Abstract
Background
Health-related quality of life (HRQOL) is an important aspect of well-being that may improve with health behavior interventions. However, health behavior change is difficult with pressure to maintain status quo.
Purpose
This report examines the effects of two lifestyle interventions and an advice-only condition on HRQOL. Effects of meeting behavioral goals and weight loss also were examined.
Methods
Participants were 295 men and 467 women (34% African American) with pre-hypertension or stage 1 hypertension from the PREMIER trial. HRQOL was assessed by the Short Form-36. Participants were assigned randomly to (1) advice only (ADVICE), (2) established guidelines for blood pressure control (EST), or (3) established guidelines plus the Dietary Approaches to Stop Hypertension (DASH) dietary pattern (EST + DASH).
Results
Assignment to EST resulted in improvement in three HRQOL subscales at 6 months and one at 18 months relative to ADVICE. EST + DASH improved in two subscales at 6 and 18 months compared with ADVICE. Across conditions, total fat, saturated fat, fruit, and vegetable intake change, along with ≥4-kg weight loss, resulted in HRQOL improvements at 6 and 18 months. No improvement was found for change in physical activity, and only a few HRQOL subscales were associated with change in sodium and low-fat dairy intake.
Conclusions
Intensive lifestyle interventions can result in improvements in HRQOL. Change in dietary intake and weight loss is also important.
Keywords: Health behavior interventions, Health-related quality of life, Randomized trials, Physical activity, DASH dietary pattern, Weight loss
Introduction
Health-related quality of life (HRQOL) is an important aspect of physical, social, and mental well-being. Measures of HRQOL describe how people perceive their health and function across physical, psychological, and social domains during their usual daily activities [1]. It is a predictor of morbidity and mortality [2, 3], and its importance is highlighted by its inclusion in both the National Health and Nutrition Examination and the Behavioral Risk Factor Surveillance System.
Disease prevention and treatment interventions often consider HRQOL outcomes when evaluating intervention efficacy. If treatments have deleterious effects on HRQOL, they are not likely to be adopted, or if they are initiated, are not maintained [4]. HRQOL measures are commonly assessed in pharmaceutical trials, but are less often included in health behavior lifestyle intervention trials [5]. While a few intervention studies have demonstrated improvements in HRQOL (e.g., [6, 7]), there have also been reports of unfavorable consequences. Physical activity interventions can result in musculoskeletal injury, which can decrease physical well-being [8, 9]. Early reports suggested that low-fat dietary patterns may increase depressive symptoms [10], and high consumption of fruits and vegetables can cause digestive symptoms that can adversely affect HRQOL [11]. Merely embarking on health behavior lifestyle change can impact interactions with family, friends, and peers, which may reduce perceptions of social well-being. Thus, it is imperative to assess the effects of lifestyle interventions on HRQOL.
This report is an analysis of PREMIER that examines the 6- and 18-month effects of health behavior lifestyle interventions on HRQOL. PREMIER was a randomized clinical trial that tested the effects of two multi-component interventions on blood pressure control among adults with pre-hypertension and stage 1 hypertension [12]. The interventions resulted in significant health behavior changes relative to an advice-only control condition, which in turn reduced weight and improved blood pressure [13–15]. For this report, we took several approaches: First, we evaluated the effects of the two interventions relative to each other and the advice-only control condition on HRQOL. Second, we evaluated how change in the health behaviors emphasized in PREMIER was associated with change in HRQOL. Finally, we examined change in HRQOL by the amount of weight lost. Because the previous literature suggests that lifestyle interventions can improve or decrease HRQOL, this study was exploratory and took a hypothesis-generating rather than hypothesis-testing approach.
Methods
PREMIER participants were men and women age 25 years or older with pre-hypertension and stage 1 hypertension who were recruited from four clinical centers (Baltimore, MD; Baton Rouge, LA; Durham, NC; and Portland, OR, USA). Kaiser-Permanente Center for Health Research served as the Coordinating Center, and the National Heart, Lung, and Blood Institute project office also participated. Participants were recruited from mass mailings, local advertisements, and local news stories. Men and women who responded to recruitment efforts were screened in person or over the telephone for eligibility and then invited to attend a series of in-person screening visits. Individuals who continued to meet all eligibility criteria and who agreed to study participation were randomized into the trial. The study was approved by each site's human subjects institutional review board, and all participants provided written informed consent. Trial methods and main results are published [13–15].
Eligibility Criteria
Blood pressure eligibility was systolic blood pressure between 120 and 159 mmHg and diastolic blood pressure between 80 and 95 mmHg [16]. Body mass index (kg/m2) (BMI) criterion was 18.5 to 45. Major exclusion criteria included use of anti-hypertensive medications, insulin, or oral hypoglycemic drugs; previous cardiovascular event or cancer diagnosis; and congestive heart failure or symptoms of angina or peripheral vascular disease.
Measures
Prior to randomization into the three treatment groups, participants underwent a series of screening visits in which baseline data were collected by trained staff. Participants underwent 6- and 18-month follow-up assessments at which HRQOL, BMI, physical activity, and dietary information were re-assessed. Data collection staff was masked to treatment group assignment.
HRQOL
The Rand 36-item Health Survey 1 [17] was used to measure HRQOL. It contains 36 items that assess eight health domains of physical and mental health. The physical health scales are physical functioning, role limitations due to physical health problems, bodily pain, and general health perceptions; the mental health scales include vitality, social functioning, role limitations due to personal or emotional health problems, and general emotional well-being. In addition, two summary scores are calculated, a physical health composite score and a mental health composite score. The items are identical to the Medical Outcomes Study Short Form-36 (SF-36) [18]. The instrument has good reliability and validity [19], and the survey subscales are robust in an obese population [20]. Internal consistency ranges from 0.78 to 0.93 [21]. Norm-based scoring was used for all scales, in which linear transformations are performed to transform scores to a mean of 50 and standard deviation of 10 for the US population [22]. This scoring method allows the scales' scores to be easily interpreted relative to the US norms. It also allows for direct comparison among each of the scales without regard to the different floor and ceiling effects of each of the individual scales. A higher score represents higher functioning for that dimension.
