Abstract
Peripheral nerve injury provokes heightened excitability of primary sensory afferents including nociceptors, and elicits ectopic activity in lesioned and neighboring intact nerve fibers. The major transmitter released by sensory afferents in the superficial dorsal horn of the spinal cord is glutamate. Glutamate is critically involved in nociceptive signaling and the development of neuropathic pain. We recorded miniature excitatory postsynaptic currents (mEPSCs) from neurons in lamina II of the rat dorsal horn to assess spontaneous synaptic activity after spared nerve injury (SNI), a model of chronic neuropathic pain. Following SNI, the frequency of mEPSCs doubled, indicating heightened glutamate release from primary afferents or spinal interneurons. Consistent with this finding, glutamate concentrations in the cerebrospinal fluid were elevated at one and four weeks after SNI. Transmitter uptake was insufficient to prevent the rise in extracellular glutamate as the expression of glutamate transporters remained unchanged or decreased. 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), an antagonist of metabotropic glutamate receptor 5 (mGluR5), reduced the frequency of mEPSCs to its preinjury level, suggesting a positive feedback mechanism that involves facilitation of transmitter release by mGluR5 activation in the presence of high extracellular glutamate. Treatment with the β-lactam antibiotic ceftriaxone increased the expression of glutamate transporter 1 (Glt1) in the dorsal horn after SNI, raised transmitter uptake and lowered extracellular glutamate. Improving glutamate clearance prevented the facilitation of transmitter release by mGluR5 and attenuated neuropathic pain-like behavior. Balancing glutamate release and uptake after nerve injury should be an important target in the management of chronic neuropathic pain.
Keywords: glutamate release, mGluR5, glutamate uptake, spinal dorsal horn, neuropathic pain
Introduction
Glutamate is the major transmitter of primary sensory afferents including nociceptors [5,39] and of excitatory interneurons in the dorsal horn of the spinal cord [58]. Maintaining glutamate homeostasis at synapses in the superficial dorsal horn, the first relay station for somatosensory input, is essential for an orderly transfer of nociceptive information to the central nervous system.
Different from other neurotransmitters, glutamate is not removed from the synaptic cleft by enzymatic degradation or direct reuptake at the presynaptic terminal. The most prominent excitatory amino acid transporter in the central nervous system, glutamate transporter 1 (Glt1) is mainly expressed by astrocytes, although it has been identified in some neurons [10]. L-glutamate/L-aspartate transporter (Glast) is found exclusively in astrocytes; only excitatory amino acid carrier 1 (Eaac1) is expressed primarily in neurons [12]. The capacity to clear glutamate from the perisynaptic space may be one factor determining the efficacy with which rising nociceptive input after inflammation or nerve injury is controlled. Increasing Glt1 in the spinal cord by local gene transfer attenuates inflammatory and nerve injury-induced pain (Maeda et al., 2008). Similar reductions of neuropathic pain have been reported after intrathecal or systemic administration of ceftriaxone, a β-lactam antibiotic that upregulates Glt1 expression [21,35,48,51].
We used spared nerve injury (SNI) to examine glutamate homeostasis in a rat model of persistent neuropathic pain. We show that insufficient glutamate uptake after SNI leads to sustained elevation of the transmitter, and that this increase in extracellular glutamate is associated with further enhancement of transmitter release, mediated through activation of metabotropic glutamate receptor (mGluR) 5.
Both ionotropic and metabotropic glutamate receptors at presynaptic terminals modulate the synaptic transmission between primary sensory afferents and dorsal horn neurons. Activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or kainate receptors suppresses sensory input, potentially by primary afferent depolarization (PAD) [25,30]. Inhibition of glutamatergic excitatory postsynaptic currents (EPSCs) has also been described following activation of presynaptic N-methyl-D-aspartate (NMDA) receptors [4]. On the other hand, activation of phosphorylated NMDA receptors at nociceptor terminals may have the opposite effect and facilitate substance P release [11,33]. Primary sensory afferents express group I, II and III mGluRs [9,26]. Group I mGluRs are involved in the development and persistence of both inflammatory and neuropathic pain [40] but appear to be silent under resting conditions in uninjured animals [27]. Evaluating the contribution of presynaptic mGluRs to synaptic plasticity after inflammation or nerve injury can be difficult, because the receptors are also present on dorsal horn neurons [23], and because dorsal horn interneurons participate in the modulation of presynaptic activity [58]. We found that insufficient removal of glutamate from the extracellular space after peripheral nerve injury causes activation of mGluR5 and paradoxically increases transmitter release in the superficial dorsal horn, providing a positive feedback mechanism that disrupts the balance between transmitter release and uptake.
Methods
Animals
We used adult male Sprague-Dawley rats (Charles River Laboratories) for all experiments. Animal procedures were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital and the Institutional Animal Care and Use Committee of Children’s Hospital, both in Boston, Massachusetts.
Peripheral nerve injury
Surgery for spared nerve injury (SNI) was performed on animals anesthetized by 3% isoflurane inhalation. We ligated two of the three peripheral branches of the sciatic nerve, the common peroneal and the tibial nerves, with silk (5-0) and transected them distally, leaving the third branch, the sural nerve, intact [13].
Drug treatment
We injected ceftriaxone (200 mg/kg body weight; Novaplus) intraperitoneally once a day, starting on the day of injury [51]. Control animals received injections of 0.9% saline (vehicle).
Glutamate in the cerebrospinal fluid
Cerebrospinal fluid (CSF) was collected from rats anesthetized by 3% isoflurane inhalation. The animals were placed in a prone position with the neck flexed. We punctured the cisterna magna through the skin and extracted 100–200 μl CSF. After centrifugation at 250 g we removed the supernatant and froze it at −80°C until analysis. Glutamate was measured using an enzyme-linked assay (BioVision). Samples visibly contaminated with blood or with erythrocytes in the pellet were discarded. We did not fix the head of the animals in a stereotactic frame to avoid artificial increases in the concentration of glutamate in the CSF [56].
Microdialysis of the spinal cord
The microdialysis probe consisted of a hollow polyacrylonitrile fiber (AN69; OD 200 μm; Hostal) with a molecular mass cutoff of approximately 40 kDa, a polyethylene inlet tube (OD 0.6 μm, ID 0.26 μm) and a micropin for insertion. We anesthetized the animals with 2–3% isoflurane and placed the microdialysis probe at the level of the L4 and L5 spinal segments through a small burr hole drilled into the Th13 vertebra [60]. The catheter was connected to a CMA 100 microdialysis pump (CMA Microdialysis) and perfused with artificial cerebrospinal fluid (ACSF) containing 141.7 mM Na, 2.6 mM K, 0.9 mM Mg, 1.3 mM Ca, 122.7 mM Cl, 21 mM HCO3, 2.5 mM HPO4, and 3.5 mM dextrose. The ACSF was gassed with 5% CO2 and 95% O2 and its pH adjusted to 7.2. The flow rate was 1 μl/min. After loosely closing the surgical wound from the catheter implantation, we took 12 baseline samples of dialysate at 5-min intervals. The average glutamate concentration in the last three samples was used as reference (100%) for relative changes after nerve lesion. Upon transection of the sciatic nerve at mid-thigh level we collected 14 test samples, also at 5-min intervals. Glutamate concentrations were measured with a CMA 600 Analyzer (CMA Microdialysis), which has a detection limit for glutamate of 1 μM and operates in a linear range up to 150 μM.
