Abstract
The authors developed a quality-of-life measure specific to long-term breast cancer survivors. Participants were women diagnosed with early-stage disease ≥7 years postdiagnosis. The final scale is the result of an iterative interview process with the 28-item scale administered to 285 participants. Factor analysis demonstrated with seven domains: physical, sexual and cognitive function, body image, coping, social support, and anxiety. Cronbach's alpha is.88. Convergent and divergent validity are also reported. The Long Term Quality of Life–Breast Cancer Scale has domains specific to breast cancer and will be useful to psychosocial and clinical researchers.
Keywords: breast cancer, oncology, quality of life, long-term survivors
INTRODUCTION
It is estimated that in 2008 there were 182,460 new cases of breast cancer diagnosed in the United States, and that 40,460 women died because of the disease (National Cancer Institute, 2009). Nonetheless, breast cancer survival rates have increased in recent years, and the 5-year survival rate is now around 86%, a 10 percentage point increase since 1976 (Warren & Devine, 2002). There are currently about 2.5 million women living with cancer in the United States (American Cancer Society, 2009). Although providing curative treatment remains the number one priority for patients and physicians, maintaining adequate long-term quality of life (QOL) is also a principal concern. Many studies (Ganz, 2006; Giedzinska, Meyerowitz, Ganz, & Rowland, 2004; Griggs et al., 2006; Hartl et al., 2003; Low, Stanton, Thompson, Kwan, & Ganz, 2006; Manne et al., 2006; Ong, Visser, Lammes, & de Haes, 2000; Shimozuma, Ganz, Peterson, & Hirji, 1999) have examined QOL issues during and shortly after treatment, but far less research has been conducted to examine QOL in breast cancer survivors 5 or more years after remission.
QOL research is especially important in the area of breast cancer survivorship, not only because there are so many survivors, but also because most patients receive some form of adjuvant therapy in addition to surgery and because of the increasing timelines for certain therapies (such as hormonal treatments), which means that women are often taking some treatment for years. In addition, the long natural history of the disease makes treatment outcomes uncertain as to whether a patient is ever cured.
Current Knowledge About QOL After Breast Cancer
In the short term, studies indicate that overall QOL appears to be rather good when considering what the survivors have only recently experienced. Although cancer can negatively affect QOL, it is well known that many cancer survivors report that the illness has brought them a new meaning to their lives and reinforced positive health behaviors and spirituality (Alfano et al., 2006). A study by Bower et al. (2005) found that after 10 years of survivorship, many women report high levels of positive meaning and healthier lifestyles after cancer. Survivors also report negative changes such as pessimistic outlook and increased feelings of vulnerability in their lives after cancer (Giedzinska et al., 2004) and report having at least one persistent physical symptom after cancer treatment. However, the cross-sectional nature of most QOL studies makes it difficult to know whether commonly reported symptoms, such as hot flashes and body image issues, are related to breast cancer and its treatment or whether they are simply a result of the aging process (Giedzinska et al., 2004). Shimozuma et al. (1999) found that although breast cancer patients experience physical problems (e.g., difficulty sleeping, less energy) and psychosocial problems (e.g., poorer mood, body image issues) shortly after surgery, most women recover from these difficulties after one year. Other studies have found similar results of minor impairment in breast cancer survivors (Hartl et al., 2003) and evidence that physical and psychological problems reported immediately after treatment tend to improve over the course of the first year (Low et al., 2006). However, studies also support the idea that between 1 and 5 years posttreatment, survivors, particularly younger ones and a significant minority of women, have continued problems including an acute fear of recurrence and lower energy (Giedzinska et al., 2004; Hartl et al., 2003). Higher vulnerability levels are also associated with poorer QOL and more negative affect (Bower et al., 2005; Gotay & Muraoka, 1998; Helgeson & Tomich, 2005). Some studies report that very few have physical and emotional difficulties, and that long-term breast cancer survivors may have comparable or improved QoL levels when compared to age-matched controls who have never had cancer (Ashing-Giwa, Ganz, & Peterson, 1998; Kendall, Mahue-Giangreco, Carpenter, Ganz, & Bernstein, 2005; Schover, 2004).
Breast cancer survivors may also have more difficulties with physical symptoms and functioning, such as lymphadema and menopausal symptoms, and perceive themselves to be in worse health than controls without cancer (Ashing-Giwa et al., 1998; Gotay & Muraoaka, 1998; Helgeson & Tomich, 2005; Kendall et al., 2005; Oh et al., 2004; Schover, 2004). Finally, studies suggest that there are certain factors that can help predict long-term QOL outcomes. For example, younger women tend to have worse QOL outcomes and more memory problems than older women (Knobf, 2007; Peuckmann et al., 2007; Stava, Lopex, & Vassilopoulou-Sellin, 2006). Poorer QOL is also associated with being single (Carver, Smith, Petronis, & An-toni, 2006; Peuckmann et al., 2007), lower optimism (Carver et al., 2006), fatigue and problems with physical functioning (Knobf, 2007; Perkins et al., 2007), receiving chemotherapy (Knobf, 2007), and a high body mass index (Peuckmann et al., 2007).
Measurement of QOL
A variety of measures have been used to assess QOL in breast cancer survivors. QOL has been measured using noncancer-specific tools, such as the (Medical Outcomes Study Short-Form 36 [MOS SF-36]; Stewart, Hays, & Ware, 1988), a general measure of physical and mental health functioning, and the Impact of Event Scale (Horowitz, Wilner, & Alvarez, 1979). Several cancer-specific measures exist that are designed to measure QOL in patients during or shortly after treatment, such as the Cancer Rehabilitation Evaluation System (CARES; Ganz, Schag, & Cheng, 1990) and the Functional Assessment for Cancer Therapy-Breast (FACT-B; Brady et al., 1997). The FACT-B is specific to breast cancer (whereas the CARES may be used for all types of cancer) but is less useful for long-term survival studies, as it focuses on treatment side effects. The Quality of Life Index (QLI) was developed for use with healthy individuals as well as individuals experiencing an illness (Ferrans & Powers, 1985). The Cancer Quality of Life Questionnaire (Sprangers et al., 1996) examines five functional areas of symptoms and obtains a global rating of health status. This instrument is widely used in Europe. A head-to-head comparison of the QLI and the Cancer Quality of Life Questionnaire found that the two instruments measure different aspects of QOL and concluded that QOL, with its many meanings, requires several measurement instruments (Gupta, Grutsch, & Lis, 2008).
