Abstract
Objectives
Determine whether assessment of left ventricular ejection fraction (LVEF) enhances prediction of new onset heart failure (HF) and cardiovascular mortality over and above N-terminal pro–B-type natriuretic peptide (NT-proBNP) level in older adults.
Background
Elevated NT-proBNP levels are common in older adults and associated with increased risk of HF.
Methods
NT-proBNP and LVEF were measured in 4,137 older adults free of HF. Repeat measures of NT-proBNP were performed 2–3 years later and echocardiography was repeated 5 years later (n=2,375) with a median follow-up of 10.7 years. The addition of an abnormal (<55%) LVEF (n=317 [7.7%]) to initially elevated or rising NT-proBNP levels was evaluated to determine risk of HF or cardiovascular mortality. Change in NT-proBNP levels were also assessed for estimating the risk of conversion from a normal to abnormal LVEF.
Results
For participants with a low baseline NT-proBNP level (<190 pg/mL) (n=2,918), addition of an abnormal LVEF didn’t improve the estimation of risk of HF and identified a moderate increase in adjusted risk for cardiovascular mortality (HR 1.69; 95%CI: 1.22 to 2.31). Among those whose NT-proBNP subsequently increased ≥ 25% to ≥190 pg/mL, an abnormal LVEF was likewise associated with an increased risk of cardiovascular mortality but not HF. Participants with an initially high NT-proBNP (≥190 pg/mL) were at greater risk overall for both outcomes, and those with an abnormal LVEF were at the highest risk. However, an abnormal LVEF did not improve model classification or risk stratification for either endpoint when added to demographic factors and change in NT-proBNP. An initially elevated NT-proBNP or rising level was associated with an increased risk of developing an abnormal LVEF.
Conclusion
Assessment of LVEF in HF free older adults based on NT-proBNP levels should be considered on an individual basis, as such assessments do not routinely improve prognostication.
Keywords: Elderly, heart failure, echocardiography, natriuretic peptides, outcomes
Introduction
Detection of depressed left ventricular function may improve prevention and treatment of progression to symptomatic heart failure (1). In adults over 50 years of age, the presence of even a mildly abnormal LVEF (LVEF≤55%) is associated with an approximately three-fold increased risk of developing heart failure and a two-fold increased risk of mortality compared to individuals with normal LVEF (2–4). Despite declines in the rates of cardiovascular deaths in the general population, more than 80% of cardiovascular deaths occur in older adults (5). However, with a relatively low prevalence (< 8%) of an abnormal LVEF even in those ≥ 65 years, it is difficult to advocate a routine imaging strategy in this population (3,6). Elevated natriuretic peptide levels are associated with depressed LVEF in the general population including older adults (7,8). Elevated NT-proBNP levels are also associated with an increased risk of new-onset heart failure in general population studies (9,10). Currently neither assessment of natriuretic peptides or LVEF is recommended for general population screening (11). However, potentially a combination of both measures would refine risk stratification to identify subjects who could benefit from therapies to reduce the risk of progression to heart failure (12). Following recommendations from recent guidelines for biomarker assessment of risk, we sought to determine the additional prognostic impact of likely downstream testing with echocardiography based on NT-proBNP results in this population (13). Second, to establish if NT-proBNP levels are a biochemical precursor to left ventricular systolic dysfunction in older adults, we investigated whether an elevated or rising NT-proBNP level identifies individuals at risk of progression from a normal to an abnormal LVEF based on sequential echocardiography.
Methods
Study Population
The CHS is a multicenter prospective observational cohort study of cardiovascular disease in independently living older adults (age ≥ 65 years) recruited from four communities and consists of the original cohort recruited in 1989 to 1990, and those enrolled in 1992 to 1993 when the study was expanded to include more African-Americans. A detailed description of the study methods has been published previously (14).
Of the 5,888 CHS participants, subjects were included if they had no prevalent heart failure, interpretable echocardiograms and sufficient serum for NT-proBNP measurement. Ultimately, 4,188 (71.1%) participants were included in this analysis (figure 1). Participants with sufficient sera volumes and an initial LVEF assessment were modestly more likely to be female and less likely to be African-American and diabetic than those without sufficient sera and/or initial EF measurement (supplemental table 1), but other factors were not different.
Figure 1. Flow chart of CHS participants included in this analysis.
Flow chart of the CHS (Cardiovascular Health Study) participants with blood samples available for amino terminal B-type natriuretic peptide (NT-proBNP) testing and echocardiography performed at baseline and again during follow-up. HF, heart failure; LVEF, left ventricular ejection fraction.
The institutional review boards of the University of Washington and the participating centers approved the CHS. The institutional review board of the University of Maryland, Baltimore, approved the current analysis.
Echocardiography
The design for the echocardiographic evaluation of CHS participants has been described previously (15). In summary, two-dimensional echocardiography was performed in 1989–1990 and again in 1994–1995. For the original cohort, this corresponded to the baseline visit and five years later. For the second cohort, this resulted in a single echocardiogram two years after the baseline visit. Global left ventricular systolic function was qualitatively assessed from the two-dimensional echocardiogram as normal (LVEF ≥ 55%), borderline (LVEF ≥ 45% to <55%) or subnormal (LVEF < 45%) ejection fraction. LVEF was qualitatively interpreted in 99% of the original CHS cohort, with inter-reader agreement of 94% and intra-reader agreement of 98% of paired studies (16). For this analysis, subjects with a borderline or subnormal LVEF were grouped together and classified as having an “abnormal” LVEF. In addition we report measures of Doppler mitral inflow peak E and peak A velocities and left atrial size measured by linear dimensions based on 2-dimensionallly directed M-mode (17).
Assay methods
NT-proBNP was measured in serum collected at baseline in the main CHS cohort (1989–90) and the second cohort (1992–93). A second measure of NT-proBNP was performed on sera collected 3 years later for the main cohort (1992–1993) and 2 years later for the second cohort (1994–1995).