Immunohistochemistry
Deeply anesthetized rats were transcardially perfused with 0.9% NaCl followed by phosphate-buffered 4% paraformaldehyde. The L4 and L5 segments of the spinal cord were dissected, postfixed for 2 hours, cryoprotected overnight in 20% sucrose and embedded in Tissue-Tek (Sakura Finetek). Transverse sections through the spinal cord were cut at 10-μm thickness on a cryostat. We blocked unspecific protein binding sites by incubating the sections for 1 hour in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (Sigma-Aldrich), 1% blocking reagent (Roche Applied Science) and 0.1% Triton X-100 (Sigma-Aldrich). The sections were immunolabeled by incubating them overnight at 4°C with primary antibodies directed against CGRP (1:2000; Peninsula Laboratories), mGluR5 (1:2000; Millipore), Glt1 (1:3000), Glast (1:4000) and Eaac1 (1:1000; all from Millipore), glial fibrillary acid protein (Gfap) (1:300; Millipore), neuronal nuclei (NeuN) protein (1:2000; Millipore), CD11b (1:500; AbD Serotec) or ionized calcium binding adaptor molecule 1 (Iba1) (1:500; Wako Chemicals), followed by 1 or 2 h of incubation with a corresponding Alexa Fluor-conjugated secondary antibody (1:250 or 1:500; Life Technologies). Axon terminals of non-peptidergic primary afferents were labelled with isolectin IB4 conjugated to Alexa Fluor 594 (1:2000; Life Technologies).
Western blotting
Western blots were performed as previously described [53]. After euthanizing the animals, we collected the ipsilateral L4 and L5 dorsal horns and homogenized the tissue by sonication in a protein extraction buffer (pH 7.5) containing 150 mM NaCl, 50 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), 2% sodiumdodecylsulphate (SDS), 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride, Complete Mini (without EDTA) protease inhibitors (Roche Applied Science) and phosphatase inhibitor cocktails 1 and 2 from Sigma-Aldrich. We employed NuPAGE Novex 4–12% Bis-Tris gels for the electrophoresis (Life Technologies) and transferred the protein to polyvinylidene fluoride (PVDF) membranes (Bio-Rad). After blocking nonspecific binding sites with 5% nonfat milk in Tris buffered saline (TBS) containing 0.1% Tween 20 for 1 h at room temperature, we incubated the membranes with a primary antibody overnight at 4°C. Antibody-protein complexes were labeled with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, followed by incubation with Super Signal West Femto Maximum Sensitivity chemiluminescent substrate (Thermo Scientific). Chemiluminescent bands were revealed by exposing Hyperfilm ECL (GE Healthcare) to the membranes. Primary antibodies were specific for Glt1a (1:10,000; Fisher Scientific, cat. no. AB1783MI) or Glt1b (1:400; kindly donated by Paul Rosenberg) [10], or directed against both isoforms of Glt1 (1:2000; Cell Signaling, cat. no. 3838). Immunolabeling for glyceraldehyde-3-phosphate dehydrogenase (Gapdh, 1:5000; Santa Cruz Biotechnology) was used for loading controls and to normalize the signal intensity of Glt1-immunoreactive bands. We employed Restore Plus (Thermo Scientific) to strip membranes between incubations with different antibodies. The results shown represent the average of dorsal horn samples from 6 animals per experimental group.
Quantitative real-time polymerase chain reaction
For quantitative real-time polymerase chain reaction (qPCR), we pooled the ipsilateral L4 and L5 dorsal horns of 3 rats per sample and processed 4 of these biologically independent samples per group. RNA was extracted from tissue homogenized in Trizol (Life Technologies) using phenol and chloroform, assessed for integrity by agarose gel electrophoresis and quantified on a NanoDrop spectrophotometer (Thermo Scientific). We employed Primer Express 3.0 (Applied Biosystems) to design primers for Glt1a (5′-CTGACTGCAGTGTTGAGGAAGAA-3′ and 5′-TGGCTGAGAATCGGGTCATT-3′), Glt1b (5′-GGGCCCAGACCATCTCACT-3′ and 5′-TTGGAGTCAGACTTGCTGATGTC-3′), and a sequence shared by both Glt1 isoforms (5′-CATTGGTGCAGCCAGTATTCC-3′ and 5′-CAGCTGTGAGAATGAGGAGCAT-3′). Genomic DNA was removed from the purified RNA samples with Turbo DNA-free DNase I (Ambion) before using 1 μg of the RNA for reverse transcription with a Superscript III First Strand Synthesis kit (Life Technologies) as previously described [1]. We performed qPCR with Power SYBR on a 7500 Fast Real-Time PCR System (Applied Biosystems). Transcript regulation was determined using the relative standard curve method according to the manufacturer’s instructions. Relative loading was compared based on the amplification of Gapdh.
Membrane preparation and in vitro glutamate uptake
Membrane preparations were isolated from spinal cord homogenate as previously described [50], with minor modifications. We euthanized the animals, dissected the ipsilateral L4 and L5 dorsal horns and used a Potter-Elvehjem teflon-glass grinder (Wheaton) to homogenize the tissue in 500 μl of an ice-cold buffer (pH 7.4) containing 0.32 M sucrose, 2 mM ethylene glycol tetra-acetic acid (EGTA), 2 mM EDTA, 20 mM HEPES and Complete Mini (without EDTA) protease inhibitors (Roche Applied Science). We centrifuged the homogenate at 3000 g and 4°C for 10 min, collected the supernatant and centrifuged it at 16,000 g and 4°C for 30 min. We resuspended the resulting pellet in sucrose buffer and added 50 μl of this membrane preparation to 450 μl Krebs buffer (pH 7.2) containing 140 mM NaCl, 2.5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 1.2 mM K2HPO4, 5 mM Tris base, 10 mM sodium-free HEPES, 10 mM dextrose, 10 μM L-glutamic acid and 0.2 μCi of L-[3,4-3H]-glutamic acid (PerkinElmer). We gently mixed the assay and incubated it at 37°C for 5 minutes. The assay was stopped by transferring the tubes on ice and filtering the tissue through Whatman GF/C paper (24 mm). We washed the filter paper 3 × with ice-cold sodium-free Krebs buffer, transferred it into scintillation vials containing 10 ml ScintiVerse II (Fisher Scientific) and measured the radioactivity retained on the paper with a liquid scintillation analyzer (Packard Instruments). We also performed a sodium-free assay for each sample to determine the uptake fraction that was independent of glutamate transporter activity. This fraction was subtracted from the total uptake; the results shown represent the sodium-dependent glutamate uptake. DL-threo-β-benzyloxyaspartate (300 μM), a blocker of glutamate transporters, was used to control for the specificity of the assay [54].