The Impact of Cancer (IOC) instrument was developed using 194 participants with lymphoma, breast, prostate, and colorectal cancers (Zebrack, Ganz, Bernaards, Peterson, & Abraham, 2006). The Quality of Life-Cancer Survivors (QOL-CS) is an instrument that assesses the concerns of cancer survivors using an 11-point scale (Ferrell, Dow, & Grant, 1995). However, Avis, Ip, and Foley (2006) pointed out a number of limitations of the QOL-CS, most notably that the validation of the scale was based on a sample of survivors ranging from 4 months to 28 years postdiagnosis.
Satisfaction with Life Domains for Breast Cancer was developed to measure patients' perceptions of their QOL (Spagnola et al., 2003). This scale is a multidimensional scale that focused on physical and socioemotional aspects of QOL and also examined some of the unique challenges of breast cancer as an illness. The scale was developed with women undergoing care for breast cancer and reports very good reliability and validity.
Avis et al. (2006) created the Quality of Life in Cancer Survivors (QLACS) scale, a 47-item instrument consisting of 12 domains developed using 94 breast cancer survivors with an 8-year follow-up. However, as with the IOC and QOL-CS, the QLACS was created for use with all types of cancer and therefore may not cover all domains necessary to adequately assess the unique experiences of long-term breast cancer survivors. Finally, the Long-Term Quality of Life (LTQL) instrument was created to assess QOL issues in long-term female cancer survivors (Wyatt & Friedman, 1996). Although the scale has good psychometric properties, the four domains of the LTQL are rather broad and thus combine aspects of QOL, such as pain problems and body image, that often do not vary together and actually assess two different areas (physical vs. emotional/psychological well-being) that should be examined separately (Avis et al., 2006).
Purpose of the Current Study
Presented in this report is a new QOL instrument specific to long-term (>7 years) breast cancer survivors. A unique aspect of this measure is that it was developed with women who were long-term survivors of breast cancer, were disease free, and, therefore, not receiving any kind of treatment. The data to construct the scale were obtained from a research project (Gordon, September 1, 2000–August 31, 2004) designed to (1) describe the prevalence and incidence of physiologic sequelae to the occurrence and treatment of breast cancer and (2) investigate the relationship of these sequelae to survival after treatment and to patients' long-term health-related QOL and associated health care needs. The challenge of such a study was to separate the effects of posttherapeutic comorbidity from those intrinsic to aging. We initially examined breast cancer patients' QOL in relation to four major areas that have been found to be related to the global health–related QOL of cancer patients' functioning: physical, social, psychological, and spiritual wellness (Dow, Ferrell, Haberman, & Eaton, 1999).
METHOD
Participants
The project's research participants were 1,546 breast cancer patients who were at least 7 years and up to 30 years postdiagnosis and who had received a modified radical mastectomy as their primary surgical treatment. All participants were diagnosed with Stage I–III disease; therefore none had distant metastases at diagnosis. The parent study's main aim was to estimate the factors that affected long-term survivorship. This substudy drew its participants from the original sample of 1,546 patients, of whom 932 (60%) were already deceased and an additional 95 died during the study. To be eligible, patients had to be cognitively able to participate in an interview. Of the long-term survivors from this population we were able to locate and invite 326 of whom 295 (90.5%) agreed to be interviewed; 10 died during the study.
Interview Participant Demographics
The mean and median age of participants at the time of interview were 68.1 years (SD = 11.0 years) and 70 years, respectively; the mean and median age at diagnosis were 49.7 years (SD = 9.1) and 49 years, respectively. The mean and median time between interview and the date of diagnosis were 19.4 years (SD = 6.8) and 20 years, respectively. Patients were well educated, 62.6% had at least one year of college education; and the median income was between $25,000 and $49,999. Most patients (91.0%) were White with 6.5% African American and 2.5% of other racial/ethnic groups. The majority of patients were married (63.0%).
Data Collection
Three separate interview schedules, each building on the previous one, were developed and administered to study participants, starting with an open-ended technique and culminating with a pool of scale items. The iterative interview technique began with obtaining breast cancer survivors' assessments of their experiences as survivors with a focus on problems they experienced since their breast cancer treatment. This approach was intended to confirm our initial list of scale domains, add or modify scale domains, and validate content validity of the scale. The second interview was a structured survey derived from the first. This wave of data collection provided yet another check on validity and allowed us to collect data on a larger sample using a more structured survey instrument. Last, one of the authors (Siminoff), led a coding team of investigators and staff researchers to read through responses from the two previous interviews to develop the final item pool and guide the wording of each scale item.
The first wave of data collection was a qualitative open-ended interview conducted with 38 breast cancer survivors residing in the Greater Cleveland metropolitan area. The sample was drawn randomly from the larger study sample pool. It consisted of an hour-long, in-person open-ended “discussion” with the patient about her breast cancer experience. The interview covered some of the patient's natural history with the illness including specifics of diagnosis (how was the disease first detected); surgical and adjuvant therapy decisions and experiences; what problems breast cancer produced for participant's physical functioning and activities of daily living (ADLs); social functioning within her family, work, and community; sexual relationship functioning; characterization of health subsequent to completion of adjuvant therapy; discussion of each major health care–related problem including the participant's attributions for the problem and how it now affects her day-to-day functioning; other major life events subsequent to the completion of breast cancer therapy and participant's response to those events; and sociodemographic information. Topics that we expected to be of special concern to breast cancer survivors were body image and balance, sexual functioning, effect of treatment on marital relations, long-term anxiety due to the “chronicity” of a breast cancer diagnosis, and anxiety about the contralateral breast. All interviews were audiotaped, transcribed, and content analyzed by one of the authors (LAS) who is an experienced qualitative researcher, and two research assistants. The analysis identified major themes and domains of relevance to patient-reported QOL issues. On the basis of this analysis, a semistructured instrument consisting of short answers and close-ended questions was constructed that could be administered by telephone.