All samples were stored at −70° to −80° C and were thawed prior to testing (maximum of three freeze-thaw cycles). NT-proBNP was measured using electrochemiluminescence immunoassay on the Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN). The coefficient of variation for the NT-proBNP assay was 2–5% during the testing period, and the analytical measurement range for NT-proBNP was 5–35,000 pg/mL. Baseline NT-proBNP levels ≥190 pg/mL (the 70th percentile for the study population), were considered elevated based on a previously identified cut-off best corresponding with increased risk of heart failure in this population (10).
Primary Outcomes
Outcomes were incident heart failure and cardiovascular mortality. Incident heart failure events were ascertained through review of medical records, by participant interview at annual study visits, and semi-annual phone calls. An expert adjudication panel determined potential heart failure events and cause of mortality (18). Cardiovascular mortality was defined as mortality related to atherosclerotic heart disease, mortality following cerebrovascular disease or mortality from other atherosclerotic and cardiovascular diseases as described in detail previously (18).
Clinical history and the electrocardiogram
Clinical characteristics and cardiovascular risk factors were obtained from the initial CHS study visit for each cohort (for the analysis of baseline NT-proBNP and outcomes) or at the study visit of the follow-up NT-proBNP (for the analysis of change in NT-proBNP and outcomes). The methodology for assessing cardiovascular risk factors has been described previously (19).
Coronary heart disease was defined as a history of angina, myocardial infarction, coronary angioplasty, or coronary artery bypass surgery. An ECG was performed annually; left ventricular mass was estimated from the ECG, and major ECG abnormalities including atrial fibrillation and left ventricular hypertrophy were defined according to previously described methods (20,21).
Statistical methods
Characteristics by baseline NT-proBNP and left ventricular functional status were compared using Chi-square tests or one-way ANOVA as appropriate. Cumulative incidence of heart failure and cardiovascular mortality were estimated using the Kaplan-Meier method. Multivariate analyses were performed using Cox proportional hazards models for new-onset heart failure and cardiovascular mortality outcomes, adjusting for demographics (age, sex, race), cardiovascular disease history, cardiovascular risk factors (systolic blood pressure, diabetes, cholesterol, creatinine, BMI), use of anti-hypertensive medications, and major ECG abnormalities. Elevated NT-proBNP was defined using a previously validated cut-off value of ≥ 190 pg/mL (10).
Change in NT-proBNP was considered as a categorical predictor among those with an initial low NT-proBNP level of <190 pg/mL. Risk of heart failure and cardiovascular mortality were examined associated with a 1) stable or decrease in NT-proBNP (i.e. no increase > 25%) and 2) an increase of at least 25% to a level ≥ 190 pg/mL. The 25% threshold for change was based on the reported intra-individual variability in NT-proBNP in stable heart failure patients (22). We then evaluated whether baseline echocardiographic information about LVEF (≥ 55% vs. < 55%) added to the predictive value of increases in NT-proBNP. Lastly, we evaluated the incremental value of LVEF as a semi-quantitative variable (<45%, 45–54% and ≥ 55%) and NT-proBNP as a continuous variable (after log-transformation) for both outcomes. The time-dependent C-statistic was used to examine the added predictive value of the LVEF assessment to (a) a demographic model with and without baseline NT-proBNP and (b) the combination of baseline and of follow-up NT-proBNP levels for incident heart failure and cardiovascular mortality. The improvement in risk classification by addition of LVEF measurement to NT-proBNP in demographic adjusted models was examined using the NRI, which represents the net percentage of subjects correctly reclassified to risk categories (23). We categorized individuals according to Cox model-based risk of 10-year HF or cardiovascular mortality of <10%, 10% to 20%, or >20%. An exploratory analysis was also performed using echocardiographic measures of diastolic function including Doppler mitral E/A ratio (categorized as <0.7, 0.8–1.5, and >1.5) and left atrial dimension added to LVEF, NT-proBNP or both.
Association between changes in NT-proBNP and subsequent new-onset LV dysfunction were evaluated using Chi-square tests. Statistical analyses were performed with Stata version 10 (Statacorp, College Station, TX) and SPSS version 17.0 (Chicago, IL); and time-dependent C-statistics were generated using R version 2.7.0.
Results
Participant characteristics
Of the 4,137 participants without prevalent heart failure and a baseline echocardiogram, 107 (2.6%) had subnormal LVEF (<45%) and 210 (5.1%) had a borderline reduced LVEF (45%–54%). The AUC for NT-proBNP to diagnose a subnormal LVEF (< 45%) was 0.85, and for any abnormal LVEF (< 55%) the AUC was 0.69. High-risk NT-proBNP levels (≥190 pg/mL) were observed in 29.5% (n=1219). Table 1 shows demographic, clinical and echocardiographic diastolic information based on the presence of a high or low NT-proBNP value, further subdivided by the presence of a normal versus abnormal LVEF. The median age of the participants was 71 years (range 65–100). NT-proBNP status (high versus low) differentiated patients with a higher prevalence of risk factors, ECG abnormalities, history of coronary heart disease, cardiovascular medication use, increased left atrial size and diastolic abnormalities. An abnormal LVEF was further associated with male gender, diabetes, coronary heart disease, ECG abnormalities, cardiovascular medication use, increased left atrial size and diastolic abnormalities.
Table 1.