Electrophysiology
We anesthetized rats by intraperitoneal injection of urethane (1.5 g/kg body weight) prior to dissecting the spinal cord for electrophysiological studies. Following a laminectomy, we removed the lumbar spinal cord and placed the tissue in an ice-cold solution containing 248 mM sucrose, 11 mM glucose, 26 mM NaHCO3, 2mM KCl, 1.25 mM KH2PO4, 2 mM CaCl2, 1.3 mM MgSO4, continuously gassed with 5% CO2 and 95% O2. We embedded the spinal cord in agarose (5%) and, using a VT1200 vibratome (Leica), cut two 600-μm thick transverse slices through the L4 and L5 segments. The slices were kept at room temperature in a chamber filled with ACSF containing 126 mM NaCl, 26 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose and continuously gassed with 5% CO2 and 95% O2. Whole-cell patch-clamp recordings were performed in the presence of 0.5 μM tetrodotoxin (TTX), 1 μM strychnine and 10 μM bicuculline. 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) and (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) (Tocris) were dissolved in water before bath application. Patch pipettes (3–5 MΩ) pulled from borosilicate glass capillaries were filled with an internal solution containing 130 mM CsCl, 2 mM MgCl2 and 10 mM HEPES. We recorded from neurons in lamina II, which is readily identified as a translucent band in the superficial dorsal horn. Voltage-clamp recordings were carried out at a fixed holding potential of −70 mV. Signals were filtered at 5 KHz and digitized at 10 kHz. We used pClamp 10 (Molecular Devices) and the WinEDR and WinWCP programs of the Strathclyde Electrophysiology Software (courtesy of John Dempster, PhD, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom) for data acquisition and analysis. Individual events were detected based on the threshold method, using a baseline-tracking amplitude-threshold algorithm.
Behavioral testing
After habituating the animals to the testing environment, we obtained two baseline measures during the week prior to SNI. Following SNI, we tested neuropathic pain-like behavior at defined intervals for a total of 30 days after surgery. All animals were examined 4 hours after drug or vehicle injections. They were placed on an elevated wire grid and stimulated on the plantar surface of the hind paw, in the territory of the “spared” sural nerve. We used calibrated von Frey monofilaments to determine the withdrawal threshold for punctate mechanical stimulation. The threshold was defined as the lowest force that provoked paw withdrawal at least twice in 10 applications. The response to cold stimulation was tested by applying a drop of acetone, which upon evaporation produces a cool sensation on the skin. We measured the time the animal spent licking, shaking, or lifting the paw during 1 min following the application of acetone [13].
Statistics
Investigators were blind to the tested conditions and treatments in all experiments. We used GraphPad Prism, version 4.00, (GraphPad Software) for statistical analysis. Biochemical and electrophysiological parameters between experimental conditions or treatments were compared using a t test. Differences in glutamate concentrations in the CSF and transporter expression at different time points after SNI were evaluated by an analysis of variance (ANOVA) followed by Dunnett’s test. To analyze pain-related behavior in rats treated with ceftriaxone or vehicle, we used a repeated-measures two-way ANOVA. Data are presented as mean ± standard error of the mean (SEM).
Results
Peripheral nerve injury causes a sustained increase in glutamate release
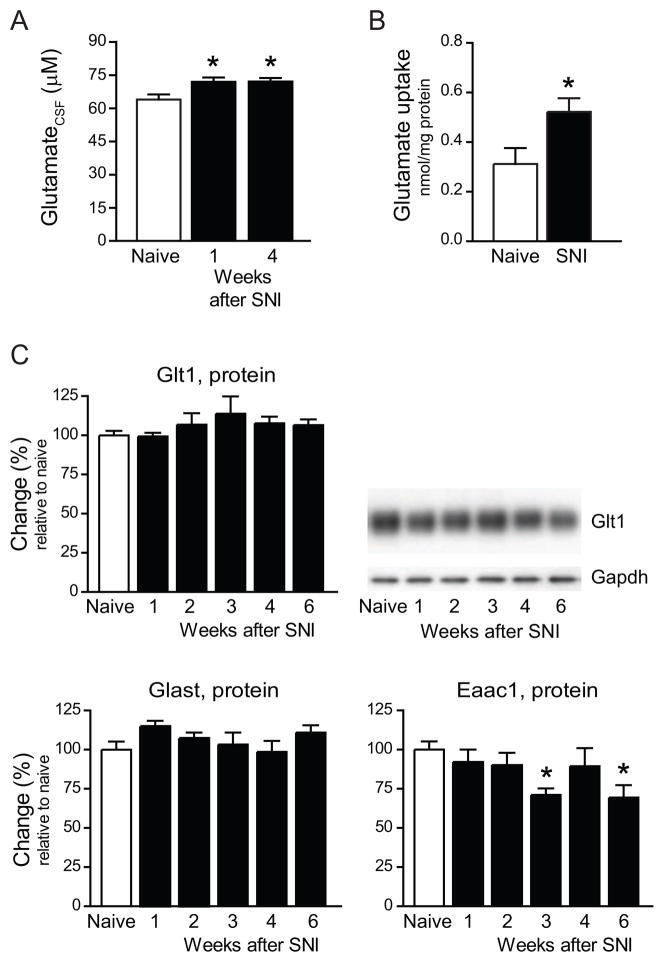
Lesion of a peripheral nerve triggers a transient burst of action potentials (injury discharge). Approximately two days later, sensory afferents develop ectopic firing which persists for weeks [32,34,37,62]. We measured glutamate levels in the CSF of rats 1 and 4 weeks after SNI to assess if ectopic activity and injury-induced enhanced excitability of primary afferents are associated with a net increase in extracellular transmitter. In uninjured (naïve) rats, glutamate concentration in the CSF was 64.0±2.3 μM (N=9). Seven days after SNI, the concentration was to 72.0±1.9 μM, an increase by 12.5% (N=13; p<0.05). Glutamate levels remained high. We measured 72.2±1.6 μM (N=14; p<0.05) at 28 days after SNI (Fig. 1A).