Two-hundred and seventy-four participants participated in the second wave of data collection. Data from this second group of interviews consisted of more discrete measurement domains. The second interview was a semistructured survey that combined close-ended questions with open-ended questions that elicited short answers. Domains measured were the same as the first interview as described above. This interview obtained a rich and detailed description of breast cancer survivors' QOL, but in a more parsimonious and structured fashion. These interviews were audiotaped for quality assurance and coding accuracy. The audiotapes served two purposes: first as a means of performing quality and reliability checks on the interviews, and second as an accurate data collection mechanism that allowed interviewers to engage the participants without the distraction of note taking or recording codes as they interviewed them. Finally, participants completed several standardized scales. Patients completed the Charlson Comorbidity Index (Charlson, Pompei, Ales, & McKenzie, 1987) the single best measure of an age-comorbidity index. We also assessed QOL/physician function using an alternative, general measure of QOL by administering the Activities of Daily Living Scale, a widely used measure that is especially valid for older populations (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963).
The last wave of data collected was the administration of a 52-item scale whose item pool was derived from the previous two waves of interviews and was designed to capture long-term breast cancer survivors' QOL. This scale was completed by 285 participants. The domains examined were physical function, social factors (including social support, sexuality, life events, and economic issues), psychological well-being (anxiety/depression, fear of recurrence, cognitive function), and spiritual well-being. This article reports on the reliability and validity of the final 28-item tool, the Long Term Quality of Life–Breast Cancer (LTQOL-BC) Scale.
Statistical Analyses
The final 28-item (LTQOL-BC) was tested and refined using data from the administration of the original pool of 52 items. Appendices A and B provide the original and final scale items. Analysis was guided by theory and prior empirical evidence to measure the phenomenon under consideration.
The first step was an exploratory factor analysis (EFA) to reduce the item pool. For a more detailed explication of the process, assumptions, and criteria for running an EFA, see Fabrigar, Wegener, MacCallum, and Strahan (1999). Before conducting the EFA, missing values were considered. Only three values were missing, and these were replaced by the median score. The EFA was conducted using principal axis factoring with promax rotation. Several criteria provided guidelines in selecting the number of factors to extract (Hatcher, 1994). Factors with eigenvalues greater than 1.0 were retained; and examination of distinct elbows in scree plots of eigenvalues and total variance explained by retained factors assisted in determining the total number of factors to retain. Items were included in a given factor if their loading was at least .40. In addition, items were checked for possible (secondary) cross loadings (>0.30) on other factors. Cross loadings indicate items that potentially measure more than one factor.
More generally, in the early stages and throughout the entire process, the number of items extracted was those that provided the “cleanest” and most “interpretable” factor loadings. Specifically, an item in a given factor must have a high primary loading greater than .4 on that factor and low secondary loadings less than .3 on other factors and appear to measure the same content as other items loading on the same factor.
Second we tested several models. The first measurement model tested consisted of an 8-factor solution. Based on examination of the factor loadings, the least acceptable item was removed, and the analysis was rerun with the remaining items. The EFA continued in this manner until a clean solution was obtained.
Finally, a Cronbach coefficient alpha was calculated for the entire scale and each individual subscale to assure internal consistency and reliability. An examination of the total scale alpha compared to the alpha when an item is deleted helped guide identifying candidate items for deletion from the scale. Further refinements were implemented through the process of confirmatory factor analysis.
RESULTS
The final factor model consisted of 28 items divided among seven unique domains within the scale: (1) physical function, (2) body image, (3) sexual function, (4) coping, (5) cognitive function, (6) social support, and (7) anxiety (see Table 1).
TABLE 1.
Final Scale Item Factor Loadings, Percent Agreement with Item, and Domain Standardized Cronbach Coefficient Alpha (N = 285)
| Subscales (Total scale α = .88) | Factor Loading | Percent Agreement |
|---|---|---|
| Physical function (α = .85) | ||
| 1. I am physically strong.a | .75 | 78.2 |
| 2. I feel fatigued all the time. | .69 | 18.3 |
| 3. I have difficulty walking up or down stairs. | .71 | 36.8 |
| 4. I find it difficult doing physical activities such as housework. | .84 | 24.2 |
| 12. I have restrictions of movement in my arms | .47 | 18.9 |
| 13. I have aching or pain in my lower body and legs. | .56 | 48.4 |
| 17. I have or had trouble getting or keeping a job/functioning as a homemaker. | .68 | 9.6 |
| Body image (α = .83) | ||
| 20. I do not feel feminine or especially attractive. | 0.67 | 15.8 |
| 21. I feel incomplete as a woman. | 0.80 | 11.9 |
| 44. Since having had breast cancer I feel cheated. | 0.69 | 11.2 |
| 45. Since having had breast cancer I feel incomplete. | 0.84 | 12.2 |
| Sexual function (α = .68) | ||
| 26. Intimate relationships are difficult for me. | 0.61 | 11.6 |
| 27. I have little sexual interest. | 0.64 | 46.7 |
| 28. Sexual intimacy is easy for me.a | 0.71 | 31.6 |
| Coping (α = .68) | ||
| 33. I take each day and make the most of it. | .69 | 94.7 |
| 36. I appreciate things and don't take them for granted. | .76 | 98.9 |
| 37. I don't sweat the small stuff. | .40 | 84.6 |
| 50. I am a stronger person since surviving my breast cancer.a | .52 | 90.2 |
| Cognitive function (α = .68) | ||
| 40. I have difficulty remembering things. | .61 | 24.6 |
| 41. I can concentrate easily.a | .67 | 87.4 |
| 42. I have difficulty thinking clearly. | .65 | 6.7 |
| Social support (α = .63) | ||
| 14. I can count on those closest to me for everyday help.a | .54 | 90.9 |
| 15. I am alone. | .45 | 30.9 |
| 16. I have friends who would help me if I had a crisis.a | .78 | 98.9 |
| Anxiety (α = .61) | ||
| 24. When I get sick I feel very anxious. | .54 | 32.6 |
| 25. I feel uncertain about things. | .73 | 23.5 |
| 30. I make very frequent visits to the doctor. | .42 | 14.7 |
| 49. I think about my breast cancer every day.a | .40 | 86.0 |
Denotes a question recoded so that it is consistent with direction of other questions.
Original scoring is 5 = strongly agree, 4 = agree, 3 = no opinion, 2 = disagree, 1 = strongly disagree, 99 = missing. Agreement in table indicates individual either agreed or strongly agreed with the scale item.
