Abstract
Objective
Weight gain and bone loss are commonly reported in breast cancer survivors. The purpose of this pilot study is to assess feasibility and explore the effect of an aerobic weight-loaded exercise intervention on bone remodeling, weight, and body composition.
Design
A one-group pre-posttest design was used to test a 16–24-week supervised walking exercise intervention among women within 2 years of menopause. Through Weeks 1–4, time and weight were progressively increased. By Week 5 and through the end of the intervention, a waist belt was loaded with 5 lb and participants spent 45 min on the treadmill 3 times/week. Bone remodeling was measured by serum biomarkers (N-terminal propeptides of type I collagen [NTX] and serum osteocalcin). Dual-energy absorptiometry scans assessed body composition. Data were collected at baseline and 16 and 24 weeks.
Results
The majority of the 26 participants were married, well educated, and employed, with a mean age of 51.3 years (SD = 6.2). The high adherence (M = 88.2%, SD = 6.8) demonstrated feasiblity. There were no significant changes in serum osteocalcin (p = .67), serum NTX (p = .31), lean muscle mass (p = .08), or percent fat mass for the group as a whole (p = .14), but fat mass increased for women on adjuvant endocrine therapy (p = .04). The women maintained their weight.
Conclusions
This novel exercise intervention for breast cancer survivors was feasible, and women otherwise at high risk for weight gain and bone loss maintained their weight and bone mass.
Keywords: breast cancer, exercise, bone loss, body composition, cancer survivors
Women with a history of breast cancer represent 40% of the 5.6 million female cancer survivors in the United States. In 2007, there was an estimated 178,480 new cases of invasive breast cancer and 62,030 cases of in situ breast cancer (Jemal et al., 2007). With advances in detection and treatment, the number of women who will enjoy long-term survival after breast cancer will substantially increase. Unfortunately, persistent and late effects of cancer treatment influence the quality of life of the survivors and may contribute to morbidity and mortality (Institute of Medicine [IOM], 2006). Cancer survivors are at risk not only for recurrence, but also for cardiovascular disease, diabetes, functional decline, and osteoporosis (Demark-Wahnefried, Pinto, & Gritz, 2006).
Weight gain during and after breast cancer treatment is common (Irwin et al., 2005; McInnes and Knobf, 2001) and has been associated with an increased risk of recurrence and lower rate of survival (Kroenke, Chen, Rosner, & Holmes, 2005). Body composition changes in women who have received chemotherapy for breast cancer include an increase in body fat and a decrease in lean muscle mass (Demark-Wahnefried et al., 2001; Ingram & Brown, 2004; Irwin et al., 2005). Treatment with Tamoxifen has been associated with increases in body fat (Ali, Al-Ghorabie, Evans, El-Sharkawt, & Hancock, 1998). Gaining weight in midlife and becoming over-weight or obese are associated with a high metabolic risk profile for cardiovascular disease, especially in younger women with induced menopause (Astma, Bartelink, Grobbee, & vander Schouw, 2006), as well as with an increased risk for diabetes (Eyre et al., 2004). Breast cancer survivors, in particular, have an increased risk for comorbid illness due to weight gain during and after treatment, changes in body composition, decreased physical functioning, and bone loss.
Adjuvant therapy for breast cancer is associated with premature-induced menopause in up to 40% of women younger than 40 years of age and 50–100% of women more than 40 years of age (Goodwin, Ennis, Pritchard, Trudeau, & Hood, 1999). Young midlife women with cancer-treatment-induced premature menopause are at high risk for bone loss, leading to osteopenia, osteoporosis, and fractures (Chen et al., 2005; Shapiro, Manola, & Leboff, 2001). Post-menopausal breast cancer survivors on aromatase inhibitors and premenopausal women who receive luteinizing hormone-releasing hormone (LHRH) or gonadotropin-releasing hormone (GnRH) analogues for gonadal quiescence or as adjuvant therapy in clinical trials have lower bone mineral density and a high predicted risk of developing osteopenia and osteoporosis (Eastell et al., 2006). Tamoxifen therapy is associated with preservation of bone mass in postmenopausal women, but the protective effect dissipates over time. In contrast, bone loss is reported in premenopausal women who take Tamoxifen (Vehmanen, Elomaa, Blomqvist, & Saarto, 2006). Low bone mass is the major risk factor for osteoporotic fractures, which are associated with acute and chronic pain, decreased functional ability, height loss, and increased mortality.