Fig. 1.
Extracellular levels of glutamate increase but glutamate transporter expression remains unchanged or decreases after sciatic nerve injury. A, Glutamate in the CSF of uninjured (naïve) rats (N=9), and rats 1 week (N=13) or 4 weeks (N=14) after spared nerve injury (SNI). B, Glutamate transport activity measured in membrane preparations of the ipsilateral dorsal horn of naïve rats and rats 1 week after SNI (N=4–6). C, Western blot analysis of Glt1, Glast and Eaac1 expression in the ipsilateral dorsal horn after SNI, N=6 per group. * p<0.05
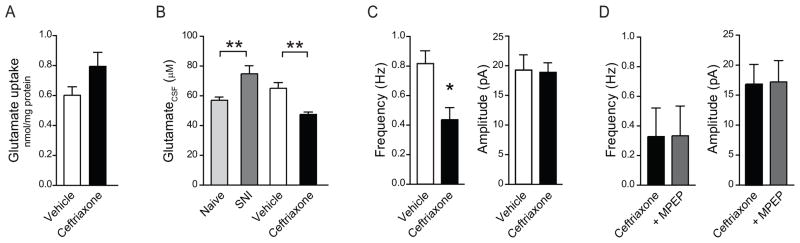
To determine glutamate transporter activity after SNI, we measured sodium-dependent uptake of glutamate in membrane preparations isolated from the ipsilateral dorsal horn of the spinal cord. Transporter-independent uptake was determined in a sodium-free assay for each preparation and subtracted from the total value. Transporter-mediated uptake was 0.31±0.06 nmol/mg protein in naïve rats (N=4) and 0.52±0.05 nmol/mg in rats 1 week after SNI (N=6; p < 0.05) (Fig. 1B). Despite this increase, transporter activity was insufficient to prevent the rise of extracellular glutamate in the CSF of rats after SNI.
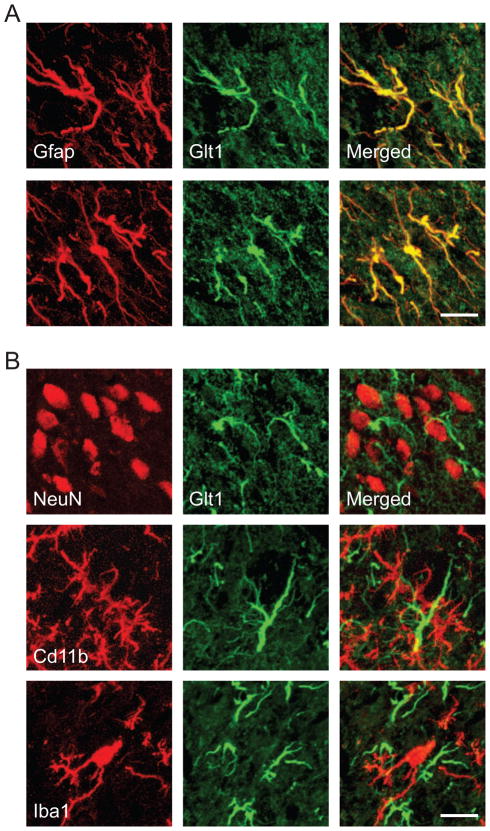
Western blots of Glt1, Glast and Eaac1, performed at weekly intervals after SNI (N=6 per time point), showed that Glt1 expression rose slightly, but only 3 weeks after SNI (not significant), whereas Glast expression remained unchanged and Eaac1 expression decreased (p < 0.05) (Fig. 1C). We found Glt1 (Fig. 2A) and Glast expressed in spinal cord astrocytes (immunolabeled for Gfap); Eaac1 colocalized with neuronal perikarya (NeuN) and astrocytes (data not shown) as previously described [49]. We did not observe Glt1 expression by microglial cells (CD11b, Iba1) (Fig. 2B), which was recently reported in a rat model of partial sciatic nerve ligation [63].
Fig. 2.
Immunohistochemical localization of Glt1 in the dorsal horn 1 week after spared nerve injury. A, Glt1 (green) was expressed by astrocytes (Gfap, red). B, Glt1 immunostaining (green) did not colocalize with neurons (NeuN, red) or microglia (CD11b, Iba1; red). The images represent z-stacks of 12–15 focal planes of 0.4 μm thickness each. Scale bars are 15 μm.
The sustained rise in extracellular glutamate must have developed after the transient transmitter increase that is triggered by an acute nerve lesion [32,34]. To evaluate the early time course of glutamate in the spinal cord after nerve injury, we measured the extracellular transmitter level in microdialysate of the rat dorsal horn during sciatic nerve transection. Because sciatic nerve transection is quick to perform, it is better suited to examine instant biochemical changes than SNI, which requires successive exposure, ligation and cutting of the common peroneal and tibial nerves [13]. Sciatic nerve transection prompted a rapid increase in intraspinal glutamate by 27.7±4.5% relative to baseline (N=5) (data not shown). However, glutamate returned to baseline levels within 50 min after the nerve lesion, reflecting the brevity of the initial injury discharge [32,34].
Activation of mGluR5 contributes to spontaneous glutamate release
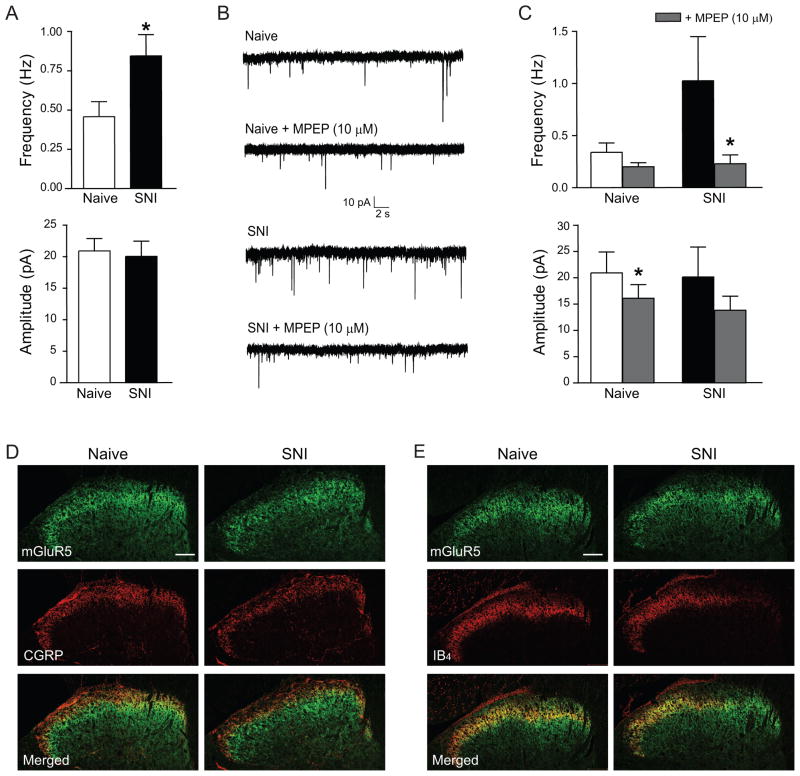
To assess spontaneous glutamate release at synapses in the superficial dorsal horn, we recorded miniature excitatory postsynaptic currents (mEPSCs) of lamina II neurons in the presence of the sodium channel blocker TTX. Seven days after SNI, the frequency of mEPSCs was markedly higher (0.84±0.14 Hz; N=15) than in naïve rats (0.46±0.09 Hz; N=15; p<0.05) (Fig. 3A and B), indicating increased presynaptic activity. Peak amplitude (Fig. 3A), rise time and decay time constant (data not shown) of the mEPSCs were unchanged.
