;5:1341–5. [PubMed] [Google Scholar]
- 12.Hunter JP, Katz J, Davis KD. The effect of tactile and visual sensory inputs in phantom limb awareness. Brain. 2003;126:579–89. doi: 10.1093/brain/awg054. [DOI] [PubMed] [Google Scholar]
- 13.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Non-painful phantom limb phenomena in amputees: incidence, clinical characteristics and temporal course. Acta Neurol Scand. 1984;70:407–14. doi: 10.1111/j.1600-0404.1984.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Katz J, Melzack R. Referred sensations in chronic pain patients. Pain. 1987;28:51–9. doi: 10.1016/0304-3959(87)91059-1. [DOI] [PubMed] [Google Scholar]
- 15.Knecht S, Henningsen H, Elbert T, Flor H, Höhling C, Pantev C, Taub E. Reorganizational and perceptual changes after amputation. Brain. 1996;119:1213–9. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- 16.Knecht S, Henningsen H, Höhling C, Elbert T, Flor H, Pantev C, Taub E. Plasticity of plasticity? Changes in the pattern of perceptual correlates or reorganization after amputation. Brain. 1998;121:717–24. doi: 10.1093/brain/121.4.717. [DOI] [PubMed] [Google Scholar]
- 17.Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain an fMRI study in upper limb amputees. Brain. 2001;124:2268–77. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- 18.Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, Haak M, Hudson LM, Priebe MM. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26:S50–56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 19.McCabe CS, Haigh RC, Halligan PW, Blake DR. Referred sensations in patients with complex regional pain syndrome type 1. Rheumatology. 2003;42:1067–73. doi: 10.1093/rheumatology/keg298. [DOI] [PubMed] [Google Scholar]
- 20.Montoya P, Ritter K, Huse E, Larbig W, Braun C, Töpfner S, Lutzenberger W, Grodd W, Flor H, Birbaumer N. The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci. 1998;10:1095–102. doi: 10.1046/j.1460-9568.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore CL, Stern CE, Dunbar C, Kostyk SK, Gehi A, Corkin S. Referred phantom sensations and cortical reorganisation after spinal cord injury in humans. Proc Natl Acad Sci USA. 2000;97:14703–8. doi: 10.1073/pnas.250348997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascual-Leone A, Peris M, Tormos JM, Pascual-Leone Pascual A, Catalá MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport. 1996;7:2068–70. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature. 1995;377:489–90. doi: 10.1038/377489a0. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran VS, Stewart M, Rogers-Ramachandran D. Perceptual correlates of massive cortical reorganization. Neuroreport. 1992;3:583–6. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandram VS, Hirstein W. The perception of phantom limbs the D.O. Hebb lecture. Brain. 1998;121:1603–30. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 26.Sathian K. Intermanual referral of sensation to anaesthesic hands. Neurology. 2000;54:1866–98. doi: 10.1212/wnl.54.9.1866. [DOI] [PubMed] [Google Scholar]
- 27.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–57. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 28.Siddall PJ, Yezierski RP, Loeser JD. Pain following spinal cord injury: clinical features, prevalence, and taxonomy. IASP Newsletter. 2000–2003 Available from: http://www.iasp-pain.org.
- 29.Soler MD, Sauri-Ruiz J, Curcoll MLI, Benito-Penalva J, Opiso-Salleras E, Vidal-Samsó J. Characteristics of chronic neuropathic pain and their relationship with psychological well-being in spinal cord injury patients. Rev Neurol. 2007;44:3–9. [PubMed] [Google Scholar]
- 30.Spiess M, Schubert M, Kliesch U. EM-SCI study group 1, Pascal Halder evolution of tibial SSEP after traumatic spinal cord injury: baseline for clinical trials. Clin Neurophysiol. 2008;119:1051–61. doi: 10.1016/j.clinph.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Turton AJ, Butler SR. Referred sensations following stroke. Neurocase. 2001;7:397–405. doi: 10.1076/neur.7.5.397.16251. [DOI] [PubMed] [Google Scholar]
- 32.Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–400. doi: 10.1093/brain/awn169. [DOI] [PubMed] [Google Scholar]
- 33.Waxman S, Hains BC. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207–15. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–9. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]