Description of Individual Domains
Table 1 exhibits the final scale and its subscales. The total scale standardized Cronbach coefficient alpha is .88 and the subscale Cronbach alphas range from 0.85 for the physical function subscale to 0.61 for the Anxiety subscale. Our analyses demonstrated a single physical function domain, but factor analysis revealed that social and psychological well-being consisted of several distinct scale factors.
1. Physical Function
Physical function refers to independent functioning as influenced by physical limitations due to age, cancer, or treatment effects. This subscale contains seven items. Items Q4, I find it difficult to do physical activities such as housework, Q1, “I am physically strong,” and Q3, “I have difficulty walking up or down stairs” have moderate to high factor loadings. Items Q2, “I feel fatigued at all times” and Q17, “I have or had trouble getting or keeping a job/functioning as a homemaker” have moderate factor loadings; and Items Q12, “I have restrictions of movement in my arms” and Q13, “I have aching or pain in my lower body and legs” have low to moderate factor loadings. Agreement with the negative aspect of these six items ranged from 9.6% to 36.8%. The subscale reliability, estimated by the standardized Cronbach coefficient alpha, is .85.
2. Body Image
The items in the body image domain focused on items that related directly to the impact of breast cancer on self images. There are four items in this subscale: Items Q20, “I do not feel feminine or especially attractive” and Q21, “I feel incomplete as a woman” had the factor loadings of .67 and .80, respectively, and agreement with these questions was 15.8% and 11.9% respectively. Similarly, Q44, “Since having had breast cancer I feel cheated” and Q45, “Since having breast cancer I feel incomplete” also had moderate and high factor loadings of .69 and .84, respectively. Agreement with these questions was 11.2% and 12.2%, respectively. Standardized Cronbach coefficient alpha for this subscale is .83.
3. Sexual Function
The items in the sexual function domain focused on intimacy issues. The three items in this scale, Q26, “Intimate relationships are difficult for me,” Q27, “I have little sexual interest,” and Q28, “Sexual intimacy is easy for me” have moderate factor loadings of .61, .64, and .71, respectively, and agreement was 11.6%, 46.7%, and 31.6%, respectively. The standardized Cronbach coefficient alpha for this subscale was .68.
4. Coping
The coping domain centered on strategies for living life subsequent to breast cancer diagnosis and treatment, and contained four items. The first, Q33, “I take each day and make the most of it” had a loading score of .69 with 94.7% of participants agreeing or strongly agreeing with this statement. Q36, “I appreciate things and don't take them for granted” had the highest percentage, 98.9% agreement than any other item; and this item has a factor loading of .76. Item Q50, “I am a stronger person since surviving my breast cancer” had a factor loading of .52 and had 90.2% agreement. Finally, 84.6% of participants agreed or agreed strongly with Q37, “I don't sweat the small stuff.” The standardized Cronbach coefficient alpha for the coping subscale is a moderate .68.
5. Cognitive Function
The cognitive function domain is composed of three items. Q41, “I can concentrate easily” had the highest factor loading of .67 and 87.4% of participants agreed or strongly agreed with the item. Item Q42, “I have difficulty thinking clearly” had a moderate factor loading (.65), and 6.7% agreement by participants. Finally, nearly one fourth (24.6%) of participants agreed with Q40, “I have difficulty remembering things.” This statement had a factor loading of .61. The standardized Cronbach coefficient alpha for this domain is .68.
6. Social Support
The social support domain contains items that related to the efficacy of the participant's support network and her orientation toward it. A large majority (90.9%) of participants agreed with Q14, “I can count on those closest to me for everyday help,” and this item has a moderate factor load score (.54). Item Q15, “I am alone” has a factor load score of .45, and almost one third (30.9%) of participants agreed with this statement. Q16, “I have friends who would help me if I had a crisis” has a factor load score of .78 and 98.9% agreed with this statement. The standardized Cronbach coefficient alpha for this domain is .63.
7. Anxiety
This domain contained statements about participants' continuing concerns with their breast cancer and anxiety about illness. The item with the highest factor loading (.73) is Q25, “I feel uncertain about things” had an agreement rate of 23.5% by participants. Item Q24, “When I get sick I feel very anxious” has the next highest factor loading at .54 and 32.6% of participants agreed with this statement. Items Q30, “I make very frequent visits to the doctor” and Q49, “I think about my breast cancer every day” had modest factor loadings. Agreement with Q39 was 14.7% and a very high rate, 86.0%, with Q49. The standardized Cronbach coefficient alpha for this domain is .61.
Convergent and Discriminant Validity
Convergent validity was assessed by reviewing t tests for the factor loadings (Table 1). All t tests had significance levels less than 1 × 10−9 indicating that all items within a factor are measuring the same construct (Anderson & Gerbing, 1988). Within the context of these data, some evidence regarding discriminant validity may be obtained by considering the chi-square difference test. To implement this test for two constructs (factors), first the standard measurement model is estimated where all factors are allowed to covary. Second, a new measurement model is created identical to the previous model, except the correlation between the two factors of interest is fixed at 1, which is equivalent to assuming that the two factors measure the same construct. The chi-square difference from these two models will then have degrees of freedom equal to 1. If the chi-square statistic is statistically significantly smaller for the first model, then the two constructs can be considered as distinct (Bagozzi & Phillips, 1982). There are seven factors in the measurement model which means that there are 21 possible comparisons between factors. The body image and sexual function factors have the highest correlation. The difference test yields a chi-square of 94.27 with 1 degree of freedom that is indicative of a significance level less than 1.0 ×−16. Comparison of other pairs of factors that have a smaller correlation than body image and sexual function factors would necessarily yield an even smaller significance level. Therefore, for the 21 comparisons each with a significance level less than or equal to 1.0 × 10−16, the overall significance level would be less than 2.3 × 10−15 which is small indeed and indicative of excellent discriminant validity.
Table 2 exhibits the correlations between the seven factors and their 95% confidence intervals. Note that none of the confidence intervals includes the value 1.00 indicating evidence for discriminant validity.
TABLE 2.
