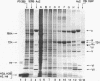
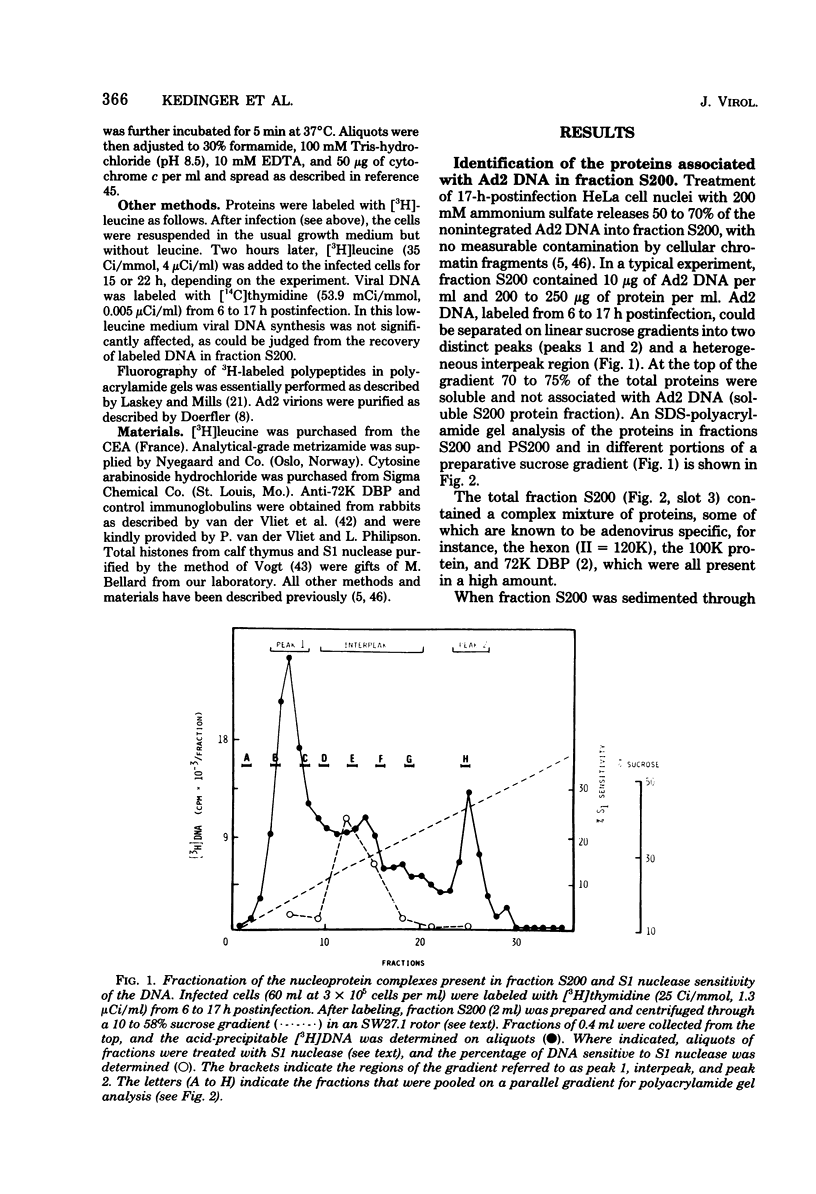
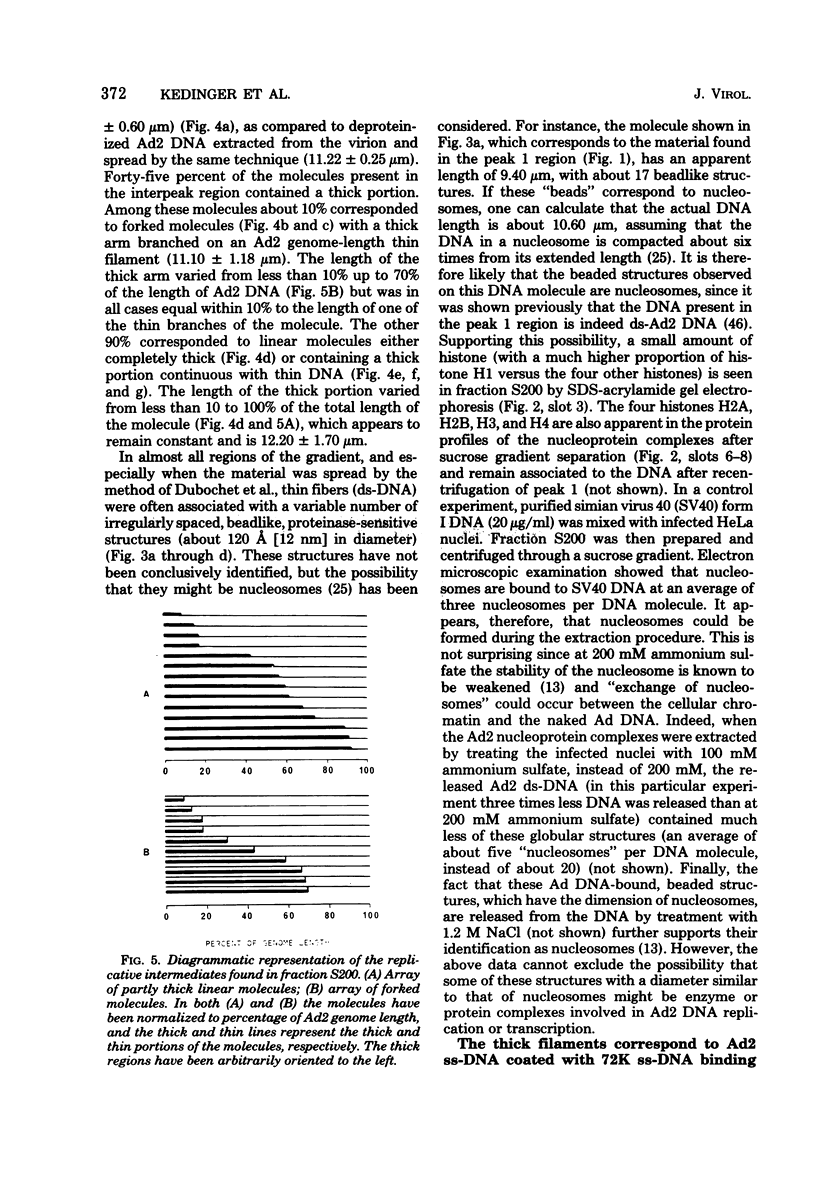
Abstract
Deoxyribonucleoprotein complexes released 17 h postinfection from adenovirus type 1 (Ad2)-infected HeLa cell nuclei were shown by electron microscopy to contain filaments much thicker (about 200 A [20 nm]) than double-stranded DNA (about 20 A [2 nm]). The complexes were partially purified through a linear sucrose gradient, concentrated, and further purified in a metrizamide gradient. The major protein present in the complexes was identified as the 72,000-dalton (72K), adenovirus-coded single-stranded DNA-binding protein (72K DBP). Three types of complexes have been visualized by electron microscopy. Some linear complexes were uniformly thick, and their length corresponded roughly to that of the adenovirus genome. Other linear genome-length complexes appeared to consist of a thick filament connected to a thinner filament with the diameter of double-stranded DNA. Forked complexes consisting of one thick filament connected to a genome-length, thinner double-stranded DNA filament were also visualized. Both thick and thin filaments were sensitive to DNase and not to RNase, but only the thick filaments were digested by the single-strand-specific Neurospora crassa nuclease, indicating that they correspond to a complex of 72K DBP and Ad2 single-stranded DNA. Experiments with anti-72K DBP immunoglobulins indicated that these nucleoprotein complexes, containing the 72K DBP, correspond to replicative intermediates. Both strands of the Ad2 genome were found associated to the 72K DBP. Altogether, our results establish the in vivo association of the 72K DBP with adenovirus single-stranded DNA, as previously suggested from in vitro studies, and support a strand displacement mechanism for Ad2 DNA replication, in which both strands can be displaced. In addition, our results indicate that, late in infection, histones are not bound to adenovirus DNA in the form of a nucleosomal chromatine-like structure.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Bellett A. J., Younghusband H. B. Replication of the DNA of chick embryo lethal orphan virus. J Mol Biol. 1972 Dec 30;72(3):691–709. doi: 10.1016/0022-2836(72)90185-4. [DOI] [PubMed] [Google Scholar]