Characteristics of participants as related to NT-proBNP and LVEF
| Variable | Total | NT-proBNP<190 | NT-proBNP<190 | NT-proBNP≥190 | NT-proBNP≥190 | p value |
|---|---|---|---|---|---|---|
| LVEF≥55% | LVEF<55% | LVEF≥55% | LVEF<55% | |||
| N=4,137 | N=2,783 | N=135 | N=1,037 | N=182 | ||
| Demographics | ||||||
| Age (years) | 72.7 ± 5.5 | 71.6 ± 4.8 | 71.7 ± 4.8 | 75.2 ± 6.2 | 75.5 ± 6.0 | <0.001 |
| Sex (female) | 2462 (59.3%) | 1689 (60.8%) | 44 (32.1%) | 686 (64.4%) | 63 (34.6%) | <0.001 |
| Race (African American) | 548 (13.2%) | 411 (14.8%) | 19 (14.1%) | 129 (12.1%) | 20 (10.9%) | 0.037 |
| Risk factors | ||||||
| SBP (mmHg) | 136.6 ± 21.4 | 133.9± 19.9 | 132.0 ± 17.1 | 143.6 ± 23.5 | 141.5 ± 24.0 | <0.001 |
| DBP (mmHg) | 70.8 ± 11.1 | 70.7 ± 10.8 | 71.07 ± 11.2 | 70.9 ± 11.7 | 71.9 ± 13.2 | 0.403 |
| Hypertension | 1823 (44.1%) | 1118 (40.2%) | 61 (45.2%) | 551 (53.2%) | 93 (51.1%) | <0.001 |
| Diabetes | 715 (17.3%) | 483 (17.4%) | 34 (25.2%) | 155 (14.9%) | 43 (23.6%) | 0.002 |
| BMI (kg/m2) | 26.6 ± 4.6 | 26.8 ± 4.6 | 27.9 ± 4.5 | 25.9 ± 4.7 | 26.5 ± 4.34 | <0.001 |
| Current Smoker | 453 (11.0%) | 311 (11.2%) | 14 (10.4%) | 113 (10.9%) | 15 (8.2%) | 0.668 |
| Cardiovascular history | ||||||
| CHD at baseline | 727 (17.6%) | 345 (12.4%) | 40 (29.6%) | 243 (23.4%) | 99 (54.4%) | <0.001 |
| ECG abnormalities | ||||||
| LVH on ECG | 172 (4.3%) | 64 (2.4%) | 5 (3.9%) | 82 (8.3%) | 21 (12.8%) | <0.001 |
| Atrial fibrillation | 89 (2.2%) | 13 (0.5%) | 1 (0.7%) | 59 (5.9%) | 16 (9.8%) | <0.001 |
| Laboratory values | ||||||
| NT-proBNP (pg/mL) | 110.5[56.4-218.8] | 76.4[43.3-117.5] | 88.8[43.4-124.5] | 314.1[236.0-522.5] | 530.6[299.8-1208.0] | * |
| eGFR (mL/min/1.73 m2) | 79.0 ± 23.2 | 81.8 ± 22.3 | 79.7 ± 23.1 | 73.1 ± 24.2 | 68.7 ± 23.4 | <0.001 |
| Cholesterol (mg/dL) | 212.3 ± 39.0 | 214.6 ± 38.3 | 211.5 ± 40.2 | 208.2 ± 39.7 | 201.1 ± 42.1 | <0.001 |
| Medications at baseline visit | ||||||
| ACE inhibitor | 258 (6.2%) | 169 (6.1%) | 9 (6.7%) | 64 (6.2%) | 18 (8.8%) | 0.516 |
| Beta blocker | 554 (13.4%) | 281 (10.1%) | 20 (14.9%) | 216 (20.8%) | 37 (20.4%) | <0.001 |
| Diuretic | 1015 (24.6%) | 623 (22.4%) | 36 (26.9%) | 305 (29.4%) | 51 (28.2%) | <0.001 |
| Antihypertensive (any) | 1889 (45.7%) | 1149 (41.3%) | 73 (54.5%) | 558 (53.8%) | 109 (60.29%) | <0.001 |
| Digoxin | 279 (6.8%) | 121 (4.4%) | 5 (3.7%) | 119 (11.5%) | 34 (18.8%) | <0.001 |
| Lipid lowering drugs | 240 (5.8%) | 170 (6.1%) | 7 (5.2%) | 51 (4.9%) | 12 (6.6%) | 0.515 |
| Echo measurements | ||||||
| Left atrial diameter (cm) | 3.9 (0.7) | 3.8 (0.6) | 4.0 (0.7) | 4.0 (0.7) | 4.3 (0.8) | <0.001 |
| E/A <0.7 | 810 (20.1%) | 499 (18.3%) | 36 (27.3%) | 209 (20.8%) | 66 (38.4%) | <0.001 |
| E/A 0.7-1.5 | 3002 (74.5%) | 2147 (78.9%) | 95 (72.0%) | 685 (68.2%) | 75 (43.6%) | |
| E/A >1.5 | 218 (5.4%) | 76 (2.8%) | 1 (0.8%) | 110 (11.0%) | 31 (18.0%) | |
p-value not calculated because NT-proBNP was used to create the grouping variable; values in median and IQR.
BMI, Body mass index; CHD, cardiovascular heart disease; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NT-proBNP, Amino terminal B-type natriuretic peptide; SBP, systolic blood pressure.
