Abstract
Background
The signed informed consent document certifies that the process of informed consent has taken place and provides research participants with comprehensive information about their role in the study. Despite efforts to optimize the informed consent document, only limited data are available about the actual use of consent documents by participants in biomedical research.
Objective
To examine the use of online consent documents in a minimal-risk genetic study.
Design
Prospective sibling cohort enrolled as part of a genetic study of hematologic and common human traits.
Setting
University of Michigan Campus, Ann Arbor, Michigan.
Participants
Volunteer sample of healthy persons with 1 or more eligible siblings aged 14 to 35 years. Enrollment was through targeted e-mail to student lists. A total of 1209 persons completed the study.
Measurements
Time taken by participants to review a 2833-word online consent document before indicating consent and identification of a masked hyperlink near the end of the document.
Results
The minimum predicted reading time was 566 seconds. The median time to consent was 53 seconds. A total of 23% of participants consented within 10 seconds, and 93% of participants consented in less than the minimum predicted reading time. A total of 2.5% of participants identified the masked hyperlink.
Limitation
The online consent process was not observed directly by study investigators, and some participants may have viewed the consent document more than once.
Conclusion
Few research participants thoroughly read the consent document before agreeing to participate in this genetic study. These data suggest that current informed consent documents, particularly for low-risk studies, may no longer serve the intended purpose of protecting human participants, and the role of these documents should be reassessed.
Primary Funding Source
National Institutes of Health.
The informed consent process is a critical component of protecting human participants in biomedical research, with the goals of informing participants of the purpose of the study and the likely risks, benefits, and alternatives (1). Ideally, this process is designed to facilitate educated decision making and promote voluntary participation in human subject research. The informed consent process honors broad ethical principles, such as respect for participant autonomy and confidentiality. Three generally accepted elements of informed consent are capacity to consent, voluntary choice, and disclosure (2-5). Furthermore, U.S. federal regulations emphasize that disclosure of information must be in language that is understandable to the participant (6). International and federal guidelines aim to enforce adherence with ethical principles for the protection of human participants in biomedical research (7-9). In the United States, the Code of Federal Regulations, 46.116 (6), requires formal review processes by local institutional review boards (IRBs) and has led to the generation of institution-specific templates for informed consent documents. In modern medical centers, use of informed consent documents is not restricted to the research setting because similar documents are also used in routine patient care, including general consent-to-treat documents, Health Insurance Portability and Accountability Act privacy statements, and surgical or procedural consent documents. These consent documents are generally incorporated into the medical record as legal evidence that the informed consent process has taken place. Designing documents to effectively communicate the key elements of informed consent and to address the diverse cultural, educational, and socioeconomic backgrounds of patients and study participants remains a major challenge (10-17).
Human genetic studies are generally considered to be low risk but almost always require formal IRB review. Because genetic studies can generate sensitive medical information about individual participants as well as their ethnic communities, ensuring confidentiality is a major consideration during review (18). Recent advances in technology have led to an explosion in human genomic research while also creating new challenges for protecting human participants. Individual genotype data can be considered private health information in light of the potential for prediction of disease risk (19), as well as family relationships (20) and ethnicity (21). Additional challenges are presented by data-sharing initiatives, such as the Database of Phenotypes and Genotypes (22), that have been developed to facilitate scientific access to large-scale genotyping information generated primarily with public research funds. To protect the privacy of research participants, safeguards have been instituted to ensure that individual genetic data are deidentified before publication or inclusion in public databases.
We measured the time taken by participants to review an online informed consent document before agreeing to participate in a genetic study and recorded the identification of a masked hyperlink inserted near the end of the online consent document.
Methods
Study Design and IRB Approval
Consent data were collected as a component of a prospective sibling cohort study to identify genetic variants influencing thrombosis and hemostasis. After completion of the consent process, participants answered a 52-question phenotyping survey online and scheduled an appointment for phlebotomy to collect 50 mL of blood. After completion of the study, participants received $30 to $50 per person, depending on sibship size. The IRB at the University of Michigan approved the study. All personal identifying data (name, e-mail address, and contact information) were removed from the study databases before analysis.
Participants
A cohort of healthy siblings was recruited from the University of Michigan by e-mails targeted to undergraduate and graduate student e-mail lists and bulletin postings on the campus of the University of Michigan, Ann Arbor, Michigan, from 12 February 2008 to 30 January 2009. Participants were directed to the study Web page, and those who completed the eligibility screening were e-mailed a username and password to securely access the study’s online informed consent document and phenotyping survey. Persons who exited the consent or survey Web page before completion were returned to the same Web page on repeated log-in. Investigators were available by e-mail to answer questions about the study. A demonstration of the study home page with links to the eligibility screen, financial compensation scheme, and consent documents, as well as the online phenotyping survey, is available at http://blood-demo.lsi.umich.edu/home/index.php.