Factor Correlations and Their 95% Confidence Intervals
| Physical Function (PF) | Body Image (BI) | Sexual Function (SF) | Coping (CO) | Cognitive Function (CF) | Social Support (SS) | Anxiety (AN) | |
|---|---|---|---|---|---|---|---|
| PF | 1.00 | .48 [0.37, 0.59] | 0.37 [0.24, 0.50] | 0.36 [0.23, 0.49] | 0.52 [0.40, 0.64] | 0.35 [0.21, 0.48] | 0.62 [0.51, 0.74] |
| BI | 1.00 | 0.65 [0.54, 0.75] | 0.33 [0.19, 0.46] | 0.57 [0.45, 0.69] | 0.35 [0.21, 0.49] | 0.67 [0.56, 0.78] | |
| SF | 1.00 | 0.25 [0.10, 0.41] | 0.47 [0.33, 0.62] | 0.17 [0.00, 0.33] | 0.48 [0.33, 0.63] | ||
| CO | 1.00 | 0.32 [0.17, 0.47] | 0.61 [0.48, 0.74] | 0.36 [0.20, 0.51]] | |||
| CF | 1.00 | 0.20 [0.04, 0.37] | 0.52 [0.37, 0.67] | ||||
| SS | 1.00 | 0.22 [0.05, 0.39] | |||||
| AN | 1.00 |
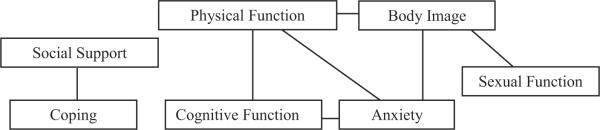
Additional evidence for the validity of the scale can be demonstrated by estimating the relationships of the individual factor's (subscale's) scores with several independent measures obtained from participants (Table 3). These associations were estimated using Pearson's correlation index, r, and are used to explore whether each factor was, in fact, measuring the constructs appropriately. The seven factors in the scale have correlations (Table 2) varying from as high as .67 for the body image and anxiety factors to as low as .17 for the social support and sexual function factors; therefore independent measures of attributes would be expected to correlate with several of the factors and to do so in a similar manner as the inter-correlations of the seven scale factors. Figure 1 below exhibits correlation coefficients greater than .5 between the seven factors.
TABLE 3.
Pearson Correlation (Significance Level for the Null Hypothesis that the Correlation is Zero)
| Physical | Body Image | Sexual Function | Coping | Cognitive Function | Social Support | Anxiety | |
|---|---|---|---|---|---|---|---|
| Charlson | 0.14 (.02) | 0.090 (.1) | 0.041 (.5) | 0.085 (.2) | 0.035 (.6) | 0.087 (.1) | 0.26 (<0.0001) |
| Major morbidity | 0.40 (<0.0001) | 0.077 (.2) | 0.076 (.2) | 0.012 (.8) | 0.049 (.4) | 0.016 (.8) | 0.25 (<0.0001) |
| Signs and symptoms of physical distress | 0.29 (<0.0001) | 0.14 (.03) | 0.13 (.05) | −0.012 (.8) | 0.15 (.02) | 0.00079 (.99) | 0.28 (<0.0001) |
| Cancer morbidity attributed to treatment | 0.14 (.03) | 0.081 (.2) | 0.15 (.02) | 0.18 (.004) | 0.16 (.01) | −0.066 (.3) | 0.19(.003) |
| Cancer Functional status attributed to treatment | 0.20 (.002) | 0.14 (.03) | 0.15 (.02) | 0.12 (.06) | 0.13 (.04) | −0.0013 (.98) | 0.25 (<0.0001) |
| ADL | 0.60 (<0.0001) | 0.24 (.0002) | 0.12 (.06) | −0.041 (.5) | 0.22 (.0006) | 0.078 (.2) | 0.28 (<0.0001) |
| Social | −0.088 (.3) | −0.23 (.003) | −0.029 (.7) | 0.22 (.004) | −0.080 (.3) | 0.25 (.008) | −0.059 (.9) |
| Sexual | −0.00089 (.99) | 0.25 (.002) | 0.22 (.006) | 0.090 (.3) | 0.055 (.5) | 0.069 (.4) | 0.25 (.001) |
| How anxious are you about your breast cancer coming back? | −0.035 (.6) | 0.11 (.08) | 0.13 (.04) | 0.12 (.05) | −.0036 (.95) | −0.089 (.9) | 0.37 (<0.0001) |
| Feeling that appearance detracts from femininity | 0.11 (.09) | 0.38 (<0.0001) | 0.20 (.002) | −0.14 (.03) | 0.076 (.2) | −0.61 (.3) | 0.24 (.0001) |
| Overall health as a result of breast cancer | −0.21 (.001) | −0.25 (<0.0001) | −0.17 (.008) | 0.063 (.3) | −.17 (.01) | −0.023 (.7) | −0.17 (.009) |
| Satisfied with cosmetic surgery | −.094 (.1) | −0.35 (<0.0001) | −0.21 (.0007) | 0.020 (.8) | −0.14 (.03) | 0.071 (.3) | −0.16 (.01) |
| Satisfied with life today | −0.29 (<0.0001) | −0.21 (.0007) | −0.16 (.01) | 0.087 (.2) | −0.086 (.2) | 0.080 (.2) | −0.26 (<0.0001) |
| Affects the way you handle stressful situations | −.079 (.2) | −0.022 (.7) | −0.0086 (.9) | 0.26 (<0.0001) | 0.040 (.5) | −0.14 (.03) | −0.058 (.4) |
| Satisfaction with Decision involving type of surgery | −0.13 (.04) | −0.17 (.008) | −0.029 (.6) | −0.044 (.5) | −0.091 (.2) | −0.027 (.7) | −0.14 (.03) |
| Satisfied with outcome of surgery | −0.18 (.004) | −0.25 (<0.0001) | −0.22 (.0005) | −0.027 (.7) | −15 (.02) | 0.10 (.1) | −0.15 (.02) |
ADL = activities of daily living.
FIGURE 1.
Schematic of Pearson correlation coefficients greater than 0.5 between the seven scale factors.