Fig. 3.
Miniature excitatory synaptic activity increases after nerve injury. A–C, Whole cell patch clamp recordings, performed on acute slices of the spinal cord, show facilitation of glutamate release through mGluR5 activation 1 week after SNI. A, Frequency and amplitude of mEPSCs in lamina II of the dorsal horn (N=15 neurons). * p<0.05 B, Representative recordings of mEPSCs. Lower traces show mEPSCs after bath application of the mGluR5 antagonist MPEP (10 μM). C, Effect of MPEP on mEPSC frequency and amplitude in naïve rats (N=5 neurons) and rats after SNI (N=6 neurons). * p<0.05 D, E, Immunohistochemical labeling of mGluR5 (green), CGRP (D, red) and IB4 (E, red) in the ipsilateral dorsal horn of naïve rats and rats 1 week after SNI. Scale bars are 100 μm.
High presynaptic activity in the dorsal horn may result from ectopic generation of action potentials or increased excitability of primary sensory afferents, or reflect enhanced activity of spinal interneurons or descending pathways from the brainstem. We hypothesized that the sustained rise in extracellular glutamate that we observed after SNI caused activation of presynaptic glutamate receptors and increased transmitter release through positive feedback. We tested this hypothesis focusing on group I mGluRs, which have recently been implicated in the modulation of glutamate release following noxious stimulation [26,27]. Primary sensory and dorsal horn neurons express the group I mGluRs 1a and 5 [7,9,61].
To examine if group I mGluRs were involved in the regulation of glutamate release after nerve injury, we recorded mEPSCs from lamina II neurons after bath application of the mGluR5 antagonist MPEP (10 μM) or the mGluR1a antagonist LY367385 (10 μM). In the spinal cord of naïve rats, MPEP did not change the frequency of mEPSCs (0.35±0.09 Hz versus 0.20±0.04 Hz in the absence of MPEP; N=5, not significant) and only slightly reduced their peak amplitude (20.92±3.96 pA versus 16.09±2.603 pA; N=5, p<0.05) (Fig. 3B and C). In the spinal cord of rats after SNI, however, MPEP markedly reduced the frequency of mEPSCs (from 1.02±0.42 Hz to 0.23±0.08 Hz; N=6, p<0.05), without changing their amplitude (from 20.14±5.69 pA to 13.82±2.67pA; N=6, not significant) (Fig. 3B and C). The mGluR1a antagonist LY367385 (10 μM) had no effect on mEPSC frequency or amplitude after SNI (data not shown).
Immunostaining for mGluR5 showed intense labeling in the superficial dorsal horn. We found colocalization with the central terminals of peptidergic nociceptors (immunostained for CGRP) in lamina I and outer lamina II (Fig. 3D) and non-peptidergic nociceptors (labeled with IB4) in inner lamina II (Fig. 3E). CGRP-immunostaining and IB4-labeling decreased after SNI, most noticeably in the medial half of the dorsal horn, where afferents from the injured tibial and peroneal nerves terminate, consistent with a previously described transient degenerative change after nerve injury [3]. In contrast, the staining intensity for mGluR5 was only slightly reduced (Fig. 3D and E). The preservation of mGluR5 immunoreactivity and our electrophysiological results indicate that mGluR5 remained expressed at a level comparable to that in naïve rats, and that its function as a modulator of glutamate release stayed intact.
Glt1 upregulation by ceftriaxone restores the balance between glutamate release and uptake
Ceftriaxone, a β-lactam antibiotic, increases Glt1 synthesis through activation of nuclear factor κB [16]. We tested if continuous treatment with ceftriaxone can improve glutamate uptake in the spinal cord sufficiently to avert the sustained elevation of extracellular glutamate and prevent the activation of mGluR5 after SNI.
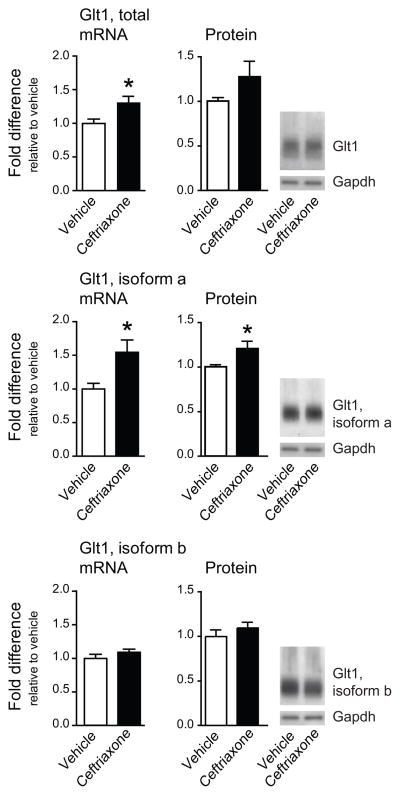
Glt1 expression rose in the ipsilateral dorsal horn of rats receiving daily intraperitoneal injections of ceftriaxone (200 mg/kg) for 7 days after SNI. The increase was attributable to an enhanced expression of isoform a, which rose 1.5-fold (p<0.05), whereas isoform b mRNA remained unchanged (Fig. 4). Western blot analysis confirmed that ceftriaxone stimulated specifically the expression of Glt1a (Fig. 4; p<0.05). Glt1a upregulation appeared to improve glutamate uptake in membrane preparations of the dorsal horn, from 0.60±0.05 nmol/mg in rats receiving vehicle injections to 0.79±0.09 nmol/mg in rats treated with ceftriaxone (N=6, not significant) (Fig. 5A). Glutamate in the CSF was reduced to 47.57±1.54 μM, compared with a concentration of 65.16±3.80 μM in rats receiving vehicle (N=12; p<0.001) (Fig. 5B).