Eligibility Criteria
Because of the nature of the genetic traits under investigation, volunteers were eligible if they were generally healthy, were aged 14 to 35 years, and had an eligible sibling. Participants who indicated that they were pregnant or had an illness requiring regular medical care were excluded. Any participant who consented was included in the analysis of consent time, including persons who did not complete the entire genetic study and persons whose sibling never participated in the study.
Informed Consent Documents
Informed consent documents were generated by using standard templates provided by the IRB at the University of Michigan (23). Participants younger than 18 years viewed and agreed to a 368-word assent document. After assent, the full informed consent document page was loaded, with instructions for a parent or guardian to view and provide consent. Two consent documents were generated: one for participants aged 18 years or older and one for parents or guardians of participants aged 14 to 17 years. These latter documents each contained 12 sections and totaled 2833 words or 2984 words, respectively. Standard reading speeds for comprehension of written texts have been established as 200 to 300 words per minute (24), corresponding to 566 to 850 seconds for the consent documents used in the current study. Flesch-Kincaid gradelevel index of readability was 9.2 for the consent documents, suggesting a 9th-grade reading level (25).
Hyperlink Tool
A hyperlink allows an online user to access linked Web sites by clicking on hyperlinked text. A hyperlink was introduced into the online consent forms on 5 May 2008 and was viewed by the 1031 persons accessing the consent documents after that date. The text of the hyperlink, “If you are reading this form, please click on this sentence,” was in the same font and color as the narrative text and was inserted into the final sentence of section 9.1. Persons who clicked on the hyperlink were directed to a small pop-up window displaying, “Thank you, you took …. seconds to find this sentence” and were then returned to the consent Web page. The PDF file of the consent document, which was available for download, did not contain the hyperlinked sentence. The hyperlink was identified by more than 1 family member in 2 sibships. In each case, the first consenting sibling had the longest time to consent for the sibship. For both sibships, the later siblings acknowledged at the time of the blood draw appointment that they had previous information about the hyperlink from the initial sibling. The consent times for all 5 of these siblings with previous knowledge of the hyperlink were excluded from hyperlink analyses but not from the overall consent-time assessment.
Measurements
Internet activity on the server hosting the study Web pages was monitored by using HTML logs. The date, time, and IP addresses of computers accessing the study home page and eligibility pages were recorded. Any IP addresses representing Web bots (repetitive Web site hits) or known IP addresses from study computers were removed before analysis of recruitment activity. The number, date, and time of informed consent Web page uploading was recorded. Consent was documented when the user clicked on a button labeled “Agree,” which was located outside of the scrolling window of the consent form. Time to consent was calculated by the difference in time between the server sending out the informed consent Web page and the server receiving an “Agree” click. For every participant who clicked “Agree,” identification of the masked hyperlink or downloading of the PDF version of the consent form was also recorded. Consent-time data, but not user IP addresses, were linked to participant and family identification codes.
Statistical Analysis
Consent times between participant groups, such as persons who identified the hyperlink and persons who did not, were compared by applying a nonparametric test of equality (the Kolmogorov-Smirnov test), and the corresponding P values were reported. P values for subgroup differences between men and women or minors and adults in study completion, printing PDFs, and identifying the hyperlink were calculated by using 2 × 2 contingency tables and the chi-square test. None of these P values were less than 0.05.
Role of the Funding Source
The National Institutes of Health funded the study. The funding source did not play a role in the design, conduct, or analysis of the study, or in the decision to submit the manuscript for publication.