Outcomes based on NT-proBNP levels and LVEF
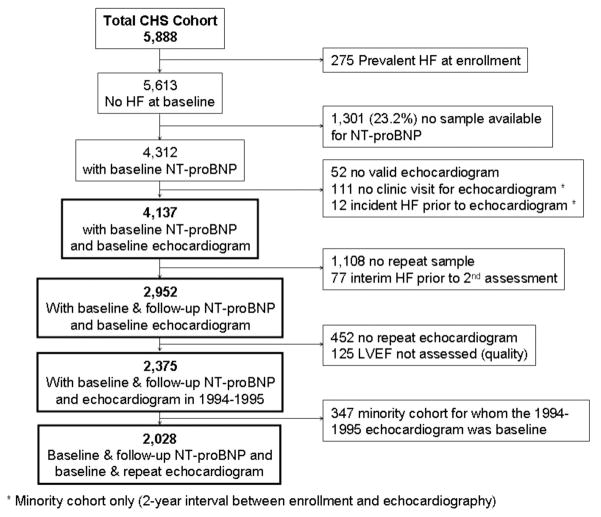
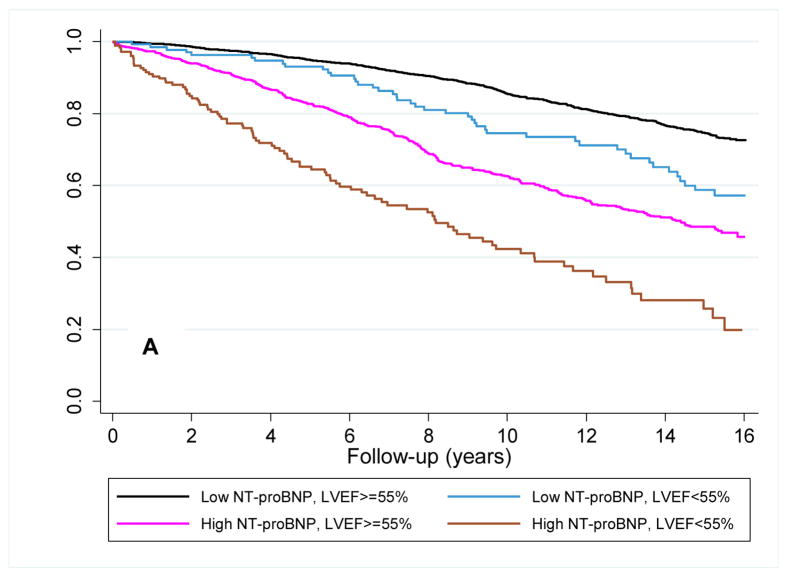
The median follow-up was 10.7 years (range 0.1 to 14.1 years) from the time of the baseline measure. There were 1,112 participants who developed heart failure and 893 who died from cardiovascular causes. The unadjusted hazard ratios for heart failure were 2.95 (95%CI=2.61–3.32) and 2.42 (95%CI=2.03–2.90) for a baseline NT-proBNP ≥190 pg/mL and LVEF <55%, respectively. Survival functions for heart failure and cardiovascular mortality based on the combination of baseline NT-proBNP level and LVEF assessment are shown in the Kaplan-Meier plots in figure 2a and 2b. Differentiation of risk occurred within the first year and continued throughout follow-up. As shown in table 2 in an unadjusted analysis, the increased risks of heart failure or cardiovascular mortality were of significant magnitude among participants with a low NT-proBNP and an abnormal LVEF (a 1.7–2.3 fold increased risk) compared to those with a normal LVEF. For participants with high baseline NT-proBNP, risks of heart failure and cardiovascular mortality were higher and this was further stratified by LVEF assessment.
Figure 2. Survival plots for outcomes utilizing baseline LVEF and NT-proBNP level.
Unadjusted Kaplan-Meier plots for (A) time to new-onset HF diagnosis and (B) time to cardiovascular mortality based on the combination of a baseline low (<190 pg/mL) or high (≥190 pg/mL) NT-proBNP and normal or abnormal LVEF. Abbreviations as in Figure 1. p<0.001 for comparison of survival curves for both HF and cardiovascular mortality.
Table 2.
Cox regression analysis for endpoints based on the initial NT-proBNP and LVEF measurements
| N (%) | Heart Failure | Cardiovascular Mortality | |||||
|---|---|---|---|---|---|---|---|
| # events | Unadjusted | Adjusted* | # events | Unadjusted | Adjusted* | ||
| Low NT-proBNP/Normal LVEF | 2783 (67.3%) | 575 | 1.00 | 1.00 | 424 | 1.00 | 1.00 |
| Low NT-proBNP/LVEF<55% | 135 (3.3%) | 44 | 1.75 (1.29, 2.38) | 1.26 (0.92, 1.73) | 43 | 2.34 (1.59, 3.45) | 1.68 (1.22, 2.31) |
| High NT-proBNP/Normal LVEF | 1037 (25.1%) | 400 | 2.75 (2.42, 3.13) | 2.05 (1.78, 2.36) | 327 | 2.67 (2.22, 3.22) | 1.92 (1.63, 2.26) |
| High NT-proBNP/LVEF<55% | 162 (4.4%) | 93 | 5.73 (4.60, 7.15) | 2.67 (2.07, 3.44) † | 99 | 5.45 (3.94, 7.54) | 2.95 (2.30, 3.79) ‡ |
Hazard Ratios adjusted for demographics (age, sex, race), CHD history, CV risk factors (systolic blood pressure, diabetes, cholesterol, body mass index, creatinine), use of anti-hypertensive medications, and major ECG abnormalities
In participants with a high NT-proBNP there was a significant difference (p=0.03) in the hazard ratios for new onset heart failure comparing participants with a normal LVEF versus LVEF < 55%
In participants with a high NT-proBNP there was a significant difference (p<0.001) in the hazard ratios for CV mortality comparing participants with a normal LVEF versus LVEF < 55%
CV, cardiovascular. Other abbreviations as per Table 1
After adjustment for clinical risk factors, BMI, ECG abnormalities and cardiovascular medications in those with low or high NT-proBNP levels, the increased risk associated with the presence of an abnormal LVEF was markedly attenuated but remained significant for both outcomes among those with an initially high NT-proBNP, and for cardiovascular mortality among those with an initially low NT-proBNP. An abnormal LVEF was no longer associated with risk of heart failure among those with an initially low NT-proBNP (table 2). In contrast, in a statistical model utilizing NT-proBNP as a continuous variable and LVEF as a semi-quantitative variable, LVEF continued to predict both outcomes after multivariate adjustment (supplemental table 2). In a separate gender-based analysis, no differences in the combined effects of LVEF and NT-proBNP were observed between men and women (supplemental table 3).