There is sound evidence to recommend exercise as a healthy lifestyle behavioral intervention to reduce the risk of persistent symptoms and late effects of cancer treatment (IOM, 2006; McNeely et al., 2006). After cancer treatment, many women view the initiation of healthy lifestyle behaviors as a means to cope with the experience, gain some control over their lives, and reduce their risks for cancer recurrence and other health conditions (Knobf, 2002; Lauver, Connolly-Nelson, & Vang, 2007). Exercise interventions with cancer patients have resulted in improved cardiovascular fitness, quality of life, psychological adjustment, physical functioning, physical strength, aerobic capacity, and muscle strength and a decrease in fatigue, depression, anxiety, and sleep disturbance (Knols, Aaronson, Uebelhart, Franson & Aufdemkampe, 2005; Schmitz, Holtzman et al., 2005). Very few studies, however, have addressed body composition outcomes (Ingram, Courneya, & Kingston, 2006; Schmitz, Ahmed, Hannan, & Yee, 2005). Furthermore, we could find only one published study that targeted bone mass as a primary outcome utilizing a home-based exercise intervention in breast cancer survivors with osteopenia (Waltman et al., 2003).
Aerobic exercise improves endurance and overall well-being in midlife women and has been shown to stabilize or prevent weight gain in healthy women (Donnelly, Jacobsen, Heelan, Seip, & Smith, 2000) and cancer survivors (Schmitz, Ahmed et al., 2005). Exercise is also recognized as an important intervention to help preserve bone mass, particularly for the early postmenopausal woman without osteopenia or osteoporosis (North American Menopause Society, 2006). However, while aerobic exercise activities improve physical and psychological well-being for midlife women, exercise as a strategy to ameliorate bone loss in postmenopausal women requires an increased osteogenic stimulus (Bemben & Fetters, 2000; Lanyon, 1996). The results of exercise trials with weight loading in healthy postmenopausal women support the hypothesis that the skeleton responds to dynamic forces, leading to preservation or increases in bone mass in women (Bemben & Fetters, 2000; Kelly, Kelly, & Tran, 2001). These types of interventions also help build and preserve muscle mass and strength and control weight. Thus, we designed an aerobic weight-loaded exercise intervention trial to evaluate the feasibility and effects of an aerobic weight-loaded exercise intervention on body composition and bone mass in midlife breast cancer survivors with early menopause (natural or induced).
Methods
We conducted a study with a one-group pre-posttest design to determine the feasibility and explore the effects of a 16–24-week aerobic weight-loaded exercise intervention on bone mass, weight, and body composition. Eligible participants were women diagnosed with Stage I or Stage II breast cancer who had completed adjuvant chemotherapy and/or radiation therapy within the past 3 years and were premenopausal or perimenopausal at diagnosis and either perimenopausal (irregular menses with amenorrhea of 2 months or more between menses in the past year; Taffe & Dennerstein, 2002) or postmenopausal (no menses for 12 months or more) at study entry. In addition, women needed to be physically able to participate in the exercise program, as determined by their physician, speak and understand English, be able to complete questionnaires, and give informed consent to participate in the study. Exclusion criteria included concurrent major health problems, specifically aortic stenosis, episodes of syncope, history of thrombolic events, tachyarrhythmias, anemia (hematocrit < 25), hypertension (>140/90 resting), uncontrolled asthma (i.e., frequent asthma attacks), or a history of shortness of breath with activity; any resistive or vigorous regular exercise program three or more times per week in the past year; or current treatment with estrogen-replacement therapy. Inclusion and exclusion criteria were verified by the participants’ physicians. Women were recruited through oncology practices, flyers, and local newspapers. The study was approved by the Human Subjects Research Review Committee, and all participants signed written informed consent.
At the time of consent, women were asked what days and times were best for them for performing the exercise intervention and what fitness center location was most convenient. Using the concept of social support to promote adherence, 2-hr blocks of time were provided 4 days a week. A weekend morning session was later added for make-ups for weather cancellations and missed days.
Measurement and Data Collection
Primary outcome measures for the study included serum biomarkers for bone re-modeling, weight, and body composition, and data were collected at baseline and at 16 and 24 weeks. Serum biomarkers included N-terminal propeptides of type I collagen as a marker for bone resorption and osteocalcin as a marker for bone formation. The Osteomark NTX serum kit is an enzyme-linked immunosorbant assay that provides a quantitative measure of cross-linked N-telopeptides of type I collagen (NTX) in serum as an indicator of bone resorption (Osteomark, 1999). For this study, the intra-assay CV was 4.6% and the interassay CV was 6.9%. Serum osteocalcin samples were analyzed via human osteocalcin radioimmunoassay (Gundberg, Looker, Niemen, & Calvo, 2002) and in this study, the intra-assay variation was 7.2% and interassay variation 3.8%. Sensitivity of serum osteocalcin has been previously reported as 0.5 ng/ml (Kemmler et al., 2004).