- Brison O., Kedinger C., Wilhelm J. Enzymatic properties of viral replication complexes isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1977 Nov;24(2):423–435. doi: 10.1128/jvi.24.2.423-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Ellens D. J., Sussenbach J. S., Jansz H. S. Studies on the mechanism of replication of adenovirus DNA. III. Electron microscopy of replicating DNA. Virology. 1974 Oct;61(2):427–442. doi: 10.1016/0042-6822(74)90279-7. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Characterization of single-stranded viral DNA sequences present during replication of adenovirus types 2 and 5. Cell. 1976 Dec;9(4 Pt 1):559–571. doi: 10.1016/0092-8674(76)90038-6. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Bellard M., Oudet P., Chambon P. Stability of nucleosomes in native and reconstituted chromatins. Nucleic Acids Res. 1976 Nov;3(11):3173–3192. doi: 10.1093/nar/3.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Bidirectional replication of adenovirus type 2 DNA. J Virol. 1976 Apr;18(1):307–315. doi: 10.1128/jvi.18.1.307-315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. C., Singer M. F. Histone synthesis during infection of monkey kidney cells with Simian Virus 40. Nucleic Acids Res. 1977 Oct;4(10):3371–3386. doi: 10.1093/nar/4.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Chambon P. Animal DNA-dependent RNA polymerases. Molecular structures and immunological properties of calf-thymus enzyme AI and of calf-thymus and rat-liver enzymes B. Eur J Biochem. 1974 May 15;44(2):421–436. doi: 10.1111/j.1432-1033.1974.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lavelle G., Patch C., Khoury G., Rose J. Isolation and partial characterization of single-stranded adenoviral DNA produced during synthesis of adenovirus type 2 DNA. J Virol. 1975 Oct;16(4):775–782. doi: 10.1128/jvi.16.4.775-782.