A measure of major comorbid conditions at the time of interview was obtained using the Charlson Comorbidity Index (Charlson et al., 1987). Higher comorbidity was associated with a higher (worse) physical function score (r = .14, p = .02) and anxiety score (r = .26, p < 0.0001). A second assessment of major comorbidity was obtained by adding the number of major comorbid conditions. Similarly, this index was associated with a higher (worse) physical function scale score (r = .40, p < 0.0001) and anxiety (r = .25, p < 0.0001). Signs and symptoms of physical distress were also elicited from participants. These included fatigue, allergies, premature aging, frequent illnesses, weight gain, eyesight problems, and changes in taste, smell, hair, or skin. A higher number of signs and symptoms was associated with worse physical function (r = .29, p < 0.0001), anxiety (r = .28, p < .0001), and to a lesser degree, body image (r = .14, p = .03), sexual function (r = .13, p = .05), and cognitive function (r = .15, p = .02). The Activities of Daily Living (ADL) instrument, completed by participants, demonstrated that worse ADL scores were highly associated with worse physical function (r = .60, p < 0.0001). Body image (r = .24, p = .0002), cognitive function (r = .22, p = .0006), and anxiety (r = .28, p < .0001) factors were also associated with the ADL score with poorer body image, worse cognitive function and higher anxiety associated with poorer ADL scores.
In Table 3, “Social” is the summed score over three questions regarding receiving social support from their husband/partner or others. Participants with more social support had lower negative body image (r = −.23, p = .003), better coping (r = .22, p = .004), and more social support (r = .25, p = .008) but did not have significant correlations with other factor scores. Participants' summed scores from five questions about changes in emotional and intimate relationships with husband/partner during the course of having breast cancer and at the present time is labeled as “Sexual” in Table 3. Higher summed score indicates worse conditions. This score has high correlations with worse body image (r = .25, p = .002), worse sexual function (r = .22, p = .006), and more anxiety (r = .25, p = .001). Participants who responded that they were anxious about their breast cancer coming back had higher anxiety scores (r = .37, p < 0.0001) and poorer sexual (r = .13, p = .04) and coping (r = .12, p = .05) scores.
The interview question, “My appearance detracts from femininity,” was correlated with worse body image (r = .38, p < 0.0001), greater anxiety (r = .24, p = .0001), and worse sexual function (r = .20, p = .002) scores. The question, “Are you satisfied with cosmetic results of surgery,” was correlated with better body image (r = −.35, p < 0.0001), better sexual function (r = −.21, p = .0007) and less anxiety (r = −.16, p = .01) scores. Participants who answered that they were satisfied with life today had better physical function (r = −.29, p < 0.0001), better body image (r = −.21, p = 0.0007), better sexual function (r = −.16, p = .01), and less anxiety (r = −.26, p < 0.0001). Participants who answered that having breast cancer affected the way they handle stressful situations tended to have better coping (r = .26, p < 0.0001).
Finally participants were asked about decisions involving the type of surgery received and the outcome of the surgery received. Those who reported satisfaction with the decision process had better physical function (r = −.13, p = .04), better body image (r = −.17, p = .008), and less anxiety (r = −.14, p = .03). Those who were satisfied with the outcome of surgery had better physical function (r = −.18, p = .004), better body image (r = −.25, p < 0.0001), and less anxiety (r = −.15, p = .02).
DISCUSSION
The LTQOL-BC Scale presented here is designed, as its name implies, to measure the QOL of long-term breast cancer survivors and is the first of its kind. As suggested by others, the instrument was designed to be multidimensional, brief, and comprehensive (Spagnola et al., 2003). Breast cancer is usually a disease diagnosed later in life, and the sample of participants for this study is typical with a median age of 49 years at diagnosis. Furthermore, because the median time to survey for these patients is 20 years after diagnosis, participants were elderly at the time of this study. Thus, separating the effects of aging from that of effects of the diagnosis and treatment of breast cancer can be difficult.
The factor labeled Physical Function consists of physical limitations. Putatively, the causes of these limitations are advanced age, cancer, or effects of cancer treatment effects. Separating the causes of aging and breast cancer is not possible within the context of this domain. This subscale, which contains the largest number of items, has a strong influence on the QOL score and has the highest reliability score (.85) of the seven scales. Body Image, Coping, and Anxiety each have four items and thus contribute equally to the total QOL score. Consistency of items within the body image subscale is excellent with a reliability score of .83 and is moderate within the Coping subscale (reliability score of .68) and Anxiety (reliability score of .61). The Body Image and Sexual Function scales clearly tap into separate qualities. The sexual function items may be due to aging or to breast cancer surgery; however, the body image items are clearly related to breast cancer and its treatments. Like Paskett et al.'s (2008) recent study of health-related QOL in long-term breast cancer survivors, we found that the most important facets of QOL for this population were physical and psychosocial factors; spiritual and economic factors were not found to be significant.
There are a number of strengths to the current study including follow-up of a large cohort of long-term breast cancer survivors, use of iterative waves of data collection to develop content valid scale items, and collection of some standard measures and chart review to examine convergent and discriminant validity. The study is limited by its cross-sectional design; future studies that follow women longitudinally would be better able to disentangle the impact of breast cancer as compared to aging or other comorbidities of QOL. Psychosocial researchers wanting to measure QOL of breast cancer patients should find the LTQOL-BC to be a useful tool. In addition, increasing attention is being paid to QOL for cancer patients treated in the context of clinical trials. This instrument will be a useful tool for those who want to assess breast cancer patients at baseline and for continued follow-up. Measures such as the LTOOL-BC should facilitate longitudinal research.
Acknowledgments
This research supported by Public Service contracts CA-78517 National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
APPENDIX A.