To complement the Cox regression analysis, the C-statistic and NRI were utilized to evaluate the incremental predictive value of LVEF assessment to NT-proBNP measurement for each outcome (table 3). For both heart failure and cardiovascular death the addition of LVEF improved prediction compared to demographics alone and resulted in a modest reclassification of risk. In contrast, the addition of LVEF assessment to demographic information and the NT-proBNP level resulted in minimal, but statistical significant improvement in the C-statistic for only the outcome of heart failure and no reclassification of risk for either outcome by the NRI statistic. When restricting the analyses to individuals with an initially elevated NT-proBNP, LVEF assessment didn’t reclassify risk of heart failure or cardiovascular mortality beyond demographic information and NT-proBNP level. (cardiovascular mortality: NRI= −0.006, p=0.7; heart failure: NRI=0.008, p=0.2). Adding echocardiographic measures of diastolic function to LVEF resulted in a significant increase in the C-statistic and reclassification by NRI. However, the addition of NT-proBNP still significantly increased the C-statistic and improved reclassification even after accounting for both LVEF and diastolic measures along with demographics.
Table 3.
Time dependent C-statistic and Net Reclassification Improvement (NRI) for progressively more complex predictive models of outcomes using LVEF and a single measure of NT-proBNP
| Heart Failure | Cardiovascular Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Compared to Model # | AUC | p value | NRI | p value | AUC | p value | NRI | p value |
| 1. Demographics | 0.667 | 0.715 | |||||||
| 2. Demographics + LVEF | 1 | 0.679 | 0.008 | .023 | 0.04 | 0.726 | 0.018 | .057 | <0.001 |
| 3. Demographics + LVEF + diastolic measurements* | 2 | 0.720 | <0.001 | .102 | <0.001 | 0.741 | 0.014 | .057 | 0.003 |
| 4. Demographics + LVEF +diastolic measurements* + baseline NT-proBNP | 3 | 0.748 | <0.001 | .082 | <0.001 | 0.774 | <.001 | .079 | <0.001 |
| 5. Demographics + NT-proBNP | 1 | 0.719 | <0.001 | .118 | <0.001 | 0.759 | <.001 | .123 | <0.001 |
| 6. Demographics + NT-proBNP + LVEF | 5 | 0.723 | 0.024 | .011 | 0.14 | 0.761 | 0.28 | .015 | 0.25 |
| 7. Demographics + NT-proBNP + LVEF + diastolic measures* | 6 | 0.748 | <0.001 | .073 | <0.001 | 0.774 | 0.004 | .042 | 0.01 |
Diastolic Measures: E/A ratio (<0.7, 0.7–1.5, >1.5), left atrial diameter.
AUC, area under the curve; LVEF, left ventricular ejection fraction; NRI, Net Reclassification Improvement; NT-proBNP, amino-terminal pro B-type natriuretic peptide
As part of a secondary analysis we also determined the number of participants that would need to undergo echocardiography to detect either one subnormal (< 45%) or abnormal (<55%) LVEF based on an initially high NT-proBNP (supplemental table 4).
Follow-up echocardiography with repeat NT-proBNP assessment
Echocardiography was available for 2,375 participants with repeat NT-proBNP levels who had not developed heart failure in the interim between measures (figure 1). LVEF < 55% (N = 202; 8.5%) was associated with increased risk of subsequent heart failure (n= 505 events, HR=2.38, 95%CI=1.80–3.13) and cardiovascular mortality (n=390 events, HR=2.93, 95%CI=2.18–3.98).
We then investigated whether LVEF assessments would add to the risk of both outcomes over and above repeated NT-proBNP assessments in participants with initially low NT-proBNP levels (< 190 pg/mL, n=1840). Participants were subdivided by comparing those whose NT-proBNP levels that increased > 25% to ≥ 190 pg/mL to those with stable or decreased NT-proBNP levels.
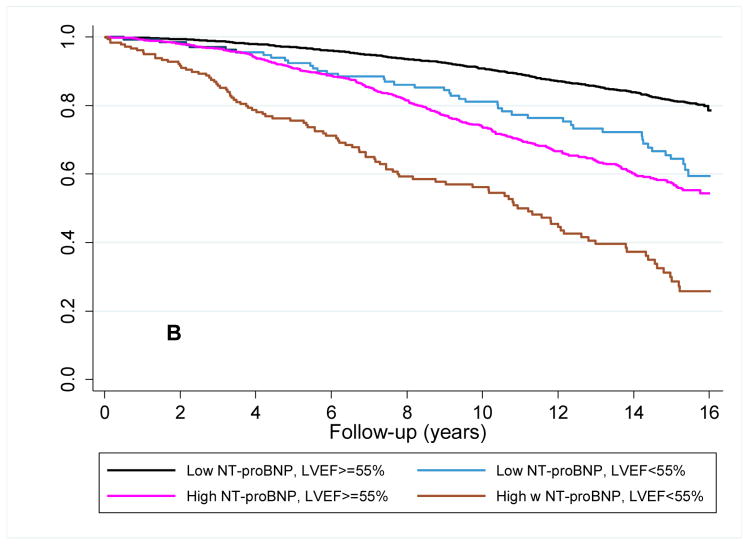
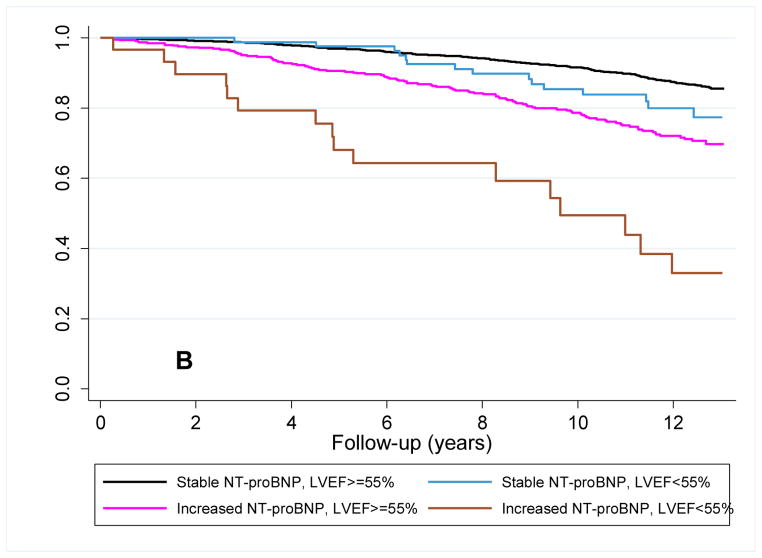
Among participants with initially low NT-proBNP levels, 361 (19.6%) had increased at follow-up. For these participants the risk of heart failure was highest among those with an abnormal LVEF, with intermediate risk being present in participants with only one characteristic (i.e., either an increase in NT-proBNP or an abnormal LVEF) (figure 3a). For cardiovascular mortality, a persistently low NT-proBNP level indicated a low-risk irrespective of LVEF. Whereas LVEF assessment further differentiated the risk of cardiovascular death in individuals with an increase in NT-proBNP level (figure 3b). By Cox regression analysis, after adjustment for covariates, LVEF only differentiated risk in the cohort of participants with a rising NT-proBNP level, and only for cardiovascular death (table 4).