Body Mass Index
Height, without shoes, was measured using a wall-mounted stadiometer. Weight, in light indoor clothes without shoes, was measured using either a balance beam scale or a high-quality digital scale. BMI was calculated as weight in kilograms/height in square meters.
Physical Activity
Total daily energy expenditure was estimated from the interviewer-administered Stanford 7-day physical activity recall [23, 24]. Participants estimated the number of hours spent over the last 7 days in sleep, and in moderate, hard, and very hard activity, with light activity calculated to total 24 h. Moderate activity was quantified as hard as a brisk walk, very hard activity was like a run, and hard activity was defined as activity in between a brisk walk and a run. Minutes spent in moderate, hard, and very hard activity over the 7 days were summed to create a moderate to vigorous physical activity minutes per week score.
Dietary Intake
The Diet Assessment Center of Pennsylvania State University conducted two unannounced 24-h recalls collected by telephone interview, one on a weekday and one on a weekend day. Nutrients and food group intake were calculated using the Nutrition Data System NDS-R 1998 [25]. Daily servings of fruits, vegetables, and low-fat dairy, along with percent kilocalories from total fat and saturated fat, were obtained from this assessment. One 24-h urine collection was obtained to measure excretion of sodium.
Demographics
Participant age, race, and educational status were collected from self-report questionnaires.
Interventions
After baseline measurements were completed, participants were randomized into one of two lifestyle interventions or an advice-only group using procedures described by Appel et al. [13]. The “established” intervention promoted four lifestyle recommendations for blood pressure control advocated by the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure VI [26]. The “established plus DASH” intervention included the same established guidelines plus the Dietary Approaches to Stop Hypertension (DASH) dietary pattern [27]. The two interventions were similar in all respects except for the DASH dietary pattern. The established intervention targeted four behaviors: increase physical activity to at least 180 min/week, reduce daily sodium intake to less than or equal to 2,300 mg, and reduce daily total fat intake to less than or equal to 30% and saturated fat to less than or equal to 10% of calories. The established plus DASH intervention targeted six behaviors, including the same physical activity and sodium requirements as the established intervention plus daily total fat and saturated fat intake goals of ≤25% and 7% of calories, respectively. Additional goals were to increase daily fruit and vegetable intake to at least nine servings and to increase low-fat dairy servings to at least two servings per day. The weight loss goal for the established and established plus DASH interventions was 6.8 kg (approximately 15 lb) at 6 months for participants who were overweight or obese at baseline (BMI values≥25 kg/m2).
Both interventions consisted of 18 face-to-face contacts during the first 6 months of the intervention and 12 face-to-face contacts during the remaining 12 months [28]. Intervention strategies and materials were identical except for the dietary recommendations. Session content included behavioral skills training, self-monitoring, social support, group accountability, and goal setting. All intervention behaviors were addressed during each intervention contact; thus, multiple behavior changes were simultaneously targeted.
Participants assigned to the advice-only comparison condition received verbal advice and materials on lifestyle modifications at the time of randomization and after completion of the 6-month data collection. Recommendations included reducing dietary sodium intake, engaging in regular physical activity, losing weight if needed, and eating a healthful diet for general cardiovascular health.
Analysis
We compared differences in change in the HRQOL domains at 6 and 18 months by treatment status using generalized linear models, controlling for baseline HRQOL, age, and indicators of clinical center, cohort, sex, and race. Thus, the HRQOL items were the dependent variables, and treatment status was the independent variable in the model. Intention-to-treat analyses were conducted for all participants providing data at the follow-up time points, irrespective of having adhered to their randomized condition. Additional models were run combining all treatment groups to examine the effects of change in the lifestyle health behaviors (i.e., 24-h sodium excretion, daily servings of dairy and fruits and vegetables, percent daily kilocalories from total and saturated fat, and weekly physical activity minutes) on change in HRQOL, irrespective of treatment status. For these models, changes in HRQOL dimensions were the dependent variables, and items related to lifestyle health behaviors were the independent variables. To detect the independent contribution of each behavior change, models included change in all other health behaviors, as well as the baseline HRQOL, age, clinical center, cohort, sex, and race.
Weight loss was categorized as <4 or ≥4 kg (approximately 10 lb) from baseline to 6 months, and from baseline to 18 months. This amount of weight loss is associated with improvements in multiple cardiovascular risk factors. The weight loss analyses included only the 653 participants who were overweight at baseline (BMI≥25 kg/m2). Change in HRQOL was the dependent variable, and weight loss status was the independent variable for these models. Models were also run using weight loss as a continuous variable. Because results were very similar, we present them by weight loss category.
All analyses were conducted using SAS version 9.1 (SAS, Cary, NC, USA). Multiple-regression analyses were based on the generalized linear modeling procedure, which produced least squared means, unstandardized coefficients, and standard errors with F test statistics for each independent variable. All p values reported are two-sided. The term “significant” was used to describe a p value less than 0.05. In addition, Cohen's d was used to compute effect size, which provides an estimate of the strength of the difference between the means of each variable that is irrespective of the variable's unit of measure [29]. A commonly used criterion to judge effect size suggests 0.20 or less is small, 0.50 is medium, and 0.80 is a large difference of magnitude [30].