Fig. 4.
Ceftriaxone treatment increases glutamate transporter expression. Expression of Glt1 mRNA (qPCR, left panels) and protein (Western blot, right panels) in the ipsilateral dorsal horn of rats treated with vehicle or ceftriaxone (200 mg/kg) for 7 days after SNI. For qPCR, N=4 dorsal horn samples per group, pooled from 3 rats each. For Western blot, N=6 individual dorsal horn samples. * p<0.05
Fig. 5.
Ceftriaxone decreases spinal glutamate and prevents the facilitation of glutamate release by mGluR5 activation in rats 1 week after SNI. A, Glutamate uptake in the ipsilateral dorsal horn of rats after SNI that were treated with vehicle or ceftriaxone (N=5–6), not significant. B, Glutamate in the CSF of naïve rats compared to untreated rats after SNI, and rats after SNI that were treated with vehicle or ceftriaxone (N=12). ** p<0.01, one-way ANOVA followed by a Bonferroni test. C–D, Whole cell patch clamp recordings, performed on acute slices of the spinal cord. C, mEPSC frequency and amplitude of rats treated with vehicle (N=7 neurons) or ceftriaxone (N=16 neurons). *p<0.05 D, Effect of MPEP (10 μM) on mEPSC frequency and amplitude in rats treated with ceftriaxone (N=4).
Next we examined if the enhanced clearance of extracellular glutamate prevented the activation of mGluR5 and decreased presynaptic activity. To test this hypothesis, we determined the frequency of mEPSCs in dorsal horn slices of rats treated with ceftriaxone for 7 days after SNI and rats receiving vehicle injections. In dorsal horn neurons of vehicle-treated rats, mEPSCs occurred at a rate of 0.82±0.09 Hz (N=7). Ceftriaxone treatment led to a decrease in the frequency of mEPSCs after SNI to 0.44±0.08 Hz (N=16; p<0.05) (Fig. 5C), a rate equivalent to that in uninjured rats (Fig. 3A and B). The amplitude of mEPSCs was unchanged (Fig. 5C). Adding the mGluR5 antagonist MPEP to the slices did not reduce the frequency of mEPSCs further, beyond the effect of ceftriaxone (0.33±0.20 Hz versus 0.33±0.19 Hz; N=4, not significant), consistent with the idea that mGluR5 is recruited only in the presence of excess levels of extracellular glutamate (Fig. 5D).
Ceftriaxone attenuates neuropathic pain-like behavior
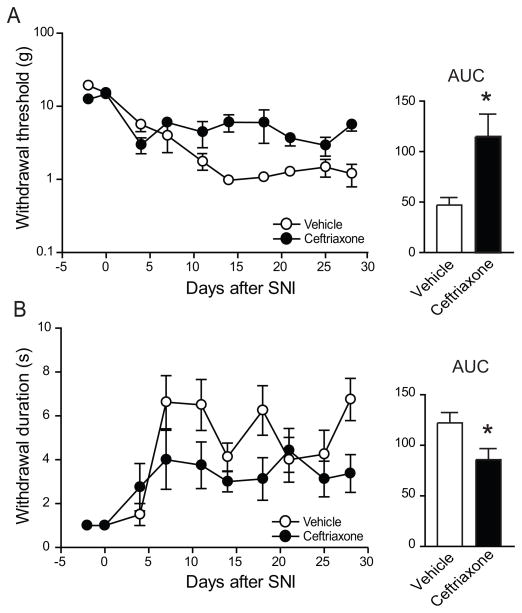
We determined the effect of ceftriaxone on neuropathic pain-like behavior by testing the responses to punctate mechanical stimulation with calibrated von Frey filaments and evaporation of a drop of acetone, which produces a cold sensation. The stimuli were applied to the plantar surface of the left hind paw, in the territory of the sural nerve which remains intact after SNI [13]. SNI reduced the withdrawal threshold for mechanical force and increased the withdrawal response to cold (Fig. 6), resulting in behavioral changes that are equivalent to mechanical and cold allodynia, respectively, in patients with neuropathic pain. Ceftriaxone attenuated the nerve injury-induced increase in sensitivity to both mechanical and cold stimulation (n=8, p<0.05, compared to vehicle treatment) (Fig. 6).
Fig. 6.
Ceftriaxone treatment reduces neuropathic pain-like behavior. Daily treatment of rats after SNI decreased A, mechanical allodynia tested with von Frey filaments, B, cold allodynia elicited by acetone evaporation. p<0.01 for mechanical allodynia, p<0.05 for cold allodynia, in two-way ANOVAs. Bar graphs in the right panels show the corresponding areas under the curves (AUCs). * p<0.05, in an unpaired Student’s t test.
Discussion
Changes in glutamate signaling contribute critically to central sensitization and the development of neuropathic pain [28,29,52]. We examined glutamate release and uptake in a model of chronic pain after peripheral nerve injury and found a severe disruption of intrinsic mechanisms that regulate glutamate homeostasis. Glutamate uptake was insufficient to prevent a long-term increase in spinal glutamate levels, and this rise in extracellular glutamate was associated with mGluR5 activation, which prompted further enhancement of glutamate release.
Consistent with our finding that group I mGluRs did not modulate miniature activity in the dorsal horn of naïve animals, mGluR5 or mGluR1a antagonists have no effect on baseline nociception [27]. In contrast, the development of enhanced pain sensitivity after inflammation [7,19,27,61,66] or nerve injury [27,31,66] clearly involves group I mGluRs. Activation of these receptors changes the firing pattern of dorsal horn neurons and is involved in long-term changes in nociceptive transmission [2,14,17]. We found that mGluR5 activation was responsible for a marked increase in miniature activity at dorsal horn synapses following peripheral nerve injury. Metabotropic GluR5 in the superficial dorsal horn colocalized with the central axons of both peptidergic and non-peptidergic nociceptors, but is also expressed on the dendrites of dorsal horn neurons [9,46]. The effect of mGluR5 activation on the frequency of mEPSCs after SNI may therefore indicate receptor recruitment at the terminals of primary sensory neurons or excitatory interneurons, involvement of mGluR5-expressing interneurons in the modulation of transmitter release, or retrograde signaling in response to mGluR5 activation on nociceptive transmission neurons. A slight decrease in mEPSC amplitudes in the presence of the mGluR5 antagonist MPEP might suggest a blockade of active postsynaptic receptors, but was not statistically significant after SNI. Considering the expression of mGluR5 on both primary sensory neurons and excitatory interneurons in the dorsal horn, glutamate release after nerve injury is likely to be regulated directly through presynaptic receptor activation.