Figure 3. Survival plots for outcomes utilizing follow-up LVEF and change in NT-proBNP level.
Unadjusted Kaplan-Meier plots for participants with baseline NT-proBNP < 190 pg/mL (A) time to new-onset HF diagnosis and (B) time to cardiovascular mortality based on the increase or absence of an increase in NT-proBNP level at follow-up and a normal or abnormal LVEF at echocardiography at follow-up. Abbreviations as in Figure 1. p<0.001 for comparison of survival curves for both HF and cardiovascular mortality.
Table 4.
Risk of new-onset heart failure and cardiovascular mortality based on follow-up LVEF and change in NT-proBNP among those with low NT-proBNP at baseline (N=1840)
| N (%) | Heart Failure | Cardiovascular Mortality | |||
|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | ||
| Stable† NT-proBNP/Normal LVEF | 1399 (58.9%) | 1.00 | 1.00 | 1.00 | 1.00 |
| Stable† NT-proBNP/LVEF<55% | 80 (3.4%) | 1.94 (1.17, 3.23) | 1.34 (0.80, 2.24) | 1.71 (0.90, 3.22) | 1.09 (0.57, 2.11) |
| Increased NT-proBNP/Normal LVEF | 319 (13.3%) | 2.77 (2.21, 3.48) | 2.15 (1.66, 2.80) | 2.24 (1.78, 3.12) | 1.83 (1.33, 2.52) |
| Increased NT-proBNP/LVEF<55% | 42 (1.8%) | 5.90 (2.91, 11.98) | 3.19 (1.46, 6.99) | 8.89 (4.68, 16.89) | 4.73 (2.37, 9.45)‡ |
CV, cardiovascular; HF, heart failure; LVEF, left ventricular ejection fraction; NT-proBNP, amino terminal B-type natriuretic peptide
Adjusted for demographics (age, sex, race), CHD history, CV risk factors (systolic blood pressure, diabetes, cholesterol, creatinine, body mass index), use of anti-hypertensive medications, major ECG abnormalities, and baseline NT-proBNP.
Stable includes participants with stable or decreased NT-proBNP levels at follow-up.
p value is <0.05 for comparison between a normal and abnormal baseline LVEF among participants with an NT-proBNP that increased between baseline and follow-up.
Abbreviations as per table 1.
The C-statistic and NRI analysis confirmed the adjusted Cox regression models’ findings. The addition of LVEF assessment to demographic information and serial NT-proBNP measurements neither significantly increased the AUC for the C-statistic nor reclassified the risk of having either outcome using the NRI statistic (table 5). Similar to models with a single measure of NT-proBNP, echocardiographic diastolic parameters provided additional prognostic and reclassification information to LVEF and serial NT-proBNP concentrations.
Table 5.