Standard venipuncture was used to obtain two redtop tubes of serum, which were spun down immediately. The supernatant was frozen at −70°C or below for storage and kept in the Clinical Research Center Core Laboratory storage freezer. Weight was recorded in pounds, with participants wearing light clothing and no shoes on a balance beam scale at the General Research Unit. Whole-body dual-energy X-ray absorptiometry (DEXA) scans (Hologic QDR 4500), which use a three-component model to assess lean body mass, fat and bone, were used in this study to determine lean body mass and body fat. A spine phantom was scanned daily to detect any drift in machine precision.
Heart rate was recorded by the on-site interventionist on a standardized intervention log before the intervention, every 5 min during the exercise session and after cool down. Women on beta blockers used the Borg scale for perceived level of exertion (Borg, 1982). Adherence was calculated as the percentage of scheduled sessions completed.
Procedures
The intervention was a supervised progressive walking exercise on a treadmill performed three times per week with a weight belt and backpack. Participants were assigned to one of three fitness centers in the local community. The sites for weight loading were the thoracic vertebrae (backpack) and the hip (waist belt). The aerobic walking occurred on treadmills, with the speed determined by a trained research assistant to be congruent with the intervention protocol. Each session throughout the intervention included a 5-min warm-up period and a 5-min cool down. Target heart rates were calculated for each woman individually, adjusting for age. All women wore a heart rate monitor watch, and those on beta-blockers used the Borg scale for perceived level of exertion (Borg, 1982).
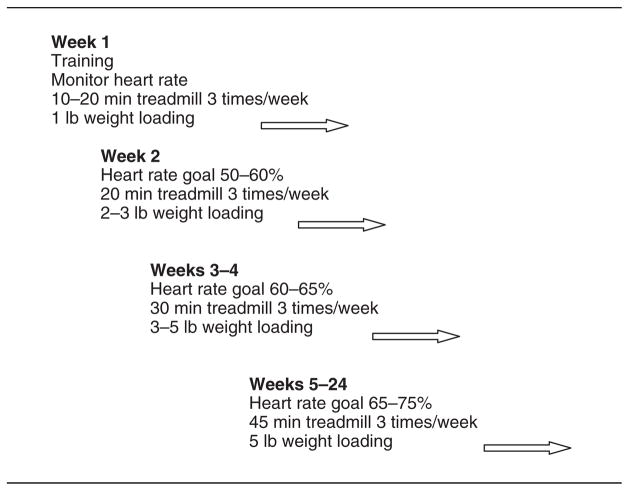
Weighted vests have been used instead of free weights or machines to provide optimal loading of the hips and allow for distribution around the torso (Going et al., 2003; Snow, Shaw, Winters, &Witzke, 2000). For this study, weight belts with slots for 0.5 and 1 lb weights were used. Weights were initially placed in slots corresponding to the sides of the hips and then equally distributed around the belt as weight progressed from 1 to 5 lb. In Week 1, women were coached on the proper use of the treadmill, how to monitor their heart rate, use of the weight belts and backpacks with 1 lb weights, and the walking intervention (Figure 1). They also completed three bouts of treadmill walking of 10–20 min duration with the 1-lb weights. In Week 2, there were three 20-min exercise periods with 1–3-lb belt and back-pack weights to achieve maximal heart rate of 50–60%. In Weeks 3–4, exercise periods consisted of three 30-min bouts with 3–5 lb belt and backpack weights to achieve maximal heart rate of 60–65%. In Weeks 5–16, there were three 45-min walking bouts using 5-lb belt and backpack weights to achieve maximal heart rate of 75%.
Figure 1.
Progressive training and intervention schedule.
The backpack was eliminated 12 weeks after the study was initiated, as 1 of the 4 participants with pre-existing arm lymphedema had an increase in arm circumference. Although it was unclear whether this was related to the intervention or to other lifestyle activities, it prompted an extensive consultation with experts in the field. Taking a conservative approach, a decision was made to eliminate the backpack based on concerns about the fit of the shoulder straps of the backpack, the hypothetical risk of exacerbating pre-existing lymphedema or contributing to the risk of developing lymphedema and the measurement challenges in identifying the presence of lymphedema (Armer, 2005).
Data were double entered into an Access database and imported into SAS for cleaning and analysis. Univariate statistics, including stratification by time, were performed to describe the sample and for continuous variables to evaluate the distribution. When necessary, variables were log-transformed to meet model assumptions. Repeated measures analysis of variance was carried out through mixed modeling using compound symmetry as the covariance matrix. Multiple comparisons were addressed through the least squared means function using Bonferroni and Tukey tests.