| Original Scale Items |
|---|
| A. Physical function |
| 1. I am physically strong. |
| 2. I feel fatigued all the time. |
| 3. I have difficulty walking up or down stairs. |
| 4. I find it difficult doing physical activities such as housework. |
| 5. I gain weight easily. |
| 6. I feel I have experienced premature aging (such as hair turning grey, early menopause). |
| 7. I experience digestive problems. |
| 8. I have uncomfortable changes in my skin, such as dryness, discoloration or tenderness. |
| 9. Things don't taste or smell the same to me anymore. |
| 10. I have problems with my eyes and my ability to see. |
| 11. I have numbness, swelling, or pain in my upper body or arms. |
| 12. I have restrictions of movement in my arms. |
| 13. I have aching or pain in my lower body and legs. |
| B. Social support |
| 14. I can count on those closest to me for everyday help. |
| 15. I am alone. |
| 16. I have friends who would help me if I had a crisis. |
| 17. I have or had trouble getting or keeping a job. |
| 18. I have had trouble obtaining health insurance. |
| 19. I have had problems obtaining long term care or life insurance. |
| C. Body image |
| 20. I do not feel feminine or especially attractive. |
| 21. I feel incomplete as a woman. |
| 22. I have problems with clothes fitting properly or am limited in the type of clothes I can wear. |
| D. Emotional function |
| 23. I am very alert to any symptom of possible illness or disease in my body. |
| 24. When I get sick I feel very anxious. |
| 25. I feel uncertain about things. |
| 26. Intimate relationships are difficult for me. |
| 27. I have little sexual interest. |
| 28. Sexual intimacy is easy for me. |
| 29. At the present time I feel sad, blue or down. |
| E. Coping |
| 30. I make very frequent visits to the doctor. |
| 31. I am very active in making decisions about my health. |
| 32. I believe healthy foods and exercise are a key part of maintaining my health. |
| 33. I take each day and make the most of it. |
| 34. My spirituality or religion is important to how I make health care decisions. |
| 35. When I get bad news I ignore it. |
| F. Future orientation |
| 36. I appreciate things and don't take them for granted. |
| 37. I don't sweat the small stuff. |
| 38. I try to avoid stressful situations and put things in perspective more. |
| 39. I live only in the present. |
| G. Cognitive function |
| 40. I have difficulty remembering things. |
| 41. I can concentrate easily. |
| 42. I have difficulty thinking clearly. |
| H. Breast cancer impact |
| 43. Since having had breast cancer I feel blessed. |
| 44. Since having had breast cancer I feel cheated. |
| 45. Since having had breast cancer I feel incomplete. |
| 46. Since having had breast cancer I feel resigned. |
| 47. I believe I conquered my breast cancer and that I am a survivor. |
| 48. Breast cancer has not changed my life. |
| 49. I think about my breast cancer every day. |
| 50. I am a stronger person since surviving my breast cancer. |
| 51. Breast cancer is ancient history for me. |
| 52. Breast cancer caused me to change my personal goals. |
APPENDIX B.
| Long Term Quality of Life–Breast Cancer Scale |
|---|
| Physical function |
| 1. I am physically strong.a |
| 2. I feel fatigued all the time. |
| 3. I have difficulty walking up or down stairs. |
| 4. I find it difficult doing physical activities such as housework. |
| 5. I have restrictions of movement in my arms |
| 6. I have aching or pain in my lower body and legs. |
| 7. I have or had trouble getting or keeping a job/functioning as a homemaker. |
| Body image |
| 8. I do not feel feminine or especially attractive. |
| 9. I feel incomplete as a woman. |
| 10. Since having had breast cancer I feel cheated. |
| 11. Since having had breast cancer I feel incomplete. |
| Sexual function |
| 12. Intimate relationships are difficult for me. |
| 13. I have little sexual interest. |
| 14. Sexual intimacy is easy for me.a |
| Coping |
| 15. I take each day and make the most of it. |
| 16. I appreciate things and don't take them for granted. |
| 17. I don't sweat the small stuff. |
| 18. I am a stronger person since surviving my breast cancer.a |
| Cognitive function |
| 19. I have difficulty remembering things. |
| 20. I can concentrate easilya |
| 21. I have difficulty thinking clearly. |
| Social support |
| 22. I can count on those closest to me for everyday help.a |
| 23. I am alone. |
| 24. I have friends who would help me if I had a crisis.a |
| Anxiety |
| 25. When I get sick I feel very anxious. |
| 26. I feel uncertain about things. |
| 27. I make very frequent visits to the doctor. |
| 28. I think about my breast cancer every day.a |
Denotes a question recoded so that it is consistent with direction of other questions.
Scoring is 5 = strongly agree, 4 = agree, 3 = no opinion, 2 = disagree, 1 = strongly disagree, 99 = missing.
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Alfano CM, McGregor BA, Kuniyuki A, Reeve BB, Bowen DJ, Smith AW, et al. Psychometric evaluation of the Brief Cancer Impact Assessment among breast cancer survivors. Oncology. 2006;70:190–202. doi: 10.1159/000094320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society What are the key statistics for breast cancer? 2009 Retrieved from http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_breast_cancer_5.asp?sitearea=
- Anderson JC, Gerbing DW. Structural equation modeling in practice: A review and recommended two-step approach. Psychological Bulletin. 1988;103:411–423. [Google Scholar]
- Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and White long-term breast carcinoma survivors. Cancer. 1998;85(2):418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Avis NE, Ip E, Foley KL. Evaluation of the Quality of Life in Adult Cancer Survivors (QLACS) scale for long-term cancer survivors in a sample of breast cancer survivors. Health and Quality of Life Outcomes. 2006;4(92):1–11. doi: 10.1186/1477-7525-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagozzi RP, Phillips LW. Representing and testing organizational theories: A holistic construal. Administrative Science Quarterly. 1982;27:459–489. [Google Scholar]
- Bower JE, Meyerowitz BE, Desmond KA, Bernards CA, Rowland JH, Ganz PA. Perceptions of positive meaning and vulnerability following breast cancer: Predictors and outcomes among long-term breast cancer survivors. Annals of Behavioral Medicine. 2005;29(3):236–245. doi: 10.1207/s15324796abm2903_10. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- Carver CS, Smith RG, Petronis VM, Antoni MH. Quality of life among long-term survivors of breast cancer: Different types of antecedents predict different outcomes. Psycho-Oncology. 2006;15:749–758. doi: 10.1002/pon.1006. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, McKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Dow RH, Ferrell BR, Haberman MR, Eaton L. The meaning of quality of life in cancer survivorship. Oncology Nursing Forum. 1999;26:519–528. [PubMed] [Google Scholar]
- Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychological Methods. 1999;4(3):272–299. [Google Scholar]
- Ferrans CE, Powers MJ. Quality of life index: Development and psychometric properties. ANS Advances in Nursing Science. 1985;8:15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995;4(6):523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- Ganz PA. Adjuvant therapy for breast cancer: No pain, no gain? Breast Diseases: A Year Book Quarterly. 2006;16(4):320–322. [Google Scholar]
- Ganz PA, Schag CA, Cheng HL. Assessing the quality of life—A study in newly-diagnosed breast cancer patients. Journal of Clinical Epidemiology. 1990;43:75–86. doi: 10.1016/0895-4356(90)90059-x. [DOI] [PubMed] [Google Scholar]
- Giedzinska AS, Meyerowitz BE, Ganz PA, Rowland JH. Health-related quality of life in a multiethnic sample of breast cancer survivors. Annals of Behavioral Medicine. 2004;28(1):39–51. doi: 10.1207/s15324796abm2801_6. [DOI] [PubMed] [Google Scholar]
- Gordon NH. Characteristics of Long-term Breast Cancer Survivors, nih/NCI, R01 CA78517. September 1, 2000–August 31, 2004 [Google Scholar]
- Gotay CC, Muraoka MY. Quality of life in long-term survivors of adult-onset cancer. Journal of the National Cancer Institute. 1998;90(9):656–667. doi: 10.1093/jnci/90.9.656. [DOI] [PubMed] [Google Scholar]
- Griggs JJ, Sorbero MES, Mallinger JB, Quinn M, Waterman M, Brooks B, et al. Vitality, mental health, and satisfaction with information after breast cancer. Patient Education and Counseling. 2006;66:58–66. doi: 10.1016/j.pec.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Gupta D, Grutsch JF, Lis CG. Comparison of two quality of life instruments for cancer patients: The Ferrans and Powers Quality of Life Index and the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire C30. Journal of the Society for Integrative Oncology. 2008;6:13–18. [PubMed] [Google Scholar]
- Hartl K, Janni W, Kastner R, Sommer H, Strobl B, Rack B, et al. Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Annals of Oncology. 2003;14:1064–1071. doi: 10.1093/annonc/mdg289. [DOI] [PubMed] [Google Scholar]
- Hatcher L. A step-by-step approach to using SAS system for factor analysis and structural equation modeling. SAS Institute Inc; Carey, NC: 1994. [Google Scholar]
- Helgeson VS, Tomich PL. Surviving cancer: A comparison of 5-year disease-free breast cancer survivors with healthy women. Psycho-Oncology. 2005;14:307–317. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilner M, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosomatic Medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. Journal of the American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Kendall AR, Mahue-Giangreco M, Carpenter CL, Ganz PA, Bernstein L. Influence of exercise activity of quality of life in long-term breast cancer survivors. Quality of Life Research. 2005;14:361–371. doi: 10.1007/s11136-004-1468-5. [DOI] [PubMed] [Google Scholar]
- Knobf MT. Psychosocial responses in breast cancer survivors. Seminars in Oncology Nursing. 2007;23(1):71–83. doi: 10.1016/j.soncn.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Low CA, Stanton AL, Thompson N, Kwan L, Ganz PA. Contextual life stress and coping strategies as predictors to adjustment to breast cancer survivorship. Annals of Behavioral Medicine. 2006;32(3):235–244. doi: 10.1207/s15324796abm3203_10. [DOI] [PubMed] [Google Scholar]
- Manne SL, Ostroff JS, Norton TR, Fox K, Grana G, Goldstein L. Cancer-specific self-efficacy and psychosocial and functional adaptation to early stage breast cancer. Annals of Behavioral Medicine. 2006;331(2):145–154. doi: 10.1207/s15324796abm3102_6. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Breast cancer. 2009 Retrieved from http://www.cancer.gov/cancertopics/types/breast.
- Oh S, Heflin L, Meyerowitz BE, Desmond KA, Rowland JH, Ganz PA. Quality of life of breast cancer survivors after a recurrence: A follow-up study. Breast Cancer Research and Treatment. 2004;87:45–57. doi: 10.1023/B:BREA.0000041580.55817.5a. [DOI] [PubMed] [Google Scholar]
- Ong LML, Visser MRM, Lammes FB, de Haes JCJM. Doctor-patient communication and cancer patients' quality of life and satisfaction. Patient Education and Counseling. 2000;41:145–156. doi: 10.1016/s0738-3991(99)00108-1. [DOI] [PubMed] [Google Scholar]
- Paskett ED, Herndon JE, II, Day JM, Stark NN, Winer EP, Grubbs SS, et al. Applying a conceptual model for examining health-related quality of life in long-term breast cancer survivors: CALGB study 79804. Psycho-Oncology. 2008;17:1108–1120. doi: 10.1002/pon.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins EA, Small BJ, Balducci L, Extermann M, Robb C, Haley WE. Individual differences in well-being in older breast cancer survivors. Critical Reviews in Oncology/Hematology. 2007;62:74–83. doi: 10.1016/j.critrevonc.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuckmann V, Ekholm O, Rasmussen NK, Møller S, Groenvold M, Christiansen P, et al. Health-related quality of life in long-term breast cancer survivors: Nationwide survey in Denmark. Breast Cancer Research and Treatment. 2007;104(1):39–46. doi: 10.1007/s10549-006-9386-6. [DOI] [PubMed] [Google Scholar]
- Schover LR. Myth-busters: Telling the true story of breast cancer survivorship. Journal of the National Cancer Institute. 2004;96(24):1800–1801. doi: 10.1093/jnci/djh346. [DOI] [PubMed] [Google Scholar]
- Shimozuma K, Ganz PA, Petersen L, Hirji K. Quality of life in the first year after breast cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Research and Treatment. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- Spagnola S, Zabora J, BrintzenhofeSzoc K, Hooker C, Cohen G, Baker F. The satisfaction with life domains scale for breast cancer (SLDS-BC) The Breast Journal. 2003;9(6):463–471. doi: 10.1046/j.1524-4741.2003.09603.x. [DOI] [PubMed] [Google Scholar]
- Sprangers MAG, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. Journal of Clinical Oncology. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- Stava CJ, Lopex A, Vassilopoulou-Sellin R. Health profiles of younger and older breast cancer survivors. Cancer. 2006;107(8):1752–1759. doi: 10.1002/cncr.22200. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey: Reliability and validity in a patient population. Medical Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Warren BS, Devine C. Diet and lifestyle and survival from breast cancer: Program on breast cancer and environmental risk factors. 2002 Retrieved from http://envirocancer.cornell.edu/FactSheet/Diet/fs44.survival.cfm.
- Wyatt G, Friedman LL. Long-term female cancer survivors: Quality of life issues and clinical implications. Cancer Nursing. 1996;19(1):1–7. doi: 10.1097/00002820-199602000-00001. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Ganz PA, Bernaards CA, Petersen L, Abraham L. Assessing the impact of cancer: Development of a new instrument for long-term survivors. Psycho-Oncology. 2006;15:407–421. doi: 10.1002/pon.963. [DOI] [PMC free article] [PubMed] [Google Scholar]