Results
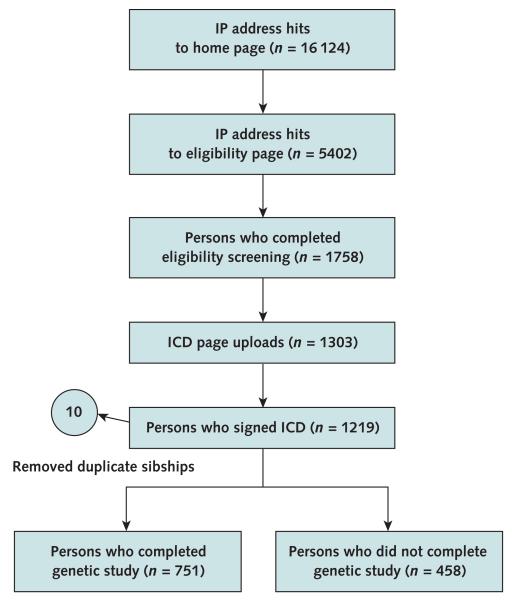
Figure 1 shows the cumulative number of hits to selected Web pages over the duration of the study. From 12 February 2008 to 31 January 2009, there were 16 124 hits to the main study home page and 5402 hits to the eligibility screen page. A total of 1758 participants completed the eligibility screen and were issued usernames and passwords. The informed consent Web page was uploaded 1303 times, and 1219 (93.6%) persons clicked “Agree” to the consent document. Ten participants were identified through genotyping to be duplicates (participating in the study for a second time and thus having previously viewed the consent document). Data for these 10 participants were removed from the analysis (median time for duplicate consent, 7.5 seconds). Of the remaining 1209 participants, 751 (62.4%) completed the entire genetic study. Age, sex, self-reported ethnicity, and additional phenotyping data were recorded for each person.
Figure 1. Study flow diagram.
Online recruitment was logged by recording Internet activity on the study home page, eligibility screen page, and log-in usernames to the informed consent document page. Internet hits to the home page and eligibility page that were from study investigators (specific static IP addresses) or Internet “bots” (>100 hits/d) were removed. A total of 1758 login usernames and passwords were sent to persons who completed the eligibility screen, allowing them to log in and view the study informed consent document. Genotyping identified 5 sibling pair duplicates, and data from these 10 participants were removed. ICD = informed consent document; IP = Internet protocol.
The Table shows the demographic characteristics and consent-time distributions among subgroups in the study. Age ranged from 14 to 35 years, with a mean of 21 years. One hundred fifty-nine consenting participants (13.1%) were younger than 18 years. Women constituted 61.9% of participants in the study compared with 48.0% for the University of Michigan student population. Self-reported ethnicity was consistent with the composition of the University of Michigan student population (26).
Table.
Participant Demographic Characteristics and Consent Times
| Variable | Participants, n (%) |
Median Time to Consent (Q1–Q3), s |
Identified Hyperlink, n (%)* |
Downloaded Consent PDF, n (%) | Completed Genetic Study, n (%) |
Sibling in Study, n (%) |
|---|---|---|---|---|---|---|
| Total participants | 1209 (100) | 53 (9–213) | 26 (2.5) | 57 (4.7) | 751 (62.1) | 982 (81.2) |
| Age † | ||||||
| 18–35 y (mean, 22 y) | 1050 (86.9) | 68 (10–228) | 24 (2.3) | 49 (4.7) | 642 (61.4) | 829 (79.0) |
| 14–17 y (mean, 16 y)‡ | 159 (13.1) | 13 (5–53) | 2 (0.2) | 8 (5.0) | 109 (68.6) | 153 (96.2) |
| Sex † | ||||||
| Male | 460 (38.1) | 36 (8–186) | 7 (1.8) | 25 (5.4) | 287 (62.4) | 368 (80.0) |
| Female | 749 (61.9) | 66 (10–230) | 19 (2.9) | 32 (4.3) | 465 (62.1) | 614 (82.0) |
| Ethnicity § | ||||||
| Non-Hispanic white | 929 (76.8) | 54 (9–225) | ||||
| Asian | 75 (6.2) | 20 (8–140) | ||||
| Spanish, Hispanic, or Latino | 61 (5.0) | 52 (10–199) | ||||
| African or African American | 44 (3.5) | 43 (10–173) | ||||
| Asian Indian | 37 (3.1) | 62 (9–260) | ||||
| Not reported | 27 (2.2) | 57 (15–200) | ||||
| Other | 24 (2.0) | 66 (13–209) | ||||
| Preferred not to answer | 12 (1.0) | 12 (4–99) | ||||
| Identified hyperlink*† | ||||||
| Yes | 26 (2.5) | 621 (369–718) | ||||
| No | 1000 (97.5) | 54 (10–209) | ||||
| Downloaded PDF † | ||||||
| Yes | 57 (4.7) | 234 (111–468) | ||||
| No | 1152 (95.3) | 45 (9–202) | ||||
| Completed genetic study | ||||||
| Yes | 751 (62.1) | 44 (9–208) | ||||
| No | 458 (37.9) | 63 (10–231) | ||||
| Participated with sibling ∥ | ||||||
| Yes | 982 (81.2) | 45 (9–205) | ||||
| No | 227 (18.8) | 90 (15–247) |
Q = quartile.