Results
Sample
Of the 33 women who consented to participate in the study, 2 never started, 3 dropped out due to injuries unrelated to the intervention (previous shoulder injury needing physical therapy, recurrent bone spur, knee injury falling on the ice), 1 dropped out due to a breast cancer recurrence, and 1, who was self employed, dropped out because she was too busy. The final sample consisted of 26 participants, the majority of whom were married, well educated, and employed, with a mean age of 51.3 years (SD = 6.2; Table 1). Body mass index (BMI) was calculated at baseline: 52% of women were overweight (BMI 25–29.9 kg/m2) and 8% were obese (BMI > 30 kg/m2). Of the 26 participants, all but the last 2 enrolled participants (due to funding limitations for continued supervision) were offered an extension of the intervention, and 19/24 (79.2%) elected to extend to 24 weeks. Sixty-five percent of participants had completed adjuvant chemotherapy, with a mean time since completion of therapy of 9.15 months. The majority of participants were on adjuvant endocrine therapy with either Tamoxifen and/or an aromatase inhibitor.
Table 1.
Demographic and Treatment Characteristics of the Sample (N = 26)
| Participant Characteristics | N | % |
|---|---|---|
| Ethnicity | ||
| Caucasian | 26 | 100 |
| Marital status | ||
| Married | 20 | 77.0 |
| Divorced/separated | 3 | 11.5 |
| Single | 3 | 11.5 |
| Educational level | ||
| High school graduate | 2 | 8.0 |
| Some college | 6 | 23.0 |
| College graduate | 10 | 38.0 |
| Graduate school | 8 | 31.0 |
| Employment status | ||
| Full time | 15 | 58.0 |
| Part time | 8 | 30.0 |
| Retired/not employed | 3 | 12.0 |
| Household income | ||
| < 40,000 | 2 | 8.0 |
| 40,000–79,999 | 5 | 19.5 |
| > 80,000 | 15 | 57.5 |
| Missing data | 4 | 15.0 |
| Primary/local treatment | ||
| Mastectomy | 3 | 11.5 |
| Mastectomy with reconstruction | 8 | 30.5 |
| Breast conservation/radiation | 15 | 58.0 |
| Adjuvant chemotherapy | ||
| AC | 5 | 19 |
| CAF/FEC | 7 | 27 |
| AC-T | 5 | 19 |
| None | 9 | 35 |
| Adjuvant endocrine therapy | ||
| Tamoxifen | 9 | 35 |
| Aromatase inhibitor | 7 | 27 |
| None | 10 | 38 |
NOTE: AC = doxorubicin, cyclophosphamide; AC-T = doxorubi-cin, cyclophosphamide, paclitaxel; CAF = cyclophosphamide, doxorubicin, flourouracil; FEC = cyclophosphamide, epirubicin, fluorouracil.
Adherence and Bone Mass
The high adherence rate (M = 88.2%, SD = 6.8, range = 75–98%) demonstrates the feasibility of this intervention. Women reported feeling empowered and positive about doing something for themselves to stay healthy. Bone remodeling was stable, as demonstrated by the absence of significant changes in serum osteocalcin levels (F = 0.40, p = .67) or serum NTX levels (F = 1.19, p = .31; Table 2). The bone mineral density (BMD) outcome variable was exploratory, as bone density is not a sensitive marker when repeated at a 6-month interval. However, when combined with the absence of change in serum biomarkers for bone remodeling, the absence of a significant change in BMD suggests that a weight-loaded aerobic exercise intervention has the potential to maintain bone mass in women at risk for bone loss. These results are particularly notable as many women in this sample were at high risk for bone loss due to recent menopause (< 2 years), 27% were on aromatase inhibitors and 1 woman was on GnRH analog for ovarian suppression.
Table 2.
Bone Mass Outcomes: Changes Over Time
| Baseline (N = 26) M (SD) |
16 Weeks (N = 25) M (SD) |
24 Weeks (N = 19) M (SD) |
Fa | Pa | |
|---|---|---|---|---|---|
| Serum biomarkers | |||||
| Osteocalcin | 9.15 (4.54) | 8.85 (3.99) | 8.70 (4.28) | 0.40 | .67 |
| NTX | 18.54 (5.9) | 18.01 (5.43) | 17.56 (4.72) | 1.19 | .31 |
| Bone mineral density | 1.14 (0.08) | 1.13 (0.08) | 1.12 (0.07) | 0.67 | .52 |
Repeated measures analysis using compound symmetry to examine time effect on outcome levels.