Time dependent C-statistic area under the curve and Net Reclassification Improvement for progressively more complex predictive models of outcomes using LVEF, diastolic measures, and repeated measures of NT-proBNP
| Compared to model # | Heart Failure | Cardiovascular Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | AUC | p value | NRI | p value | AUC | p value | NRI | p value | |
| 1. Demographics | 0.661 | 0.700 | |||||||
| 2. Demographics + LVEF | 1 | 0.670 | 0.06 | .022 | 0.08 | 0.714 | 0.015 | .055 | 0.006 |
| 3. Demographics + LVEF + diastolic measures* | 2 | 0.702 | <0.001 | .079 | <0.001 | 0.734 | 0.004 | .047 | 0.052 |
| 4. Demographics + LVEF + diastolic measures* + baseline and second NT-proBNP | 3 | 0.771 | <0.001 | .152 | <0.001 | 0.779 | <0.001 | .144 | <0.001 |
| 5. Demographics + baseline and second NT-proBNP | 1 | 0.755 | <0.001 | .212 | <0.001 | 0.762 | <.001 | .170 | <0.001 |
| 6. Demographics + baseline and second NT-proBNP + LVEF | 5 | 0.758 | 0.23 | .000 | 0.9 | 0.766 | 0.22 | .023 | 0.11 |
| 7. Demographics + baseline and second NT-proBNP + LVEF + diastolic measures* | 6 | 0.771 | 0.009 | .034 | 0.04 | 0.779 | 0.003 | .046 | <0.001 |
Diastolic Measures: E/A ratio (<0.7, 0.7–1.5, >1.5), left atrial diameter
AUC, area under the curve; LVEF, left ventricular ejection fraction; NRI, Net Reclassification Improvement; NT-proBNP, amino-terminal pro B-type natriuretic peptide
As part of a secondary analysis based on change in NT-proBNP level we determined the number of participants that would need to undergo echocardiography to detect either one subnormal (< 45%) or abnormal (<55%) LVEF based on an initial NT-proBNP level of < 190 pg/mL that increased > 25% to ≥ 190 pg/mL at follow-up (supplemental table 4)
Predicting a decline in LVEF based on serial NT-proBNP levels
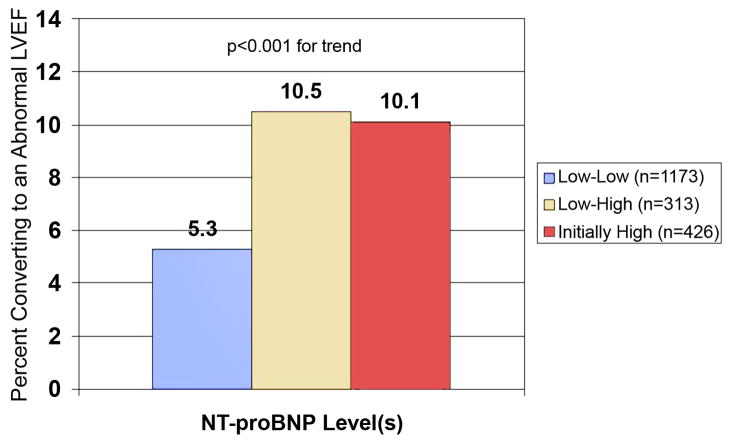
Participants in the main cohort with an NT-proBNP < 190 pg/mL and a normal LVEF had repeat echocardiograms two years after their second measure of NT-proBNP (N=1,486). An abnormal LVEF developed in 95 (6.4%). Participants with a rise in NT-proBNP were significantly more likely to have subsequent decline in their LVEF compared to participants with a stable low NT-proBNP level. Those who started with a high NT-proBNP and a normal LVEF (n=426) had a similar proportion who developed an abnormal LVEF as those with an initially normal, but rising NT-proBNP (figure 4).
Figure 4. Participants whose LVEF changed from normal to abnormal as predicted by NT-proBNP levels.
Participants whose LVEF changed from normal to abnormal (LVEF< 55%) from baseline to follow-up echocardiogram as predicted by serial NT-proBNP levels or an initial high level. Low indicates NT-proBNP <190 pg/mL. High indicates an initial NT-proBNP level ≥190 pg/mL or a rise > 25% to ≥190 pg/mL. Abbreviations as in Figure 1.
Discussion
The results from this study demonstrate in ambulatory older adults without heart failure, the addition of LVEF assessment to either a single NT-proBNP assessment or sequential measures adds little to risk assessment for new onset heart failure or cardiovascular mortality. Furthermore, in contrast to NT-proBNP levels, LVEF alone only modestly reclassifies risk when considering just demographic characteristics. Confirming the limited utility of a natriuretic peptide level to “screen” for subnormal (< 45%) LVEF, 14 participants with a high baseline and 34 participants with rising NT-proBNP levels would need to be screened to detect one subnormal LVEF. Despite limited accuracy to detect a subnormal LVEF, a high baseline or an increasing NT-proBNP level identified individuals at greatest risk of developing a new abnormal LVEF on follow-up echocardiography. This latter finding is potentially intriguing as many CHS participants with initially normal LVEF who develop symptoms of heart failure are found to have an abnormal LVEF at the time of presentation (24).
In CHS and other community population studies an abnormal LVEF is an independent predictor of both new heart failure hospitalizations and cardiovascular mortality (2–4,25). Yet in this analysis, once adjusted for comorbidities, LVEF assessment adds little additional predictive benefit beyond the measurement of NT-proBNP. There are several potential reasons for this new finding. First, an abnormal LVEF is a relatively infrequent finding in community dwelling older adults (<8%) compared to an elevated NT-proBNP level (approximately 30%) (3,6,10). The low prevalence accounts in part for the weak influence of LVEF in reclassifying risk of heart failure or cardiovascular death (Tables 3, 5). The lack of specificity of natriuretic peptides for increased left ventricular volumes or pressure in asymptomatic subjects can explain the “false-positive” results when using NT-proBNP as a screening tool for an abnormal LVEF in the general population (26–28).
Assessment of LVEF to refine prognostication in community dwelling older adults based on an elevated natriuretic peptide level should be approached cautiously. Despite a prior study suggesting that natriuretic peptide measurement could be cost effective in select populations to screen for abnormal LVEF, recent guidelines don’t recommend measuring either natriuretic peptides or LVEF as part of a screening strategy (11,12). It may be tempting to consider combining natriuretic peptide levels and LVEF to identify those at greatest risk, and by unadjusted analysis this appears to be present. With introduction and dispersion of inexpensive handheld ultrasound imaging devices, rapid and less expensive assessment of LVEF will become prevalent (29). However, once comorbidities are considered, the additional prognostication of LVEF to an NT-proBNP level is markedly attenuated. Furthermore, the addition of LVEF provides insignificant information to improve discrimination and reclassify individuals into lower or high-risk groups even when considering only participants with initially high NT-proBNP. Our findings should be contrasted to earlier findings in the post myocardial infarction setting where natriuretic peptide levels and LVEF have prognostic synergism for both heart failure and death (30). However, reflective of the differences between a post myocardial infarction population and screening “at-risk” community based subjects, the prevalence of an abnormal LVEF was approximately 10 times higher in the post myocardial infarction setting (30). In an older adults without known heart failure clinicians will need to individualize decision-making with respect to echocardiography even in the presence of a high NT-proBNP level indicating an increased risk of developing heart failure symptoms, while also considering the importance of knowing diastolic filling patterns, left atrial size or other cardiac pathology in specific cases.