Percentages were calculated on the subgroup of 1026 participants who viewed a consent document containing the hyperlink.
P < 0.001 for difference between subgroups in consent time by using the Kolmogorov–Smirnov test.
Represents time to consent, identification of hyperlink, and downloading of the PDF by parents or guardians consenting for a minor.
Data were self-reported.
P < 0.05 for difference between subgroups in consent time by using the Kolmogorov–Smirnov test.
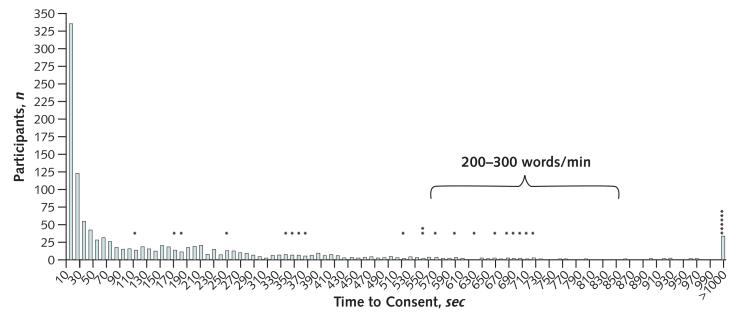
The distribution of consent times for 1209 participants (Figure 2) shows a peak at 10 seconds (n = 341 [28%]) and a right-sided tail consistent with a heterogeneous choice of consent times. Among all participants, the median consent time was 53 seconds. Eightyone (6.7%) participants took longer than the minimum estimated reading time (566 seconds) to indicate consent. Times to consent greater than 1000 seconds were recorded for 34 persons (2.8%) (grouped into the last bin in Figure 2).
Figure 2. Consent-time distribution.
The number of participants in each 10-second time bin is shown. The interval for expected reading times for comprehension at 200 to 300 words per minute (566 to 850 seconds) is indicated with a bracket. Each asterisk indicates a person who identified the hyperlink within the corresponding time bin.
A total of 26 participants independently identified the informed consent document hyperlink (2.5% of study participants, each indicated with an asterisk in Figure 2). The median consent time for the participants who identified the hyperlink was significantly longer than the median time for the 1000 who did not (621 vs. 54 seconds; P < 0.001) (Table).
A total of 159 parents or guardians consented for their child (13.1% of all participants); median time to consent was 13 seconds, compared with 68 seconds for the 1050 participants of consenting age (P < 0.001). Because the participants were unobserved and consent Web pages were loaded immediately after minors agreed to the assent form, we cannot exclude the possibility that some minors may have clicked “Agree” without parent or guardian agreement. Consenting parents or guardians were less likely to identify the hyperlink than participants aged 18 to 35 years, although a direct role of the minor cannot be excluded. Women took longer to consent than men (median time, 66 vs. 36 seconds; P < 0.001, Kolmogorov-Smirnov test) but were not more likely to identify the hyperlink (P > 0.24), download the consent PDF (P > 0.35), or complete the entire genetic study (P > 0.79).
The 57 (4.7%) participants who downloaded the consent PDF file took significantly longer to consent (median, 234 seconds) than the 1152 who did not (median, 45 seconds; P < 0.001). Participants with other siblings who completed the consent did so with faster median times than participants without siblings (median, 45 vs. 90 seconds; P < 0.012). There were no significant differences in time to consent between participants who completed the entire genetic study and persons who completed only the online consent and survey (median, 44 vs. 63 seconds; P > 0.144).
Discussion
Our results demonstrate that most participants in our study (93.6%) provided consent without spending sufficient time to thoroughly read and comprehend the informed consent document. The 81 (6.7%) participants with consent times greater than the minimum predicted reading time (566 seconds) may overestimate the number of participants who actually read the consent document because participants were not directly observed during the informed consent process and the consent interval could include time spent on other distracting activities. The latter might include multitasking on cell phones, audio players, and other electronic devices, activities which seem to be ubiquitous in our study demographic (27). This hypothesis is supported by about one third of this group, which had consent times greater than 1000 seconds (range, 1007 to 69 407 seconds)—far longer than the time necessary to read the consent document. Overall, these data suggest that the 2.5% of total participants who identified the hyperlink may provide a more accurate estimate of careful readers than the 6.7% of participants with consent times greater than the minimum predicted reading time.