Weight and Body Composition
The women had no significant change in weight (F = 0.54, p = .59) over the 4–6-month intervention (Table 3). One woman, who went on a diabetic diet prescribed by her physician, lost 33 lb (197.9 lb at baseline and 164 lb at 24 weeks) and was excluded from the body composition analysis. Generally, the women maintained their body composition, as demonstrated by the absence of significant changes in lean muscle mass over time (p = .08) and percent fat mass for the group as a whole over time (p = .14). Despite the absence of change in fat mass in the group as a whole, there was an interaction effect for endocrine treatment over time (p = .04), and fat mass increased significantly more for women on Tamoxifen (p = .006) and women on an aromatase inhibitor (p = .05) compared to women who were not on adjuvant endocrine therapy. The mean percent fat mass for women on Tamoxifen was 38.5 at baseline and 40.2 at 24 weeks. Similarly, women on an aromatase inhibitor had a baseline percent fat mass of 36.8, which increased to 39.1 at 24 weeks. In contrast, the mean percent fat mass for women on no endocrine therapy remained relatively stable (baseline = 37.8; 24 weeks = 38.1).
Table 3.
Weight and Body Composition Changes Over Time
| Baseline (N = 25) M (SD) | 16 Weeks (N = 25) M (SD) | 24 Weeks (N = 19) M (SD) | Fa | Pa | |
|---|---|---|---|---|---|
| Body composition | |||||
| Lean muscle mass (g) | 41157 (4942) | 41048 (4316) | 40143 (4131) | 2.84 | .08 |
| Fat mass (%) | 37.38 (5.23) | 37.90 (5.10) | 38.05 (5.69) | 1.59 | .14 |
| Weight (lb) | 155.5 (21.5) | 155.9 (19.0) | 153.5 (19.7) | 0.54 | .59 |
Repeated measures analysis using compound symmetry to examine time effect on outcome levels.
Discussion
This novel exercise intervention for breast cancer survivors is feasible, and the participants, at high risk for weight gain and bone loss, had stable weight and bone mass. Our adherence rate compares favorably with other reported studies with breast cancer survivors (Schmitz, Ahmed et al., 2005). Cancer survivors appear motivated to engage in healthy lifestyle behaviors, and even previously sedentary individuals are willing to engage in physical-activity interventions following cancer treatment (Rabin, Pinto, Trunzo, Frierson, & Bucknam, 2006). Structured group exercise interventions for breast cancer survivors provide emotional and social support and enhance women’s confidence in their ability to exercise to their capacity (Campbell, Mutrie, White, McGuire, & Kearney, 2004).
Weight gain is common and well documented in midlife breast cancer survivors (Irwin et al., 2005; McInnes & Knobf, 2001). Decline in physical activity during cancer treatment likely contribute to the weight gain observed in breast cancer survivors (Demark-Wahnefried et al., 1997), and increased physical activity is known to attenuate weight gain in healthy midlife women (Sternfeld, Bhat, Wang, Sharp, & Quesenberry, 2005). In this study, weight was maintained, which was a positive outcome as women with breast cancer are known to gain weight during and after cancer treatment. Exercise interventions of a similar dose intensity (30–60 min 3 times per week) have also resulted in weight maintenance (McNeely et al., 2006; Schmitz, Ahmed et al., 2005). Many breast cancer survivors are psychologically distressed by weight gain (Knobf, 2001), which can further contribute to body-image concerns. Thus, maintaining weight through increased physical activity helps to reduce physical and psychological effects of cancer treatment and to improve quality of life (Burnham & Wilcox, 2002).
With or without weight gain, women with breast cancer have been reported to experience changes in body composition, specifically increases in percent body fat and decreases in lean muscle mass (Freedman et al., 2004; Ingram et al., 2006). Body composition in this study was unchanged during the intervention except for a subset of women on adjuvant endocrine therapy. The findings of stable body composition in this study are similar to the findings from a 12-week physical activity intervention (Matthews et al., 2007) and a 16-week strength training/cardiovascular fitness intervention for women with breast cancer (Ligibel et al., 2006). However, when researchers compared outcomes of an exercise intervention group to a control group of breast cancer survivors, improvement in body composition was reported (Ingram et al., 2006; Schmitz, Ahmed, et al., 2005).
Weight gain in women on adjuvant endocrine therapy with Tamoxifen has been reported (Fallowfield et al., 2001; Fisher et al., 1996; McInnes & Knobf, 2001), but there was not a statistically significant difference between the Tamoxifen and control participants for weight gain (Fallowfield et al., 2001; Fisher et al., 1996). However, similar to our findings related to body composition changes, Ali and colleagues (1998) used dual-energy absorptiometry to assess body composition in women on Tamoxifen and reported increases in fat mass. Our data support women’s complaints from clinical practice about increases in central fat associated with weight gain on Tamoxifen.