Results
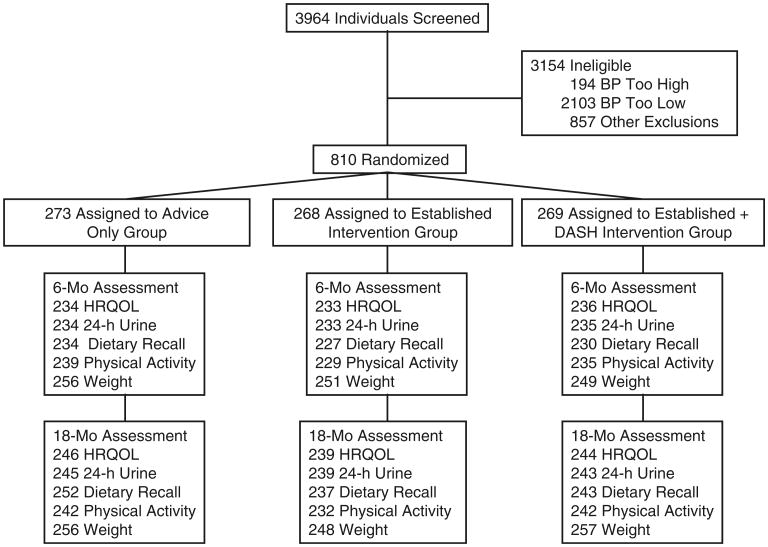
PREMIER randomized 810 participants into the trial. The flow of participants through the trial is displayed in Fig. 1. Of the total sample, 703 (87%) and 729 (90%) participants provided HRQOL information at 6 and 18 months, respectively. Approximately two thirds (61%) of participants are female, and 34% are African American. Baseline age was 50.2±8.9 years. Over half the sample attained at least a college degree. Table 1 displays the participant characteristics and baseline behavioral data, which did not differ by treatment status.
Fig. 1. The flow of participants through the trial.
Table 1. Baseline participant characteristics and behavioral data by treatment assignment.
| Advice only (N=273) | Established (N=268) | Established + DASH (N=269) | |
|---|---|---|---|
| Age (years)a | 49.5±8.8 | 50.2±8.6 | 50.2±9.3 |
| Female (%) | 63 | 65 | 57 |
| African American (%) | 37 | 37 | 29 |
| Education (% college graduate) | 54 | 64 | 56 |
| Body mass index (kg/m2)a | 32.9±5.6 | 33.0±5.5 | 33.3±6.3 |
| Sodium (mEq/24 h)a | 174.9±68.1 | 168.9±71.5 | 179.9±78.2 |
| Dairy (servings/day)a | 1.6±1.2 | 1.7±1.4 | 1.7±1.3 |
| Fruits and vegetables (servings/day)a | 4.4±2.2 | 4.5±2.3 | 4.7±2.4 |
| Total fat (% kcal)a | 32.5±7.3 | 33.5±8.0 | 33.2±7.7 |
| Saturated fat (% kcal)a | 10.8±3.3 | 11.0±3.2 | 10.9±3.1 |
| Moderate to vigorous physical activity (min/week)a | 207.7±261.0 | 224.3±291.7 | 216.0±370.4 |
Mean ± SD
At baseline, HRQOL scores indicated means slightly above the US population (i.e., mean scores above 50). Group means in the HRQOL subscales and composite scores are displayed in Table 2. Mean HRQOL scores at baseline did not differ by treatment assignment. At 6 and 18 months, adjusted mean change from baseline of the general health problems and vitality subscales were greater in the established + DASH groups compared with advice only. The established group also had greater change scores for the vitality subscale than advice only at 6 and 18 months. At 6 months, the role limitations–emotional scale significantly declined in the established group relative to the advice-only and established + DASH groups. The established group had a greater improvement in the physical composite score compared with advice only at 6 months. For the comparisons that reached statistical significance, effect sizes ranged from 0.19 comparing the mean difference in the established + DASH to the established group on 6-month role limitation–emotional scores to 0.31 for the comparison of the mean difference in the established + DASH to the advice-only group for the vitality subscale at 6 months. These effect sizes associated with these values are considered small.
Table 2. Baseline, 6-month change, and 18-month change in quality of life measures by intervention group.
| Quality of life measures (adjusted mean/mean change ± SE) | Intervention assignment | ||
|---|---|---|---|
|
| |||
| Advice only (N=219) | Established (N=221) | Established + DASH (N=219) | |
| Health-related quality of life (SF-36) | |||
| Physical functioning | |||
| Baseline | 52.1±0.4 | 51.9±0.4 | 52±0.4 |
| 6 months | −0.8±0.5 | 0±0.5 | 0.8±0.5a |
| 18 months | −0.4±0.5 | 0±0.5 | 0.3±0.5 |
| Role limitations–physical | |||
| Baseline | 52.0±0.5 | 51.3±0.5 | 51.9±0.5 |
| 6 months | 0±0.6 | 0.7±0.6 | −0.6±0.6 |
| 18 months | 0±0.7 | 0.4±0.7 | −0.4±0.7 |
| Bodily pain | |||
| Baseline | 54.6±0.4 | 54.2±0.4 | 53.8±0.5 |
| 6 months | −0.5±0.5 | 0.4±0.5 | 0.1 ±0.5 |
| 18 months | 0±0.5 | −0.7±0.5 | 0.6±0.5 |
| General health problems | |||
| Baseline | 51.7±0.5 | 50.8±0.5 | 50.5±0.5 |
| 6 months | −1 ±0.5 | 0.1 ±0.5 | 0.9±0.5b |
| 18 months | −1.3±0.5 | 0.1 ±0.5a | 1.3±0.5b |
| Vitality | |||
| Baseline | 51.2±0.6 | 49.7±0.6 | 49.7±0.6 |
| 6 months | −1.9±0.6 | 0.1 ±0.6b | 1.7±0.6b,c |
| 18 months | −1.2±0.6 | 0.7±0.6b | 0.6±0.6a |
| Social functioning | |||
| Baseline | 53.2±0.5 | 53.2±0.5 | 52.9±0.5 |
| 6 months | −0.3±0.6 | −0.2±0.6 | 0.6±0.6 |
| 18 months | 0.4±0.7 | −0.7±0.7 | 0.3±0.7 |
| Role limitations–emotional | |||
| Baseline | 52.3±0.5 | 52.2±0.5 | 51.6±0.6 |
| 6 months | 0.9±0.6 | −1.5±0.6b | 0.5±0.6d |
| 18 months | 0.3±0.7 | −0.3±0.7 | 0±0.7 |
| General mental health | |||
| Baseline | 53.5±0.5 | 52.7±0.5 | 52.4±0.5 |
| 6 months | −0.2±0.5 | −0.2±0.5 | 0.4±0.5 |
| 18 months | 0.2±0.5 | −0.3±0.5 | 0.1 ±0.5 |
| Physical composite score | |||
| Baseline | 52.3±0.4 | 51.8±0.4 | 52.0±0.5 |
| 6 months | −1 ±0.5 | 0.8±0.5b | 0.2±0.5 |
| 18 months | −0.6±0.5 | 0.1 ±0.5 | 0.5±0.5 |
| Mental composite score | |||
| Baseline | 52.6±0.5 | 52.0±0.5 | 51.5±0.6 |
| 6 months | 0.1±0.6 | −1±0.6 | 0.9±0.6c |
| 18 months | 0.1±0.6 | −0.3±0.6 | 0.2±0.6 |
All analyses adjusted for baseline value, clinical center, cohort, age, and race/ethnicity
p<0.10 change in comparison with change in advice only
p<0.05 change in comparison with change in advice only
p<0.10 change in comparison with change in established