Limitations
This is a large well-characterized cohort of community dwelling older adults with serial NT-proBNP levels and echocardiography. However, there are limitations to the study design. The addition of the second cohort of African American older adults provides for a balanced demographic reflective of older adults in the United States. For this group, baseline NT-proBNP was measured 2 years prior to an echocardiogram. We have shown that LVEF will change over time, but only in a minority of participants even in the presence of an abnormal NT-proBNP level.
In CHS, LVEF was not quantified as a percent. Interpretation was performed in a semi-quantitative manner with excellent intra and inter-reader reproducibility (16). It is noteworthy that poor outcomes have been associated with even a borderline LVEF (estimated at 45–55%) (3). Lastly, this study doesn’t incorporate all echocardiographic measures of diastolic function, but we do show that diastolic measures can assist in reclassifying risk beyond LVEF and NT-proBNP levels. It remains complex as to how to best integrate diastolic measures into an individual’s care.
Conclusions
Older adults comprise the majority of new cases of heart failure, yet most live many years without the diagnosis. Elevated NT-proBNP levels likely reflect an ongoing pathologic process that can initially manifest as progression to an abnormal LVEF prior to symptoms or as symptoms in the presence of preserved LVEF. Once adjusting for the multiple comorbidities often present in ambulatory older adults, we were unable to demonstrate that an assessment of LVEF could further stratify prognosis after measurement of NT-proBNP. In the presence of an elevated NT-proBNP level in this population a tailored approach to cardiac imaging appears most appropriate.
Supplementary Material
Acknowledgments
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01 AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging, R01 HL-075366 from the National Heart, Lung and Blood Institute. Additional funding was provided by Roche Diagnostics.
We would like to thank Simona Barlera, M.Sc., Medical Statistician, Laboratory of Medical Statistics, Department of Cardiovascular Research Istituto MARIO NEGRI, for the programming code to run the time dependant C-statistic.
Abbreviations and Acronyms
- AUC
Area under the curve
- BNP
B-type natriuretic peptide
- BMI
Body Mass Index
- CHS
Cardiovascular Health Study
- CI
Confidence interval
- ECG
Electrocardiogram
- LVEF
Left ventricular ejection fraction
- NRI
Net reclassification improvement
- NT-proBNP
N-terminal pro B-type natriuretic peptide
Footnotes
Disclosures
Christopher deFilippi receives grant support, consulting and speaking honorarium from Roche Diagnostics and Siemens Healthcare Diagnostics, manufactures of the NT-proBNP assay.
Robert Christenson grant support and consulting honorarium from Siemens Healthcare Diagnostics and grant support, consulting and speaking honorarium Roche Diagnostics and, manufactures of the NT-proBNP assay.
Stephen Seliger has received grant support though Roche Diagnostics in support of this current and other CHS ancillary biomarker studies. He also has received consulting fees from Roche Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327:685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 2.Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES) Eur Heart J. 2007;28:1128–34. doi: 10.1093/eurheartj/ehm102. [DOI] [PubMed] [Google Scholar]
- 3.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of Systolic and Diastolic Ventricular Dysfunction in the Community: Appreciating the Scope of the Heart Failure Epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Ewald B, Ewald D, Thakkinstian A, Attia J. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38:101–113. doi: 10.1111/j.1445-5994.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 8.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JG, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:2182–99. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43:1019–26. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for Evaluation of Novel Markers of Cardiovascular Risk: A Scientific Statement From the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann of Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 16.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–48. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 17.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left Atrial Volume, Geometry, and Function in Systolic and Diastolic Heart Failure of Persons >=65 Years of Age (The Cardiovascular Health Study) Am J Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 18.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann of Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 19.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann of Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 20.Furberg CD, Manolio TA, Psaty BM, et al. Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group. Am J Cardiol. 1992;69:1329–35. doi: 10.1016/0002-9149(92)91231-r. [DOI] [PubMed] [Google Scholar]
- 21.Rautaharju PM, Manolio TA, Siscovick D, et al. Utility of new electrocardiographic models for left ventricular mass in older adults. The Cardiovascular Health Study Collaborative Research Group. Hypertension. 1996;28:8–15. doi: 10.1161/01.hyp.28.1.8. [DOI] [PubMed] [Google Scholar]
- 22.Schou M, Gustafsson F, Kjaer A, Hildebrandt PR. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28:177–182. doi: 10.1093/eurheartj/ehl449. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 24.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 25.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-Type Natriuretic Peptides and Cardiovascular Risk: Systematic Review and Meta-Analysis of 40 Prospective Studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 26.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma Brain Natriuretic Peptide to Detect Preclinical Ventricular Systolic or Diastolic Dysfunction: A Community-Based Study. Circulation. 2004;109:3176–3181. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 27.de Lemos JA, McGuire DK, Khera A, et al. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: Results from the Dallas Heart Study. Am Heart J. 2009;157:746–753.e2. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Eggers KM, Lindahl B, Venge P, Lind L. B-Type Natriuretic Peptides and Their Relation to Cardiovascular Structure and Function in a Population-Based Sample of Subjects Aged 70 Years. Am J Cardiol. 2009;103:1032–1038. doi: 10.1016/j.amjcard.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Atherton JJ. Screening for Left Ventricular Systolic Dysfunction: Is Imaging a Solution? J Am Coll Cardiol Img. 2010;3:421–428. doi: 10.1016/j.jcmg.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Richards AM, Nicholls MG, Espiner EA, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–92. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.