This is the first known study of a weight-loaded exercise intervention in breast cancer survivors who are at high risk for bone loss due to early menopause. Furthermore, 27% of the participants were on aromatase inhibitors, which are associated with increased risk of bone loss (Chen et al., 2005). Using serum biomarkers as indicators of skeletal health, bone remodeling was found to be stable over the course of the 4–6-month intervention. Although this is a relatively short duration to assess bone turnover, serum osteocalcin as a marker of bone formation has been reported in studies of longer durations (12–26months) (Judge et al., 2005; Kemmler et al., 2004). These studies suggest that the outcome of moderate exercise may reflect decreases in bone resorption versus increases in bone formation in the context of increased or stable bone mass as measured by bone mineral density. In our study, there was a slight but nonsignificant decrease in bone resorption. Moderate-intensity resistive exercise interventions with adequate durations (i.e., 12 months) in healthy early postmenopausal women have demonstrated the ability to preserve or increase bone mass (Kemmler et al., 2004;Wallace & Cumming, 2000), but high-intensity resistance exercise may be needed to effect change in markers of bone formation (Bemben & Fetters, 2000; Judge et al., 2005). The findings from this pilot study on bone outcomes warrant examination in a larger randomized trial of longer duration.
Symptom management, health promotion, and risk reduction are integral to cancer nursing practice, especially for patients who are transitioning to survivorship. The IOM report (2006) highlights the critical importance of intervention for persistent treatment effects, minimizing the risk of late effects, and enhancing quality of life for survivors. Breast cancer diagnosis and treatment are associated with psychological and physical symptom distress, which gradually improve over time. However, persistent and late effects of cancer therapy, such as weight gain, changes in body composition, and bone loss place women at higher risk for the development of comorbid conditions and may influence survival outcomes. Over the past decade, a significant body of evidence has established the physical and psychological benefits of exercise in breast cancer survivors, and specifically interventions to reduce the risks associated with weight gain and negative changes in body composition. The data from this pilot study further identify the potential benefit of a weight-loaded exercise intervention on reducing the risks associated with cancer treatment. Breast cancer survivors are interested in health promotion, not only related to reducing their risk of cancer recurrence but also to reduce risks of other health conditions, such as osteoporosis (Knobf, 2002; Lauver, et al., 2007).
Limitations
This pilot study was designed to assess feasibility and generate preliminary data on study outcomes. Small sample size is an inherent limitation of pilot studies. The other major limitation of this study was the one-group pre-posttest nonrandomized design. The limitation of lack of a control group is particularly relevant in exercise studies that assess bone and body composition. In larger randomized trials, it is common that the intervention group remains stable or slightly improves whereas the control group loses bone mass and has negative changes in body composition (e.g., increased fat mass, loss of lean muscle mass). The lack of a control group in this study prevents us from drawing any definitive conclusions about the findings of stability of bone and body composition in our sample. The participants were White, well educated, and middle class by reported income, which may influence the adherence rate observed. Also, data on calcium and vitamin D intake, which are important components of bone health in post-menopausal women, were not collected. Despite the need for caution related to the study limitations, the data are encouraging and support further investigation in a larger randomized control trial.
Conclusion
In summary, we established that the design of the aerobic weight-loaded exercise intervention was acceptable, implementation at fitness centers in the local community was feasible, and women had high adherence. Additionally, women did not gain weight or lose bone mass and they maintained body composition throughout the 4–6-month intervention period. Furthermore, women felt supported and empowered and reported improved physical and emotional functioning (Knobf et al., 2006).
The end of cancer treatment has been described as a “teachable moment” and one that should be used by providers to promote healthy lifestyle behaviors to minimize late effects of cancer treatment and possibly influence survival (Demark-Wahnefried, Aziz, Rowland, & Pinto, 2005, p. 5827). Yet there is much we still do not know about the role and outcomes of exercise in cancer (Demark-Wahnefried et al., 2006). To minimize the treatment effects in breast cancer survivors, specifically to address weight gain, negative changes in body composition, decline in physical activity, menopause and bone loss, future exercise intervention research needs to consider duration (>12 months), types of resistance, inclusion of ground and reaction forces (for osteogenic stimulus), and behavioral components to foster adherence and sustainability (Kemmler et al., 2004). The initial benefits reported for exercise on physical, psychological, social, and quality of life outcomes in cancer survivors need to be linked to the type, duration, and timing of interventions, and longitudinal research is needed to address the potential impact on survival outcomes for a broad spectrum of cancer survivors (Demark-Wahnefried et al., 2006).
In addition, supervised structured interventions are costly in money, time, and personnel. Exploration of self-directed, supported, or supervised versus some “best mixture” of approaches warrants investigation. Similarly, the dose of the intervention needs to be determined and linked specifically to outcomes of interest. For breast cancer survivors, determination of the type and dose of exercise needs to be made in the context of the target outcomes that are associated with risk for the development of chronic illness (e.g., weight gain, negative changes in body composition, bone loss). General nutrition and physical activity guidelines are recommended for cancer survivors (Doyle et al., 2006). Additional research is indicated, however, to identify the optimal type and duration of diet and exercise to promote better health outcomes in cancer survivors, especially those at higher risk for late effects of treatment or other chronic illness.