p<0.05 change in comparison with change in established
Dietary change, particularly percent daily caloric intake in total fat and saturated fat, was significantly associated with change in HRQOL scores (Table 3). Decrease in total and saturated fat intake was significantly associated with improvement in physical functioning (18 months), role limitations–physical (18 months), general health problems (6 and 18 months), vitality (6 and 18 months), and physical composite scores (6 and 18 months). Additionally, a decrease in saturated fat intake was significantly associated with increased physical functioning and role limitations–physical at 6 months and bodily pain and social functioning scores at 18 months. The coefficients indicate that a ten-unit change in percent kilocalories from total fat and saturated fat intake was associated with an increase in physical functioning scores of approximately 0.6 (p=0.04) and 1.8 units (p=0.01), respectively, from baseline to 18 months independent of other dietary and physical activity changes.
Table 3. Mean change in quality of life measures by change in health behaviors.
| Sodium (mEq/24h) Unstandardized parameter estimate ± SE | Low-fat dairy (servings/day) Unstandardized parameter estimate ± SE | Fruits and vegetables (servings/day) Unstandardized parameter estimate ± SE | Total fat (% kcal) Unstandardized parameter estimate ± SE | Saturated fat (% kcal) Unstandardized parameter estimate ± SE | Physical activity (min/week) Unstandardized parameter estimate ± SE | |
|---|---|---|---|---|---|---|
| Health-related quality of life(SF-36) | ||||||
| Physical functioning | ||||||
| 6-month change | 0±0.004 | 0.059±0.184 | 0.128±0.077c | −0.043±0.026 | −0.147±0.065a | 0±0.001 |
| 18-month change | 0±0.003 | 0.190±0.19 | 0.137±0.075c | −0.058±0.027a | −0.179±0.069b | 0.001 ±0.001 |
| Role limitations–physical | ||||||
| 6-month change | −0.01±0.004a | −0.445±0.226a | −0.114±0.094 | −0.051 ±0.032 | −0.194±0.08a | 0±0.001c |
| 18-month change | 0±0.004 | −0.133±0.255 | 0.051±0.101 | −0.076±0.037a | −0.303±0.093b | 0±0.001 |
| Bodily pain | ||||||
| 6-month change | 0±0.004 | −0.218±0.184 | −0.082±0.077 | 0±0.026 | −0.014±0.065 | 0±0.001 |
| 18-month change | 0.006±0.003c | 0.275±0.195 | 0.252±0.077b | −0.049±0.028c | −0.170±0.071a | 0±0.001 |
| General health problems | ||||||
| 6-month change | −0.01 ±0.004c | −0.160±0.185 | 0.094±0.077 | −0.091 ±0.026b | −0.241±0.065b | 0.001 ±0.001 |
| 18-month change | 0±0.003 | 0.068±0.189 | 0.236±0.075b | −0.079±0.027b | −0.270±0.068b | 0±0.001 |
| Vitality | ||||||
| 6-month change | −0.01 ±0.004 | −0.466±0.224a | 0.217±0.093a | −0.085±0.032b | −0.252±0.079b | 0±0.001 |
| 18-month change | 0.001±0.004 | −0.305±0.228 | 0.159±0.09c | −0.077±0.033a | −0.257±0.082b | 0.002±0.001c |
| Social functioning | ||||||
| 6-month change | 0.001±0.005 | −0.152±0.232 | 0.062±0.097 | −0.031 ±0.033 | −0.085±0.082 | 0±0.001 |
| 18-month change | −0.002±0.004 | 0.021±0.241 | −0.010±0.096 | −0.043±0.035 | −0.208±0.087a | 0.002±0.001 |
| Role limitations–emotional | ||||||
| 6-month change | −0.003±0.005 | −0.376±0.232 | −0.005±0.097 | −0.012±0.033 | −0.038±0.083 | −0.001±0.001 |
| 18-month change | 0.001±0.004 | −0.077±0.244 | −0.031 ±0.097 | −0.044±0.035 | −0.116±0.089 | 0±0.001 |
| General mental health | ||||||
| 6-month change | −0.004±0.004 | −0.074±0.191 | 0.083±0.08 | −0.017±0.027 | −0.063±0.068 | 0±0.001 |
| 18-month change | 0±0.003 | −0.107±0.2 | 0.048±0.079 | −0.020±0.029 | −0.089±0.073 | 0±0.001 |
| Physical composite score | ||||||
| 6-month change | −0.005±0.004 | −0.179±0.186 | −0.002±0.078 | −0.053±0.027a | −0.185±0.066b | 0±0.001 |
| 18-month change | 0±0.003 | 0.168±0.199 | 0.211±0.078b | −0.071 ±0.029b | −0.256±0.072b | 0.001 ±0.001 |
| Mental composite score | ||||||
| 6-month change | −0.003±0.004 | −0.266±0.221 | 0.100±0.092 | −0.028±0.032 | −0.074±0.078c | 0±0.001 |
| 18-month change | 0±0.004 | −0.186±0.225 | −0.020±0.089 | −0.031 ±0.032 | −0.122±0.082 | 0±0.001 |
Analyses were completed after combining treatment groups. Analyses were adjusted for baseline value, other health behavior variables included in the table, clinical center, cohort, age, sex, and race/ethnicity
p<0.05
p<0.01
p<0.10
Increase in daily servings of fruits and vegetables was associated with improvement in bodily pain (18 months), general health problems (18 months), vitality (6 months), and physical composite scores (18 months) (Table 3). The improvements were modest: An increase in one daily serving of fruits and vegetables resulted in increased HRQOL scores of magnitudes between 0.21 and 0.25 units. A decrease in one daily serving of low-fat dairy intake at 6 months was associated with a 0.44 higher score in role limitations–physical (p=0.05) and a 0.47 higher vitality score (p=0.04). Change in physical activity was not independently associated with change in any of the HRQOL component scores.