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., van der Vliet P. C., Rosenwirth B., Anderson C., Rabek J., Levinson A., Anderson S. Characterization of an adenovirus early protein required for viral DNA replication: a single strand specific DNA binding proteins. Mol Cell Biochem. 1976 Apr 28;11(2):79–95. doi: 10.1007/BF01792789. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U. Letter: Some unusual properties of replicating adenovirus type 2 DNA. J Mol Biol. 1973 Dec 25;81(4):521–527. doi: 10.1016/0022-2836(73)90521-4. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin J., Bourgaux-Ramoisy D., Bourgaux P. Single-stranded regions in replicating DNA of adenovirus type 2. J Gen Virol. 1973 Aug;20(2):233–237. doi: 10.1099/0022-1317-20-2-233. [DOI] [PubMed] [Google Scholar]
- Schilling R., Weingärtner B., Winnacker E. L. Adenovirus type 2 DNA replication. II. Termini of DNA replication. J Virol. 1975 Oct;16(4):767–774. doi: 10.1128/jvi.16.4.767-774.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Arens M., Green M. DNA binding proteins in the cytoplasm and in a nuclear membrane complex isolated from uninfected and adenovirus 2 infected cells. Biochemistry. 1975 Jan 28;14(2):332–337. doi: 10.1021/bi00673a020. [DOI] [PubMed] [Google Scholar]
- Tolun A., Pettersson U. Termination sites for adenovirus type 2 DNA replication. J Virol. 1975 Oct;16(4):759–766. doi: 10.1128/jvi.16.4.759-766.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Vliet P. C., Zandberg J., Jansz H. S. Evidence for a function of the adenovirus DNA-binding protein in initiation in DNA synthesis as well as in elongation of nascent DNA chains. Virology. 1977 Jul 1;80(1):98–110. doi: 10.1016/0042-6822(77)90383-x. [DOI] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Weingärtner B., Winnacker E. L., Tolun A., Pettersson U. Two complementary strand-specific termination sites for adenovirus DNA replication. Cell. 1976 Oct;9(2):259–268. doi: 10.1016/0092-8674(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Brown D. D., Reeder R. H. The molecular basis for length heterogeneity in ribosomal DNA from Xenopus laevis. J Mol Biol. 1976 Aug 25;105(4):461–486. doi: 10.1016/0022-2836(76)90229-1. [DOI] [PubMed] [Google Scholar]