Acknowledgments
Funding for the study was provided by the Yale School of Nursing Center for Self and Family Management (P20NRR07806), Office of Research/Scholarship, Center of Excellence in Chronic Illness Care; Yale Cancer Center; and American Cancer Society Professorship in Oncology Nursing (PON-99–11–01). This work was also supported by the Yale Bone Center (NIH RR00125), which supports the Mineral Metabolism Core Laboratory of the Yale General Clinical Research Center (NIH/NCRR/CTSA 1 UL RR024139).
References
- Ali PA, Al-Ghorabie FH, Evans CJ, El-Sharkawt AM, Hancock DH. Body composition measurements using DXA and other techniques in Tamoxifen-treated patients. Applied Radiation Isotope. 1998;49:643–645. doi: 10.1016/s0969-8043(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Armer J. The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer Investigation. 2005;1:76–83. [PubMed] [Google Scholar]
- Astma F, Bartelink ML, Grobbee DE, vander Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause. 2006;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- Bemben DA, Fetters NI. The independent and additive effects of exercise training and estrogen on bone metabolism. Journal of Strength Conditioning Research. 2000;14:114–120. [Google Scholar]
- Borg GV. Rating of perceived exertion scale. Medicine Science Sports & Exercise. 1982;14:377–387. [PubMed] [Google Scholar]
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine Science Sports & Exercise. 2002;34:1863–1867. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise progamme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. European Journal of Oncology Nursing. 2004;9:56–63. doi: 10.1016/j.ejon.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Chen Z, Maricic M, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, et al. Osteoporosis and rate of bone loss among postmenopausal survivors of breast cancer. Cancer. 2005;104:1520–1530. doi: 10.1002/cncr.21335. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Aziz N, Rowland J, Pinto B. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Hars V, Conaway MR, Havlin K, Rimer BK, McElveen G, et al. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy. American Journal Clinical Nutrition. 1997;65:1495–1501. doi: 10.1093/ajcn/65.5.1495. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcan PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving chemotherapy. Journal of Clinical Oncology. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: Potential for prevention and questions that remain. Journal of Clinical Oncology. 2006;24:5125–5131. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Jacobsen J, Heelan KS, Seip R, Smith S. The effects of 18 months of intermittent vs. continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary moderately obese females. Journal of Obesity Related Metabolic Disorders. 2000;24:560–572. doi: 10.1038/sj.ijo.0801198. [DOI] [PubMed] [Google Scholar]
- Doyle C, Kusihi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. Cancer A Journal for Clinicians. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clark G, Adams JE, et al. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen alone or in combination (ATAC) trial (18233230) Journal of Bone Mineral Research. 2006;21:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- Eyre H, Kahn R, Robertson R, Clark NG, Doyle C, Hong Y, et al. Preventing cancer, cardiovascular disease and diabetes. A common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109:3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Fleissig A, Edwards R, West A, Powles TJ, Howell A, et al. Tamoxifen for the prevention of breast cancer: Psychosocial impact on women participating in two randomized controlled trials. Journal of Clinical Oncology. 2001;19:1885–1892. doi: 10.1200/JCO.2001.19.7.1885. [DOI] [PubMed] [Google Scholar]
- Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham L, Wolmark N, et al. Five versus more than five years of Tamxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. Journal of the National Cancer Institute. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- Freedman RJ, Aziz N, Albanes D, Hatman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. Journal of Clinical Endocrinology & Metabolism. 2004;89:2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- Going S, Lohman T, Houtkooper L, Metcalfe L, Flint-Wagner H, Blew R, et al. Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporosis International. 2003;14:637–643. doi: 10.1007/s00198-003-1436-x. [DOI] [PubMed] [Google Scholar]
- Goodwin P, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. Journal of Clinical Oncology. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Looker AC, Nieman SD, Calvo MS. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender and race of ethnicity. Bone. 2002;31:703–708. doi: 10.1016/s8756-3282(02)00902-x. [DOI] [PubMed] [Google Scholar]