Mean HRQOL domains by weight loss status at 6 months are displayed in Table 4. At baseline, there was no difference in HRQOL scores between those categorized as losing at least 4 kg weight versus not at either time point. However, those who lost at least 4 kg at 6 months had significant improvements in physical functioning, role limitations–physical, role limitations–emotional, and mental composite score at 6 months, and general health problems, vitality, general mental health, and physical composite score relative to those who did not lose at least 4 kg from baseline to 6 months. For example, those who lost at least 4 kg at 6 months had, on average, a 2.5-unit increase in physical functioning score between baseline and 6 months compared with a mean 0.6 increase for participants who lost less than 4 kg. The group that lost more weight also had a 2.3-unit increase in general mental health, whereas the group that lost less weight had a mean change of zero (p<0.0001). Effect sizes for the significant differences in HRQOL scores between participants who did and did not lose 4 kg at 6 months ranged from 0.16 (small magnitude) for the 18-month physical functioning score change to 0.47 (medium magnitude) for 6-month vitality score change.
Table 4. Change in quality of life measures by achievement of weight loss goal.
| 6-month weight loss | 18-month weight loss | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| (Adjusted mean/mean change ± SE) | >4kg (N=270) | ≤4kg (N=389) | Effect size | >4kg (N=255) | ≤4kg (N=429) | Effect size |
| Health-related quality of life (SF-36) | ||||||
| Physical functioning | ||||||
| Baseline | 51.9±0.4 | 52.0±0.3 | 51.8±0.4 | 52.0±0.3 | ||
| 6-month change | 2.5±1.4 | 0.6±1.4a | 0.273 | 2.4±1.5 | 1.0±1.4a | 0.214 |
| 18-month change | 0.8±1.5 | 0.2±1.5b | 0.157 | 1.6±1.5 | −0.2±1.5a | 0.272 |
| Role limitations-physical | ||||||
| Baseline | 52.0±0.5 | 51.9±0.4 | 52.0±0.5 | 52.0±0.4 | ||
| 6-month change | 1.9±1.9 | 0.1±1.9b | 0.248 | 1.5±2.0 | 0.5±1.9 | 0.153 |
| 18-month change | −0.1 ±2.1 | −1.1±2.0 | 0.141 | 0.3±2.1 | −1.0±2.0c | 0.172 |
| Bodily pain | ||||||
| Baseline | 53.8±0.4 | 54.3±0.3 | 54.2±0.4 | 54.2±0.3 | ||
| 6-month change | −0.6±1.4 | −1.6±1.4c | 0.167 | −0.3±1.5 | −1.5±1.4b | 0.150 |
| 18-month change | −0.2±1.6 | −1.2±1.5c | 0.174 | 0.8±1.6 | −1.2±1.5a | 0.276 |
| General health problems | ||||||
| Baseline | 50.3±0.5 | 51.2±0.4 | 50.3±0.5 | 51.3±0.4 | ||
| 6-month change | 4.2±1.4 | 1.3±1.4a | 0.410 | 4.0±1.5 | 1.9±1.4a | 0.297 |
| 18-month change | 4.2±1.5 | 1.6±1.4a | 0.372 | 5.0±1.5 | 1.9±1.4a | 0.470 |
| Vitality | ||||||
| Baseline | 50.2±0.5 | 50.3±0.4 | 49.7±0.6 | 50.8±0.4 | ||
| 6-month change | 4.7±1.7 | 0.9±1.7a | 0.474 | 4.6±1.8 | 1.6±1.7a | 0.395 |
| 18-month change | 0.8±1.5 | 0.2±1.5a | 0.256 | 4.5±1.8 | 1.3±1.7a | 0.428 |
| Social functioning | ||||||
| Baseline | 53.0±0.5 | 53.0±0.4 | 53.3±0.4 | 53.1±0.4 | ||
| 6-month change | 4.3±1.8 | 3.5±1.8 | 0.160 | 3.9±1.9 | 3.7±1.8 | 0.018 |
| 18-month change | 3.0±1.9 | 2.8±1.9 | 0.048 | 3.8±1.9 | 2.6±1.9c | 0.147 |
| Role limitations-emotional | ||||||
| Baseline | 52.4±0.5 | 51.7±0.4 | 51.8±0.5 | 52.2±0.4 | ||
| 6-month change | 2.1 ±2.0 | 0.3± 1.9b | 0.225 | 0.9±2.0 | 0.9±1.9 | 0.058 |
| 18-month change | 0.5±2.0 | 0.5±2.0 | 0.012 | 0.1±2.1 | 0.6±2.0 | 0.004 |
| General mental health | ||||||
| Baseline | 53.0±0.5 | 53.1±0.4 | 52.4±0.5 | 53.5±0.3 | ||
| 6-month change | 2.3±1.4 | 0.0±1.4a | 0.319 | 1.5±1.1 | 0.6±1.4c | 0.146 |
| 18-month change | 2.4±1.5 | 1.2±1.5b | 0.143 | 2.2±1.5 | 1.5±1.5 | 0.115 |
| Physical composite score | ||||||
| Baseline | 51.6±0.4 | 52.2±0.3 | 52.0±0.5 | 53.5±0.3 | ||
| 6-month change | 1.8±1.5 | 0.0±1.4a | 0.262 | 2.0±1.5 | 0.3±1.4a | 0.242 |
| 18-month change | 0.8±1.5 | −0.8±1.5a | 0.246 | 1.9±1.6 | −0.8±1.5a | 0.377 |
| Mental composite score | ||||||
| Baseline | 52.3±0.5 | 52.0±0.3 | 51.7±0.5 | 52.5±0.4 | ||
| 6-month change | 3.5±1.7 | 1.3±1.7a | 0.284 | 2.5±1.7 | 1.9±1.7 | 0.102 |
| 18-month change | 2.7±1.7 | 2.1±1.7 | 0.057 | 2.6±1.8 | 2.3±1.7 | 0.075 |
Only the participants with a BMI>25 kg/m2 at baseline were included in this analysis (N=653). Analyses were completed after combining intervention group. Analyses were adjusted for baseline value, clinical center, cohort, age, and race/ethnicity
p<0.01
p<0.05
p<0.10
Table 4 also displays mean change in HRQOL by weight loss at 18 months. For those who lost at least 4 kg at 18 months, improvements in physical functioning, bodily pain, general health problems, vitality, and physical composite score were noted at 6 and 18 months compared with those who did not lose weight at 18 months. For example, those who lost at least 4 kg at 18 months showed, on average, a 4.6- and 4.5-unit increase in vitality score from baseline to 6 and 18 months, respectively, compared with a mean 1.9-unit increase over the same time periods in participants who lost less than 4 kg at 18 months (moderate effect sizes).
Discussion