Group I mGluRs are coupled through Gq/11 protein to phospholipase C activation, inositol trisphosphate generation and intracellular calcium mobilization, which may be one mechanism through which they facilitate neurotransmitter release [44,45]. Coupling of mGluR5 to Trpv1 provides for an additional increase in calcium influx [26]. Activation of mGluR5 may further modulate neuronal excitability through mitogen-activated protein kinase-mediated phosphorylation of voltage-gated potassium channel subunit Kv4.2 [20]. Expression and function of mGluR5 remained stable after SNI despite an injury-induced transient degenerative change in non-peptidergic nociceptor terminals that is characterized by diminished IB4 binding and a decrease in the varicosities of type Ia synaptic glomeruli [3]. Nerve injury appears to even induce upregulation of mGluR5 in primary sensory afferents and dorsal horn neurons [22]. We did not find evidence supporting an involvement of mGluR1a, which was recently described following formalin injection into the rat hind paw after chronic constriction of the sciatic nerve [27]. The larger impact of mGluR1 activation on glutamate release in this combined model may be related to the additional nociceptive Trpa1 activation by formalin [36]. According to electron microscopic studies, mGluR1 in the dorsal horn is predominantly postsynaptically located, on the dendrites of lamina V neurons, and integrated into the plasma membrane after peripheral tissue inflammation [46].
Our results suggest that a high level of extracellular glutamate is responsible for mGluR5 activation on presynaptic terminals. Normally, Glt1, Glast and Eaac1 remove glutamate rapidly from the extracellular space by buffering and uptake [59]. Glutamate uptake in the spinal cord is an important regulator of physiological somatosensory transmission and pain [57]. But elevated glutamate concentrations in the CSF at one and four weeks following SNI showed that the transmitter uptake was insufficient, despite a modest increase in glutamate transport that we observed in our in vitro studies. Glt1 and Glast expression did not change over six weeks after SNI, and Eaac expression decreased. Whereas a previous report suggested a transient rise in spinal glutamate transporters after sciatic nerve constriction [55], long-term reduction of the transporter proteins including Glt1 has been demonstrated in several models of peripheral nerve injury [8,41,55], corresponding to our finding that the net clearance of glutamate is insufficient. Studies describing an analgesic effect of glutamate transporter inhibition indicate that these proteins may also contribute to increased pain sensitivity, for example, if experimental conditions lead to a reversal of glutamate transport or a suppression of afferent input through presynaptic glutamate receptors [43,64].
Increased miniature activity in the presence of already high extracellular glutamate concentrations represents an abnormal facilitation of spontaneous transmitter release. Rising afferent input from ectopic activity and enhanced nociceptor excitability after nerve injury [32,34,37,62] should rather be met with a compensatory reduction of presynaptic transmitter release as a means of homeostatic adaptation [47]. The facilitating effect of mGluR5 activation after nerve injury contrasts with the suppression of presynaptic activity that is mediated by activation of group II mGluRs and kainate receptors on the central terminals of primary afferents, or by AMPA receptor activation on inhibitory interneurons [15,25,40]. But it may be reinforced by glutamate causing kainate receptor-dependent suppression of inhibitory transmission [24].
We used continuous treatment with ceftriaxone, a β-lactam antibiotic that increases Glt1 in astrocytes through activation of nuclear factor κB, as a tool to enlarge the capacity for glutamate uptake [38,51]. Following treatment with ceftriaxone, we found a specific increase in isoform Glt1a, which is expressed not only by astrocytes but also on the presynaptic terminals of excitatory neurons [10], suggesting that ceftriaxone may modulate glutamate uptake by both astrocytes and neurons. Glt1b was not induced, in contrast with a previously described rise in Glt1b expression in the hippocampus of rats treated with ceftriaxone [51]. However, Glt1b expression in this study was examined in uninjured rats, implying that astrocytes were in a resting state. Nerve injury, on the other hand, provokes morphological and molecular changes in spinal astrocytes that may be associated with altered Glt1b regulation. Considering the regional heterogeneity of astrocytes with respect to development, gene expression and function [65], it is also possible that modulation of the two Glt1 isoforms differs between brain and spinal cord, similar to other regional differences in the regulation of Glt1a and Glt1b [6,18]. We demonstrated specificity for Glt1a induction in the dorsal horn of the spinal cord at both RNA and protein levels.
The induction of Glt1a expression with ceftriaxone reduced extracellular glutamate to preinjury levels and interrupted the positive feedback mediated by mGluR5 activation. The balance between transmitter release and uptake was restored and pain-related behavior equivalent to mechanical and cold allodynia in humans decreased, as previously described [21,48]. A similar attenuation of neuropathic pain has been achieved with minocycline-induced upregulation of glutamate transporters or Glt1 gene transfer [35,42], underscoring the importance of glutamate homeostasis for the transmission of nociceptive input in the spinal cord. Clinical management of neuropathic pain involves pharmacological interventions to reduce glutamate release, for example, pregabalin and ziconotide, or block postsynaptic NMDA receptors, for example, dextromethorphan and memantine. Tolerable doses of these medications usually provide only partial pain relief. Our results suggest that the enhancement of glutamate uptake provides a complementary strategy to reestablish glutamate homeostasis at dorsal horn synapses. Ceftriaxone is currently clinically tested for the treatment of amyotrophic lateral sclerosis (NCT00349622) and refractory psychosis (NCT00591318). The drug needs to be administered intravenously, limiting its use in a chronic condition such as neuropathic pain, which requires sustained treatment. However, if these ongoing trials demonstrate long-term safety, targeting glutamate transport should be considered a promising approach to pain management.
Summary.
Following peripheral nerve injury, insufficient neurotransmitter uptake in the spinal cord causes activation of metabotropic glutamate receptors and further enhancement of glutamate release.
Acknowledgments
We thank Paul A. Rosenberg, PhD, and Geraldine Gomez, PhD, for their advice and for generously providing us with the antibody against Glt1b. We also thank Wei Chao, MD, PhD, for his support and Annett Häussler for technical assistance. This work was supported by grant R01 NS050408 (to J. S.) from the National Institute of Neurological Disorders and Stroke and grant SFB 815, A12 (to I. T.) from the Deutsche Forschungsgemeinschaft.