Although these data suggest that only a very small proportion of participants carefully reviewed the consent document, limitations of our study should be noted. Participants had the opportunity to read about the study on the home page and through Web links that described eligibility requirements and payment for participation. Because participants were not observed, discussion of the issues raised in the consent document with a sibling, friend, or parent or guardian who had more thoroughly read the document cannot be excluded. However, only 81 (6.7%) of persons had consent times greater than 566 seconds, 6 in sibships in which both participants had consent times greater than 566 seconds and 57 in sibships in which all of the other members had consent times less than 566 seconds. Of the latter group, only 34 (60%) were the first to consent in their family, suggesting that the potential bias from family members who read the consent on the other family members’ reading time was minimal. The 57 participants who downloaded the consent document PDF are another subgroup that may have more thoroughly read or shared the consent document with their siblings who had short online consent times. Forty-seven participants who downloaded the consent PDF also had siblings in the study. However, participants who had a member of the sibship download the PDF had a median time to consent of 49 seconds, compared with 38 seconds for sibships in which no one downloaded the PDF. Thus, sharing of printed consent documents does not seem to contribute to a shorter consent time. Finally, there was a discrepancy between the number of informed consent Web pages viewed (n = 1303) and the number returned to the server as “Agree” (n = 1219). A subset of these participants could have clicked “Disagree” or exited the Web site without agreeing. Although we cannot exclude more than 1 login session for as many as 84 participants, which could have led to an underestimate of total consent time, the high mean consent time for persons who identified the hyperlink (621 seconds) suggests that careful reading of the consent document over several sessions was uncommon.
This analysis used a Web-based consent form, in contrast to the more common face-to-face consent process, which could result in differences in the decision to carefully read the consent document. One previous report suggested better reading of the informed consent document in a clinical setting, where face-to-face consenting was done (28). In contrast, 69% (172 of 250) of patients consenting to intrathoracic, intraperitoneal, or vascular surgery in another study admitted to not reading the consent document before signing (29), and another analysis concluded that online informed consent is as effective as traditional printed documents in terms of reading and recall of information (30). By avoiding observation bias from study investigators, our online approach may have allowed participants more freedom to choose how much time to spend reading the informed consent document.
Taken together, these results suggest that the consent by participants to participate in this and many other studies that rely on informed consent documents for communication is unlikely to have been as truly informed as originally intended by the investigators and the IRB. This deficiency in the informed consent process may be particularly relevant to low-risk research studies, which represent a large proportion of the new protocols under review by most academic IRBs, including our own (7). In addition, this lack of truly informed consent is likely to extend beyond research studies, to include informed consent documents used for treatment in the clinical care setting (29).
For the persons who chose to participate in this and many other studies, the length of the standard informed consent document and the marked imbalance with perceived risk may seem equivalent to the frequently encountered end-user license agreement (EULA) that is routinely invoked to protect a software company against unlawful use of its product. The intentions behind an informed consent document and a EULA differ, however: The former is designed to educate and inform study participants and record their consent, and the latter is a legal document that protects a vendor. However, the increasing similarity in the appearance of these documents may contribute to a perception among study participants that the informed consent document exists to protect the investigators and institution, not the research participant (14, 31). Although careful review of EULAs by consumers may be rare, with few exceptions the EULA remains an enforceable legal contract (32). A recent news story reported that 7500 online shoppers inadvertently “sold their souls” to a video game company by failing to select the “opt out” check box when granting consent to the online EULA (33). Remarkably, the 12% of customers who opted out of this provision exceeds the 2.5% of participants in our study who successfully identified the masked hyperlink.
Local IRB involvement in multi-institutional studies can reduce participation and delay data collection (34). Waiver of the need for consent documents seems to improve participation in minimal-risk research and medical testing rates (35, 36). Although our study was designated as minimal-risk research by the University of Michigan IRB, the final approved consent documents, based on standard templates provided by the IRB, were considerably longer (>2800 words) than many published articles and more than twice the length of a typical chemotherapy protocol consent document (average, 1087 words in 2005 to 2007 [range, 399 to 2345 words]) (37, 38). The length and complexity of informed consent documents for many types of human research has steadily increased over the past 2 decades (37-39). In fact, the consent document for the current study has increased from 2736 words in 2006 to 3179 words (14%) in 2011. The length of informed consent documents seems to be directly associated with decreased rates of participation in research, the introduction of recruitment bias, and increased costs of research (40-42).