Relative to advice only, both intervention groups modestly improved in one physical health HRQOL dimension—vitality—at both 6 and 18 months. The established + DASH group also had 6- and 18-month improvement in the general health problems subscale. Regardless of intervention status, change in percent daily calories from saturated fat at 6 and 18 months was independently associated with change in most of the HRQOL physical dimensions, with similar results for change in percent daily calories from total fat, although fewer dimensions were significant. Improvements in a few HRQOL physical dimensions were also noted with increased servings of fruits and vegetables. Losing at least 4 kg at either 6 or 18 months resulted in improvement in most physical health dimensions.
This is one of the first reports to document positive changes in HRQOL resulting from multi-component lifestyle interventions relative to a control condition. Toobert et al. did not observe differences in HRQOL from a multi-component lifestyle intervention compared with controls [31]. In a subsample of participants from the DASH trial, changes from the nutritional interventions were noted only in the health change subscale relative to controls [32]. Steptoe and colleagues found significant improvements in physical and mental health composite scores from baseline to 8 weeks and 1 year follow-up in two interventions matched by contact visits but differing in content (e.g., behavioral counseling versus fruit and vegetable nutritional education counseling interventions) [5]. The study design did not include a control group. Using different HRQOL assessment measures that were more specific to possible changes resulting from a dietary intervention, Corle and colleagues found improvements from a low-fat, high-fiber and fruits and vegetables intervention that persisted for 4 years in self-care, health action, and health beliefs domains compared with controls [4]. Our results support the notion that multi-component lifestyle interventions can improve general physical health QOL outcomes.
We found that decrease in total and saturated fat resulted in a number of HRQOL improvements, with fewer HRQOL associations for increasing fruits and vegetables intake. Steptoe et al. [5] noted that 8-week change in fruit and vegetable intake was associated with change in physical health composite score, but they did not measure dietary fat intake. It is generally thought that reducing daily fat intake concomitantly increases fruit and vegetable consumption. In PREMIER, however, we previously reported only modest correlations between change in total fat and fruits and vegetable intake (r=−0.31) [33]. Change in daily fat intake may confer unique benefits on physical health QOL. The relationship between low-fat dairy consumption and QOL requires further study. Although the DASH diet emphasizes increasing low-fat dairy to ≥2 servings a day, we found that decreased consumption of low-fat dairy was associated with short-term improvements in physical role limitations and vitality.
Increased physical activity did not improve HRQOL when controlling for concomitant dietary changes, although others have found positive associations. Cross-sectional BRFSS data indicate that those who met recommended levels of physical activity had higher levels of HRQOL than those who did not [34]. Wendel-Vos and colleagues reported higher SF-36 scores for most dimensions with higher physical activity quintile [35]. Five-year change in physical activity was associated with improvement in social functioning in men and women and bodily pain, general health perceptions, and the physical health composite score for men. Our preliminary analyses, without controlling for dietary changes, resulted in a number of significant HRQOL associations. However, we found that change in physical activity had low correlations with change in dietary patterns (total fat (r=−0.09), fruit and vegetable servings (r=0.04), and sodium intake (r = −0.12)) [33], so it is perplexing that associations were no longer significant in our final models. Potential associations among physical activity and HRQOL are clearly complex and deserve continued investigation, particularly how other health behaviors may change concurrently with physical activity.
For those who lost more than 4 kg, physical health QOL improved at 6 and 18 months for almost all subscales. In a report of randomized, controlled trials of sibutramine versus control, Samsa et al. also found improvements across weight loss category in the physical health subscales at medium (24–28 weeks) and long-term (52 weeks) follow-up [36]. Others have reported improvements in physical health QOL with weight loss interventions [37–39], and fewer studies also have noted sustained improvements in mental health sub-scales [38, 39]. Weight loss interventions appear to improve the physical health QOL subscales, with the vitality scale showing the most consistent improvement [40].