Footnotes
Conflicts of interest statement
J. S. has served as consultant for Convergence Pharmaceuticals, GlaxoSmithKline, Pfizer, Saint-Jude Medical and Sanofi-Aventis and has received grant support from Pfizer and GlaxoSmithKline for unrelated research projects.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amaya F, Samad TA, Barrett L, Broom DC, Woolf CJ. Periganglionic inflammation elicits a distally radiating pain hypersensitivity by promoting COX-2 induction in the dorsal root ganglion. Pain. 2009;142(1–2):59–67. doi: 10.1016/j.pain.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azkue JJ, Liu XG, Zimmermann M, Sandkühler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106(3):373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Bailey AL, Ribeiro-da-Silva A. Transient loss of terminals from non-peptidergic nociceptive fibers in the substantia gelatinosa of spinal cord following chronic constriction injury of the sciatic nerve. Neuroscience. 2006;138(2):675–690. doi: 10.1016/j.neuroscience.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24(11):2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492(1):78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4(4):417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- 8.Binns BC, Huang Y, Goettl VM, Hackshaw KV, Stephens RL., Jr Glutamate uptake is attenuated in spinal deep dorsal and ventral horn in the rat spinal nerve ligation model. Brain Res. 2005;1041(1):38–47. doi: 10.1016/j.brainres.2005.01.088. [DOI] [PubMed] [Google Scholar]
- 9.Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501(5):780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24(5):1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Zhang G, Marvizon JC. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 166(3):924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 14.Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6(3):274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- 15.Engelman HS, Anderson RL, Daniele C, Macdermott AB. Presynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors modulate release of inhibitory amino acids in rat spinal cord dorsal horn. Neuroscience. 2006;139(2):539–553. doi: 10.1016/j.neuroscience.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear factor-kappaB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. J Neurosci. 2011;31(25):9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinke B, Sandkühler J. Group I metabotropic glutamate receptor-induced Ca(2+)-gradients in rat superficial spinal dorsal horn neurons. Neuropharmacology. 2007;52(3):1015–1023. doi: 10.1016/j.neuropharm.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162(4):1055–1071. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Hu HJ, Bhave G, Gereau RW. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22(17):7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HJ, Gereau RW. Metabotropic glutamate receptor 5 regulates excitability and Kv4.2-containing K channels primarily in excitatory neurons of the spinal dorsal horn. J Neurophysiol. 105(6):3010–3021. doi: 10.1152/jn.01050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Li W, Lu L, Cai J, Xian X, Zhang M, Li Q, Li L. An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain. 2010;148(2):284–301. doi: 10.1016/j.pain.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Hudson LJ, Bevan S, McNair K, Gentry C, Fox A, Kuhn R, Winter J. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22(7):2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 1999;410(4):627–642. [PubMed] [Google Scholar]
- 24.Kerchner GA, Wang GD, Qiu CS, Huettner JE, Zhuo M. Direct presynaptic regulation of GABA/glycine release by kainate receptors in the dorsal horn: an ionotropic mechanism. Neuron. 2001;32(3):477–488. doi: 10.1016/s0896-6273(01)00479-2. [DOI] [PubMed] [Google Scholar]
- 25.Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci. 2001;21(1):59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci. 2009;29(32):10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar N, Laferriere A, Yu JS, Poon T, Coderre TJ. Metabotropic glutamate receptors (mGluRs) regulate noxious stimulus-induced glutamate release in the spinal cord dorsal horn of rats with neuropathic and inflammatory pain. J Neurochem. 2010;114(1):281–290. doi: 10.1111/j.1471-4159.2010.06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16(11):1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 29.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35(1):135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- 31.Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem. 112(1):162–172. doi: 10.1111/j.1471-4159.2009.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85(3):503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386(6626):721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000;84(2–3):309–318. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- 35.Maeda S, Kawamoto A, Yatani Y, Shirakawa H, Nakagawa T, Kaneko S. Gene transfer of GLT-1, a glial glutamate transporter, into the spinal cord by recombinant adenovirus attenuates inflammatory and neuropathic pain in rats. Mol Pain. 2008;4:65. doi: 10.1186/1744-8069-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaelis M, Liu X, Janig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20(7):2742–2748. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153(1):329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 130(3):283–309. doi: 10.1016/j.pharmthera.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neugebauer V. Metabotropic glutamate receptors--important modulators of nociception and pain behavior. Pain. 2002;98(1–2):1–8. doi: 10.1016/s0304-3959(02)00140-9. [DOI] [PubMed] [Google Scholar]
- 41.Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 2010;103(5):2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nie H, Zhang H, Weng HR. Minocycline prevents impaired glial glutamate uptake in the spinal sensory synapses of neuropathic rats. Neuroscience. 2010;170(3):901–912. doi: 10.1016/j.neuroscience.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niederberger E, Schmidtko A, Rothstein JD, Geisslinger G, Tegeder I. Modulation of spinal nociceptive processing through the glutamate transporter GLT-1. Neuroscience. 2003;116(1):81–87. doi: 10.1016/s0306-4522(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 44.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9(6):423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 46.Pitcher MH, Ribeiro-da-Silva A, Coderre TJ. Effects of inflammation on the ultrastructural localization of spinal cord dorsal horn group I metabotropic glutamate receptors. J Comp Neurol. 2007;505(4):412–423. doi: 10.1002/cne.21506. [DOI] [PubMed] [Google Scholar]
- 47.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience. 2010;169(4):1888–1900. doi: 10.1016/j.neuroscience.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 50.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 51.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 52.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89(2):707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 53.Scholz J, Abele A, Marian C, Haussler A, Herbert TA, Woolf CJ, Tegeder I. Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats. Pain. 2008;138(1):130–142. doi: 10.1016/j.pain.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53(2):195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 55.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23(7):2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takasugi Y, Shirai T, Futagawa K, Koga Y, Egawa K, Watanabe S, Umeda T. Transcutaneous cisternal puncture for sampling of cerebrospinal fluid in awake rat. Exp Anim. 2005;54(2):193–196. doi: 10.1538/expanim.54.193. [DOI] [PubMed] [Google Scholar]
- 57.Tao YX, Gu J, Stephens RL., Jr Role of spinal cord glutamate transporter during normal sensory transmission and pathological pain states. Mol Pain. 2005;1:30. doi: 10.1186/1744-8069-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8(12):935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 60.Vetter G, Geisslinger G, Tegeder I. Release of glutamate, nitric oxide and prostaglandin E2 and metabolic activity in the spinal cord of rats following peripheral nociceptive stimulation. Pain. 2001;92(1–2):213–218. doi: 10.1016/s0304-3959(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 61.Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini F, Kuhn R, Urban L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40(1):10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 62.Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116(3):243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xin WJ, Weng HR, Dougherty PM. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Mol Pain. 2009;5:15. doi: 10.1186/1744-8069-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaster M, Guan X, Petralia RS, Rothstein JD, Lu W, Tao YX. Effect of inhibition of spinal cord glutamate transporters on inflammatory pain induced by formalin and complete Freund’s adjuvant. Anesthesiology. 2011;114(2):412–423. doi: 10.1097/ALN.0b013e318205df50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20(5):588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, 3rd, Wade CL, Decker MW, Honore P. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur J Pharmacol. 2004;506(2):107–118. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]