Minimal-risk research is currently estimated to constitute greater than 50% of the direct costs to IRBs, representing a significant administrative burden that potentially compromises the capacity for thorough evaluation of higher-risk studies (7). These concerns have led to calls for major reform to the regulatory process, including exemptions from IRB review or dramatically simplified consent documents for some forms of minimal-risk research (7, 13, 43, 44). Our data highlight the need for such reform and suggest that Web-based approaches may provide a useful tool for monitoring the informed consent process.
Acknowledgment
The authors thank Joseph Antin, MD; David Hanauer, MD; and Eric D. Kodish, MD, for their comments and suggestions.
Grant Support: By National Institutes of Health grants R37HL039693 (Dr. Ginsburg) and K12HD028820 (Dr. Desch). Dr. Ginsburg is a Howard Hughes Investigator, and Dr. Kim is a Greenwall Faculty Scholar in Bioethics.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M10-2665.
Reproducible Research Statement: Study protocol: Available from Dr. Ginsburg (ginsburg@umich.edu). Statistical code: Available from Dr. Li (junzli@umich.edu). Data set: Available to eligible investigators through dbGaP at www.ncbi.nlm.nih.gov/gap.
Current author addresses and author contributions are available at www.annals.org.
ACP Chapter Meetings
For information on upcoming ACP chapter meetings, including scientific programs and registration forms, please visit www.acponline.org/meetings/chapter.
Current Author Addresses: Drs. Desch and Laventhal: University of Michigan, Department of Pediatrics, Division of Neonatal-Perinatal Medicine, Mott Children’s Hospital, Floor 5, Room 5790, 1500 East Medical Center Drive, 5254, Ann Arbor, MI 48109.
Dr. Li: Department of Human Genetics, 5789A Medical Science II, Box 5618, Ann Arbor, MI 48109-5618.
Dr. Kim: Bioethics Program, University of Michigan, 300 North Ingalls Street, 7C27, Ann Arbor, MI 48109.
Ms. Metzger: Chicago Department of Public Health, 2160 West Ogden Avenue, Chicago, IL 60612.
Dr. Siemieniak: University of Michigan, Life Sciences Institute, 210 Washtenaw Avenue, Ann Arbor, MI 48109.
Dr. Ginsburg: 5028 Life Sciences Institute Building, 210 Washtenaw Avenue, Ann Arbor, MI 48109.
Author Contributions: Conception and design: K. Desch, D. Siemieniak. Analysis and interpretation of the data: K. Desch, J. Li, S. Kim, K. Metzger, D. Siemieniak, D. Ginsburg.
Drafting of the article: K. Desch, N. Laventhal, K. Metzger, D. Siemieniak. Critical revision of the article for important intellectual content: K. Desch, J. Li, S. Kim, N. Laventhal, K. Metzger, D. Siemieniak, D. Ginsburg.
Final approval of the article: K. Desch, J. Li, S. Kim, N. Laventhal, D. Siemieniak, D. Ginsburg.
Provision of study materials or patients: K. Desch, D. Siemieniak. Statistical expertise: J. Li, D. Siemieniak.
Obtaining of funding: D. Ginsburg.
Administrative, technical, or logistic support: K. Metzger, D. Siemieniak. Collection and assembly of data: K. Desch, K. Metzger, D. Siemieniak.
References
- 1.Jefford M, Moore R. Improvement of informed consent and the quality of consent documents. Lancet Oncol. 2008;9:485–93. doi: 10.1016/S1470-2045(08)70128-1. PMID: 18452859. [DOI] [PubMed] [Google Scholar]
- 2.Kim S. Evaluation of Capacity to Consent to Treatment and Research. Oxford Univ Pr; New York: 2010. [Google Scholar]
- 3.Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357:1834–40. doi: 10.1056/NEJMcp074045. PMID: 17978292. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp T, Childress J. Principles of Biomedical Ethics. 5th ed. Oxford Univ Pr; New York: 2001. pp. 63–79. [Google Scholar]
- 5.Meisel A, Roth LH, Lidz CW. Toward a model of the legal doctrine of informed consent. Am J Psychiatry. 1977;134:285–9. doi: 10.1176/ajp.134.3.285. PMID: 842705. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Federal Government . Code of Federal Regulations Title 45, Part 46.116. [11 July 2011]. Protection of Human Subjects. Accessed at http://edocket.access.gpo.gov/cfr_2002/octqtr/45cfr46.116.htm on. [Google Scholar]
- 7.Kim S, Ubel P, De Vries R. Pruning the regulatory tree. Nature. 2009;457:534–5. doi: 10.1038/457534a. PMID: 19177111. [DOI] [PubMed] [Google Scholar]
- 8.Legemaate J. The CIOMS guidelines for biomedical research involving human subjects. Eur J Health Law. 1994;1:161–5. doi: 10.1163/157180994x00268. PMID: 11654540. [DOI] [PubMed] [Google Scholar]
- 9.Protection of human subjects Belmont Report: notice of report for public comment. Fed Regist. 1979;44:23191–7. PMID: 10241035. [PubMed] [Google Scholar]