The differences in HRQOL scores we found, although statistically significant, were modest. It is difficult to compare results across studies to determine if the effects we found are clinically significant. Although the SF-36 is a widely used instrument, HRQOL is reported in the extant literature using a variety of instruments. The current norm-based SF-36 scoring method precludes comparisons of previous studies using older scoring methods. In a comparison of long-term mortality outcome in patients after cardiac arrest, Steinberg and colleagues found between seven- and 13-point baseline differences in the SF-36 subscales between those who survived through follow-up and those died [3]. In contrast, the greatest between-group difference we found was less than two points for the vitality subscale at 6 months between the established plus DASH and the advice-only groups. HRQOL is a relatively new construct to be included in lifestyle intervention measurement protocols. Because the extant literature is so scarce, it remains unclear how HRQOL may change from interventions, particularly in healthy populations. Our data and those from others, however, clearly indicate that weight loss is likely to positively influence HRQOL [33–37], which we found even in a generally healthy sample. To be able to establish the effects of individual lifestyle behavioral components on HRQOL, we recommend that a standard measure of HRQOL, particularly the SF-36, be regularly included in behavior change interventions.
It is important to develop a better understanding of the association among dietary and physical activity change and HRQOL. Elavsky and colleagues propose that associations between increased physical activity and QOL may be mediated by improved self-efficacy, self-esteem, and positive affect [41]. Blissmer et al. found improvements in some HRQOL domains after weight loss that remained above baseline levels at 24 months even after weight regain [38]. They suggest that the social interaction and social support offered from the treatment may serve to increase HRQOL. It is important to examine potential psychosocial and cognitive mediating effects to understand how lifestyle change can improve HRQOL.
This study has a number of strengths. PREMIER used widely disseminated behavioral recommendations for blood pressure control—hypertension and pre-hypertension are present in approximately two thirds of the US population [42]. HRQOL is rarely reported in trials [43]. We report these results and use the SF-36 instrument, which is a well-established instrument that is valid in a number of population subgroups. Other measures used in the trial are also valid and reliable. Our sample size was large and sufficient to examine treatment differences and differences among those who did and did not achieve behavioral and weight loss goals. The trial continued for 18 months with an outstanding 93% follow-up.
There are limitations as well. Clinical trials typically enroll highly motivated individuals, which limits generalizability. Generalizability is also limited by HRQOL scores that are higher than the average population. The changes in HRQOL were notably modest; however, researchers have a poor understanding of clinically significant/relevant changes in this instrument. Further work is needed to understand the associated benefit of modest HRQOL changes in generally healthy populations. While it is a strength that we included the 18-month data, this was during an active intervention phase. Future research should determine if there are sustained QOL improvements after the intervention is terminated.
In conclusion, our results suggest that more intensive lifestyle interventions can result in notable physical health improvement in HRQOL, particularly for general health problems and vitality domains, among generally healthy individuals with higher than optimal blood pressure. Change in dietary intake appears to play a key role in improving physical HRQOL, and weight loss improves both the physical and mental HRQOL components. While controlled studies consider HRQOL outcomes when evaluating efficacy of interventions, few studies have examined the effects of multi-component lifestyle interventions on HRQOL outcomes. This study provides unique results compared with others found in the HRQOL literature by providing evidence of improved physical well-being with regard to multi-component lifestyle interventions.
Acknowledgments
This work was supported by NIH grants UO1 HL60570, UO1 HL60571, UO1 60573, UO1 HL60574, and UO1 HL62828. We thank Dr. Chuhe Chen and Ms. Gayle Meltesen for conducting the statistical analyses, and the participants from all the clinical centers for participating in the trial.
Footnotes
Conflict of interest statement: The authors have no conflict of interest to disclose.
Contributor Information
Deborah Rohm Young, Email: dryoung@umd.edu, University of Maryland School of Public Health, 2234 SPH, College Park, MD 20742, USA.
Janelle Coughlin, Johns Hopkins Medical Institutions, Baltimore, MD, USA
Gerald J. Jerome, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Towson University, Baltimore, MD, USA
Valerie Myers, Pennington Biomedical Research Center, Baton Rouge, LA, USA
Soo Eun Chae, University of Maryland School of Public Health, 2234 SPH, College Park, MD 20742, USA
Phillip J. Brantley, Pennington Biomedical Research Center, Baton Rouge, LA, USA
References
- 1.Centers for Disease Control and Prevention CDC. Health-related quality-of-life measures—United States, 1993. MMWR Morb Mortal Wkly Rep. 1995;44:195–200. [PubMed] [Google Scholar]
- 2.O'Loughlin C, Murphy NF, Conlon C, O'Donovan A, Ledwidge M, McDonald K. Quality of life predicts outcome in a heart failure disease management program. Int J Cardiol. 2010;139:60–67. doi: 10.1016/j.ijcard.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg JS, Joshi S, Schron EB, Powell J, Hallstrom A, McBurnie M. Psychosocial status predicts mortality in patients with life-threatening ventricular arrhythmias. Heart Rhythm. 2008;5:361–365. doi: 10.1016/j.hrthm.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Corle DK, Sharbaugh C, Mateski DJ, et al. Self-rated quality of life measures: Effect of change to a low-fat, high-fiber, fruit and vegetable enriched diet. Ann Behav Med. 2001;23:198–207. doi: 10.1207/S15324796ABM2303_7. [DOI] [PubMed] [Google Scholar]