- Ingram C, Brown J. Patterns of weight and body composition change in premenopausal women with early stage breast cancer. Cancer Nursing. 2004;27:483–490. doi: 10.1097/00002820-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Ingram C, Courneya KS, Kingston D. The effects of exercise on body weight and composition in breast cancer survivors: An integrative systematic review. Oncology Nursing Forum. 2006;33:937–950. doi: 10.1188/06.ONF.937-950. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. From cancer patient to cancer survivor: Lost in transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- Irwin M, McTiernan A, Baumgartner RN, Baumgartner K, Bernstein L, Gilliland FD, et al. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic and lifestyle factors. Journal of Clinical Oncology. 2005;23:774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Seigal R, Ward E, Murray T, Xu J, Thun M. Cancer statistics 2007. CA A Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Judge JO, Kleppinger A, Kenny A, Smith J, Biskop B, Marcella G. Home-based resistance training improves femoral bone mineral density in women on hormone therapy. Osteoporosis International. 2005;16:1096–1108. doi: 10.1007/s00198-004-1816-x. [DOI] [PubMed] [Google Scholar]
- Kelly GA, Kelly KS, Tran ZV. Resistance training and bone mineral density in women. A metaanalysis of controlled trials. American Journal Physical Medicine & Rehabilitation. 2001;80:65–77. doi: 10.1097/00002060-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Kemmler W, Lauber D, Weineck J, Hensen J, Kalendar N, Engelke K, et al. Benefits of 2 years of intense exercise on bone density, physical fitness and blood lipids in early postmenopausal osteopenic women. Archives of Internal Medicine. 2004;164:1084–1091. doi: 10.1001/archinte.164.10.1084. [DOI] [PubMed] [Google Scholar]
- Knobf MT. The menopausal symptom experience in young mid-life women with breast cancer. Cancer Nursing. 2001;24:201–210. [PubMed] [Google Scholar]
- Knobf MT. Carrying on: The experience of premature menopause in women with early stage breast cancer. Nursing Research. 2002;51:9–17. doi: 10.1097/00006199-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Knobf MT, Avila D, Woodward P, Fennie K, DiPietro L, Thompson AS. The effect of an exercise intervention on QOL and symptoms in breast cancer survivors. Oncology Nursing Forum. 2006;33:463, Abstract No. 243. [Google Scholar]
- Knols R, Aaronson NK, Uebelhart D, Franson J, Aufdemkampe KL. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain and survival after breast cancer diagnosis. Journal of Clinical Oncology. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- Lanyon LE. Using functional loading to influence bone mass and architecture: Objectives, mechanisms and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18:37S–43S. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- Lauver D, Connolly-Nelson K, Vang P. Healthrelated goals in female cancer survivors after treatment. Cancer Nursing. 2007;30(1):9–15. doi: 10.1097/00002820-200701000-00002. [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Chen W, Keshaviah K, Adloff L, Partridge A, Salinardi T, et al. The impact of an exercise intervention on body composition, fat distribution and weight in breast cancer survivors. Journal of Clinical Oncology. 2006;24(18S):590. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Wilcox S, Hanby CL, Der Ananian C, Heiney SP, Gebretsadick T, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Supportive Cancer Care. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- McInnes J, Knobf MT. Weight gain and quality of life in women treated with adjuvant therapy for breast cancer. Oncology Nursing Forum. 2001;28:675–684. [PubMed] [Google Scholar]
- McNeeley ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Canadian Medical Association Journal. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society. Management of osteoporosis in postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause. 2006;13:340–367. doi: 10.1097/01.gme.0000222475.93345.b3. [DOI] [PubMed] [Google Scholar]
- Osteomark. Osteomark NTX test booklet. Seattle, Washington: Ostex International; 1999. [Google Scholar]
- Rabin CS, Pinto BM, Trunzo JJ, Frierson GM, Bucknam LM. Physical activity among breast cancer survivors: Regular exercisers versus participants in a physical activity intervention. Psycho-Oncology. 2006;15:344–354. doi: 10.1002/pon.961. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiology Biomarkers Prevention. 2005;14:1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Holtzman J, Courneya K, Maase LC, Duval S, Kane R, et al. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology Biomarkers Prevention. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early stage breast cancer. Journal of Clinical Oncology. 2001;19:3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. Journal of Gerontology. 2000;55A:M489–M491. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP. Menopause, physical activity and body composition/fat distribution in mid-life women. Medicine Science Sports & Exercise. 2005;37:1195–1202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- Taffe J, Dennerstein L. Time to final menstrual period. Fertility & Sterility. 2002;78:397–403. doi: 10.1016/s0015-0282(02)03231-4. [DOI] [PubMed] [Google Scholar]
- Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. Journal of Clinical Oncology. 2006;24:675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- Wallace BA, Cumming RG. Systematic review of randomized trials of the effects of exercise on bone mass in pre- and postmenopausal women. Calcified Tissue International. 2000;67:10–18. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- Waltman N, Twiss JJ, Ott CD, Gross GG, Lindsay A, Moop TE, et al. Testing an intervention for preventing osteoporosis in postmenopausal breast cancer survivors. Image: Journal of Nursing Scholarship. 2003;35:333–338. doi: 10.1111/j.1547-5069.2003.00333.x. [DOI] [PubMed] [Google Scholar]