- 10.Paris A, Brandt C, Cornu C, Maison P, Thalamas C, Cracowski JL. Informed consent document improvement does not increase patients’ comprehension in biomedical research. Br J Clin Pharmacol. 2010;69:231–7. doi: 10.1111/j.1365-2125.2009.03565.x. PMID: 20233193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–6. doi: 10.1056/NEJMsa021212. PMID: 12594317. [DOI] [PubMed] [Google Scholar]
- 12.Sharp SM. Consent documents for oncology trials: does anybody read these things? Am J Clin Oncol. 2004;27:570–5. doi: 10.1097/01.coc.0000135925.83221.b3. PMID: 15577434. [DOI] [PubMed] [Google Scholar]
- 13.Stunkel L, Benson M, McLellan L, Sinaii N, Bedarida G, Emanuel E, et al. Comprehension and informed consent: assessing the effect of a short consent form. IRB. 2010;32:1–9. PMID: 20853797. [PMC free article] [PubMed] [Google Scholar]
- 14.Cassileth BR, Zupkis RV, Sutton-Smith K, March V. Informed consent—why are its goals imperfectly realized? N Engl J Med. 1980;302:896–900. doi: 10.1056/NEJM198004173021605. PMID: 7360175. [DOI] [PubMed] [Google Scholar]
- 15.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358:1772–7. doi: 10.1016/S0140-6736(01)06805-2. PMID: 11734235. [DOI] [PubMed] [Google Scholar]
- 16.Howard JM, DeMets D. How informed is informed consent? The BHAT experience. Control Clin Trials. 1981;2:287–303. doi: 10.1016/0197-2456(81)90019-2. PMID: 6120794. [DOI] [PubMed] [Google Scholar]
- 17.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–601. doi: 10.1001/jama.292.13.1593. PMID: 15467062. [DOI] [PubMed] [Google Scholar]
- 18.Mello MM, Wolf LE. The Havasupai Indian tribe case—lessons for research involving stored biologic samples. N Engl J Med. 2010;363:204–7. doi: 10.1056/NEJMp1005203. PMID: 20538622. [DOI] [PubMed] [Google Scholar]
- 19.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–76. doi: 10.1056/NEJMra0905980. PMID: 20647212. [DOI] [PubMed] [Google Scholar]
- 20.Weir BS, Anderson AD, Hepler AB. Genetic relatedness analysis: modern data and new challenges. Nat Rev Genet. 2006;7:771–80. doi: 10.1038/nrg1960. PMID: 16983373. [DOI] [PubMed] [Google Scholar]
- 21.Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319:1100–4. doi: 10.1126/science.1153717. PMID: 18292342. [DOI] [PubMed] [Google Scholar]
- 22.Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet. 2007;39:1181–6. doi: 10.1038/ng1007-1181. PMID: 17898773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.University of Michigan Medical School . Informed consent templates: what’s new. [21 June 2011]. Accessed at http://med.umich.edu/irbmed/ict/ict_new.htm on. [Google Scholar]
- 24.Legge GE, Pelli DG, Rubin GS, Schleske MM. Psychophysics of reading—I. Normal vision. Vision Res. 1985;25:239–52. doi: 10.1016/0042-6989(85)90117-8. PMID: 4013091. [DOI] [PubMed] [Google Scholar]
- 25.FLESCH R. A new readability yardstick. J Appl Psychol. 1948;32:221–33. doi: 10.1037/h0057532. PMID: 18867058. [DOI] [PubMed] [Google Scholar]
- 26.Michigan University . Ethnicity of University of Michigan students. [21 June 2011]. Accessed at www.umich.edu/~regoff/enrollment/ethnicity.html on. [Google Scholar]
- 27.Foerh U. Kaiser Family Foundation. [21 June 2011]. Accessed at www.kff.org/entmedia/upload/7593.pdf on. [Google Scholar]
- 28.Vila JJ, Jiménez FJ, Iñarrairaegui M, Prieto C, Nantes O, Borda F. Informed consent document in gastrointestinal endoscopy: understanding and acceptance by patients. Rev Esp Enferm Dig. 2006;98:101–11. doi: 10.4321/s1130-01082006000200005. PMID: 16566642. [DOI] [PubMed] [Google Scholar]
- 29.Lavelle-Jones C, Byrne DJ, Rice P, Cuschieri A. Factors affecting quality of informed consent. BMJ. 1993;306:885–90. doi: 10.1136/bmj.306.6882.885. PMID: 8490411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varnhagen CK, Gushta M, Daniels J, Peters TC, Parmar N, Law D, et al. How informed is online informed consent? Ethics Behav. 2005;15:37–48. doi: 10.1207/s15327019eb1501_3. PMID: 16127857. [DOI] [PubMed] [Google Scholar]
- 31.Henry J, Palmer BW, Palinkas L, Glorioso DK, Caligiuri MP, Jeste DV. Reformed consent: adapting to new media and research participant preferences. IRB. 2009;31:1–8. PMID: 19402337. [PMC free article] [PubMed] [Google Scholar]
- 32.Granick J. Court Turns Against Abusive Clickwrap Contracts. Wired. 2007 [Google Scholar]
- 33. [21 June 2011];7,500 online shoppers unknowingly sold their souls. FOXNews.com. Accessed at www.foxnews.com/scitech/2010/04/15/online-shoppers-unknowingly-sold-souls/ on.