- 5.Steptoe A, Perkins-Porras L, Hilton S, Rink E, Cappuccio FP. Quality of life and self-rated health in relation to changes in fruit and vegetable intake and in plasma vitamins C and E in a randomised trial of behavioural and nutritional education counselling. Br J Nutr. 2004;92:177–184. doi: 10.1079/BJN20041177. [DOI] [PubMed] [Google Scholar]
- 6.Block G, Sternfeld B, Block CH, et al. Development of Alive! (A Lifestyle Intervention Via Email), and its effect on health-related quality of life, presenteeism, and other behavioral outcomes: Randomized controlled trial. J Med Internet Res. 2008;10:e43. doi: 10.2196/jmir.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian Y, Zhang J, Lin Y, et al. A tailored target intervention on influence factors of quality of life in Chinese patients with hypertension. Clin Exp Hypertens. 2009;31:71–82. doi: 10.1080/10641960802409804. [DOI] [PubMed] [Google Scholar]
- 8.Ory M, Resnick B, Jordan PJ, et al. Screening, safety, and adverse events in physical activity interventions: Collaborative experiences from the behavior change consortium. Ann Behav Med. 2005;(29 Suppl):20–28. doi: 10.1207/s15324796abm2902s_5. [DOI] [PubMed] [Google Scholar]
- 9.Goodrich DE, Larkin AR, Lowery JC, Holleman RG, Richardson CR. Adverse events among high-risk participants in a home-based walking study: A descriptive study. Int J Behav Nutr Phys Act. 2007;4:20. doi: 10.1186/1479-5868-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: A quantitative review of primary prevention trials. BMJ. 1990;301:309–314. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Warner SA, Elmer PJ, Tharp TM, et al. Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol Biomarkers Prev. 2000;9:307–317. [PubMed] [Google Scholar]
- 12.Svetkey LP, Harsha DW, Vollmer WM, et al. Premier: A clinical trial of comprehensive lifestyle modification for blood pressure control: Rationale, design and baseline characteristics. Ann Epidemiol. 2003;13:462–471. doi: 10.1016/s1047-2797(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 14.Svetkey LP, Erlinger TP, Vollmer WM, et al. Effect of lifestyle modifications on blood pressure by race, sex, hypertension status, and age. J Hum Hypertens. 2005;19:21–31. doi: 10.1038/sj.jhh.1001770. [DOI] [PubMed] [Google Scholar]
- 15.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-Month results of a randomized trial. Ann Intern Med. 2006;144:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- 20.Corica F, Corsonello A, Apolone G, Lucchetti M, Melchionda N, Marchesini G. Construct validity of the Short Form-36 Health Survey and its relationship with BMI in obese outpatients. Obesity. 2006;14:1429–1437. doi: 10.1038/oby.2006.162. [DOI] [PubMed] [Google Scholar]
- 21.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Konsinski M, Seller SD. SF-36 physical and mental health summary scales: A user manual. Boston: The Health Institute; 1994. [Google Scholar]
- 23.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 24.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 25.Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products - A research perspective. Journal of Food Composition and Analysis. 2001;14:315–322. [Google Scholar]
- 26.The sixth report of the Joint National Committee on prevention, detection evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 27.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 28.Funk KL, Elmer PJ, Stevens VJ, et al. PREMIER—A Trial of Lifestyle Interventions for Blood Pressure Control: Intervention Design and Rationale. Health Promot Pract. 2008;9:271–280. doi: 10.1177/1524839906289035. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis. Current Direction Psychological Science. 1992;1:98–101. [Google Scholar]
- 30.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Toobert DJ, Glasgow RE, Strycker LA, et al. Biologic and quality-of-life outcomes from the Mediterranean Lifestyle Program: A randomized clinical trial. Diabetes Care. 2003;26:2288–2293. doi: 10.2337/diacare.26.8.2288. [DOI] [PubMed] [Google Scholar]
- 32.Plaisted CS, Lin PH, Ard JD, McClure ML, Svetkey LP. The effects of dietary patterns on quality of life: A substudy of the Dietary Approaches to Stop Hypertension trial. J Am Diet Assoc. 1999;99:S84–S89. doi: 10.1016/s0002-8223(99)00421-6. [DOI] [PubMed] [Google Scholar]
- 33.Young DR, Vollmer WM, King AC, et al. Can individuals meet multiple physical activity and dietary behavior goals? Am J Health Behav. 2009;33:277–286. doi: 10.5993/ajhb.33.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown DW, Balluz LS, Heath GW, et al. Associations between recommended levels of physical activity and health-related quality of life. Findings from the 2001 Behavioral Risk Factor Surveillance System (BRFSS) survey. Prev Med. 2003;37:520–528. doi: 10.1016/s0091-7435(03)00179-8. [DOI] [PubMed] [Google Scholar]
- 35.Wendel-Vos GC, Schuit AJ, Tijhuis MA, Kromhout D. Leisure time physical activity and health-related quality of life: Cross sectional and longitudinal associations. Qual Life Res. 2004;13:667–677. doi: 10.1023/B:QURE.0000021313.51397.33. [DOI] [PubMed] [Google Scholar]
- 36.Samsa GP, Kolotkin RL, Williams GR, Nguyen MH, Mendel CM. Effect of moderate weight loss on health-related quality of life: An analysis of combined data from 4 randomized trials of sibutramine vs placebo. Am J Manag Care. 2001;7:875–883. [PubMed] [Google Scholar]
- 37.Fontaine KR, Barofsky I, Bartlett SJ, Franckowiak SC, Andersen RE. Weight loss and health-related quality of life: Results at 1-year follow-up. Eat Behav. 2004;5:85–88. doi: 10.1016/S1471-0153(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 38.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: Intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4:43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification—a 2-y follow-up study. Int J Obes Relat Metab Disord. 2003;27:1233–1241. doi: 10.1038/sj.ijo.0802379. [DOI] [PubMed] [Google Scholar]
- 40.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 41.Elavsky S, McAuley E, Motl RW, et al. Physical activity enhances long-term quality of life in older adults: Efficacy, esteem, and affective influences. Ann Behav Med. 2005;30:138–145. doi: 10.1207/s15324796abm3002_6. [DOI] [PubMed] [Google Scholar]
- 42.National Center for Health Statistics. Chartbook on the Health of Americans. Hyattsville: National Center for Health Statistics; 2008. [Google Scholar]
- 43.Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF-36 outcomes in randomised trials: Systematic review. BMJ. 2009;338:a3006. doi: 10.1136/bmj.a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]