- 34.Finch SA, Barkin SL, Wasserman RC, Dhepyasuwan N, Slora EJ, Sege RD. Effects of local institutional review board review on participation in national practice-based research network studies. Arch Pediatr Adolesc Med. 2009;163:1130–4. doi: 10.1001/archpediatrics.2009.206. PMID: 19996050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krousel-Wood M, Muntner P, Jannu A, Hyre A, Breault J. Does waiver of written informed consent from the institutional review board affect response rate in a low-risk research study? J Investig Med. 2006;54:174–9. doi: 10.2310/6650.2006.05031. PMID: 17152856. [DOI] [PubMed] [Google Scholar]
- 36.Zetola NM, Klausner JD, Haller B, Nassos P, Katz MH. Association between rates of HIV testing and elimination of written consents in San Francisco. JAMA. 2007;297:1061–2. doi: 10.1001/jama.297.10.1061. Letter. PMID: 17356026. [DOI] [PubMed] [Google Scholar]
- 37.Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. 2007;25:e13–4. doi: 10.1200/JCO.2006.10.3341. Letter. PMID: 17369564. [DOI] [PubMed] [Google Scholar]
- 38.Berger O, Grønberg BH, Sand K, Kaasa S, Loge JH. The length of consent documents in oncological trials is doubled in twenty years. Ann Oncol. 2009;20:379–85. doi: 10.1093/annonc/mdn623. PMID: 18922881. [DOI] [PubMed] [Google Scholar]
- 39.Albala I, Doyle M, Appelbaum PS. The evolution of consent forms for research: a quarter century of changes. IRB. 2010;32:7–11. PMID: 20590051. [PubMed] [Google Scholar]
- 40.Ness RB. Joint Policy Committee, Societies of Epidemiology. Influence of the HIPAA Privacy Rule on health research. JAMA. 2007;298:2164–70. doi: 10.1001/jama.298.18.2164. PMID: 18000200. [DOI] [PubMed] [Google Scholar]
- 41.Tu JV, Willison DJ, Silver FL, Fang J, Richards JA, Laupacis A, et al. Investigators in the Registry of the Canadian Stroke Network. Impracticability of informed consent in the Registry of the Canadian Stroke Network. N Engl J Med. 2004;350:1414–21. doi: 10.1056/NEJMsa031697. PMID: 15070791. [DOI] [PubMed] [Google Scholar]
- 42.Marco CA. Impact of detailed informed consent on research subjects’ participation: a prospective, randomized trial. J Emerg Med. 2008;34:269–75. doi: 10.1016/j.jemermed.2007.06.026. PMID: 18022787. [DOI] [PubMed] [Google Scholar]
- 43.Infectious Diseases Society of America Grinding to a halt: the effects of the increasing regulatory burden on research and quality improvement efforts. Clin Infect Dis. 2009;49:328–35. doi: 10.1086/605454. PMID: 19566438. [DOI] [PubMed] [Google Scholar]
- 44.Lantos J. It is time to professionalize institutional review boards. Arch Pediatr Adolesc Med. 2009;163:1163–4. doi: 10.1001/archpediatrics.2009.225. Editorial. PMID: 19996057. [DOI] [PubMed] [Google Scholar]