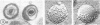Abstract
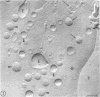
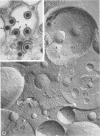
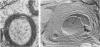
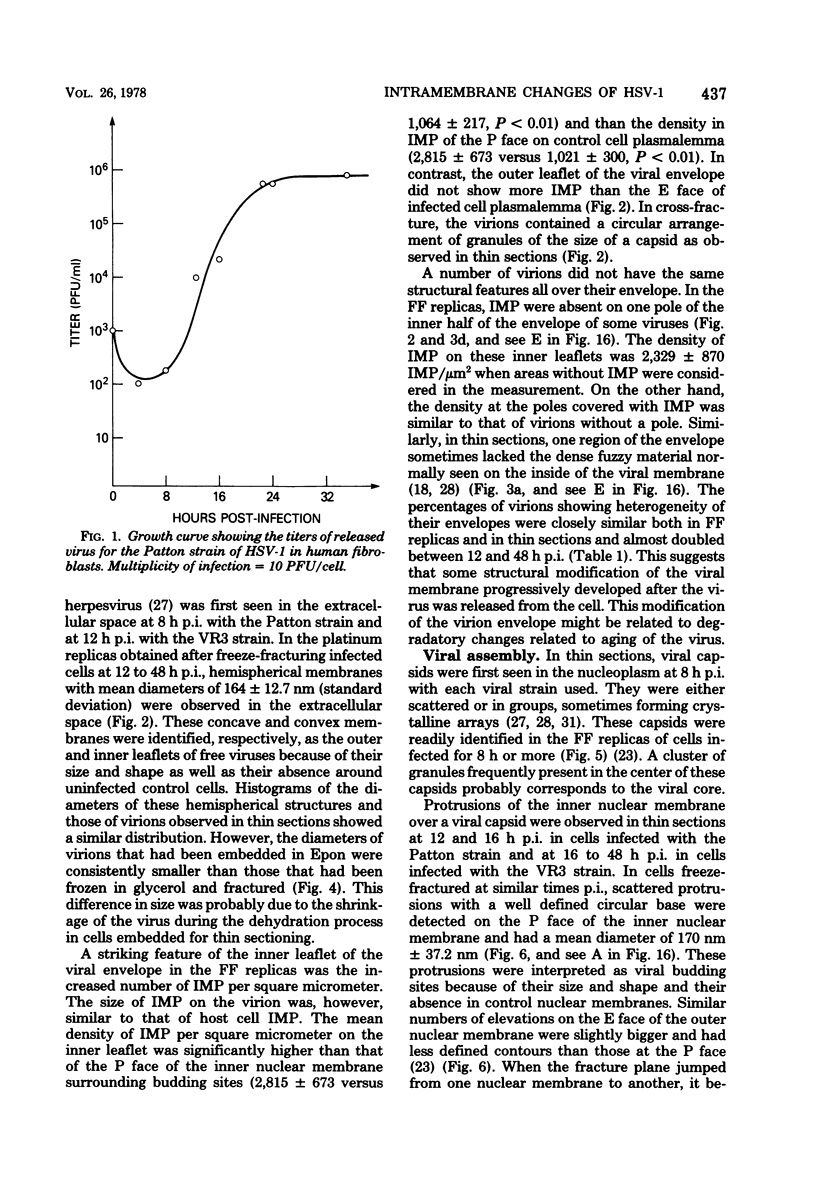
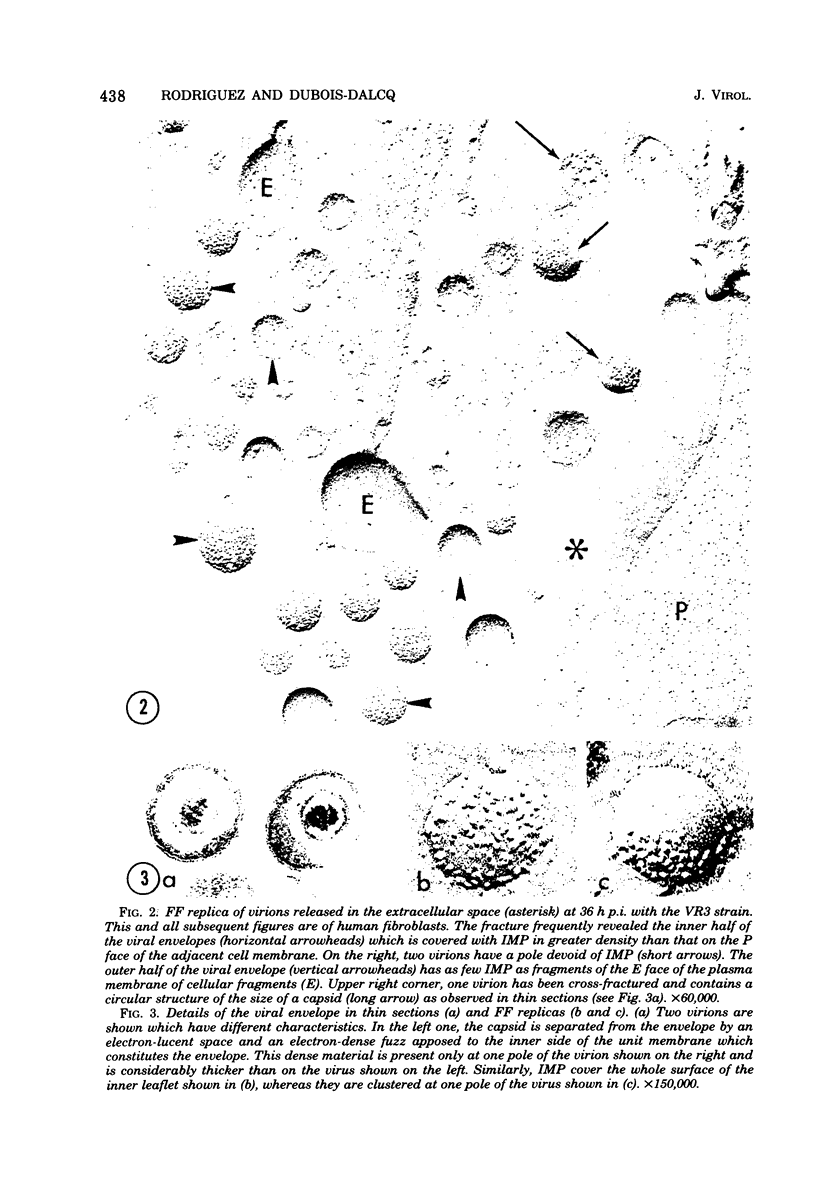
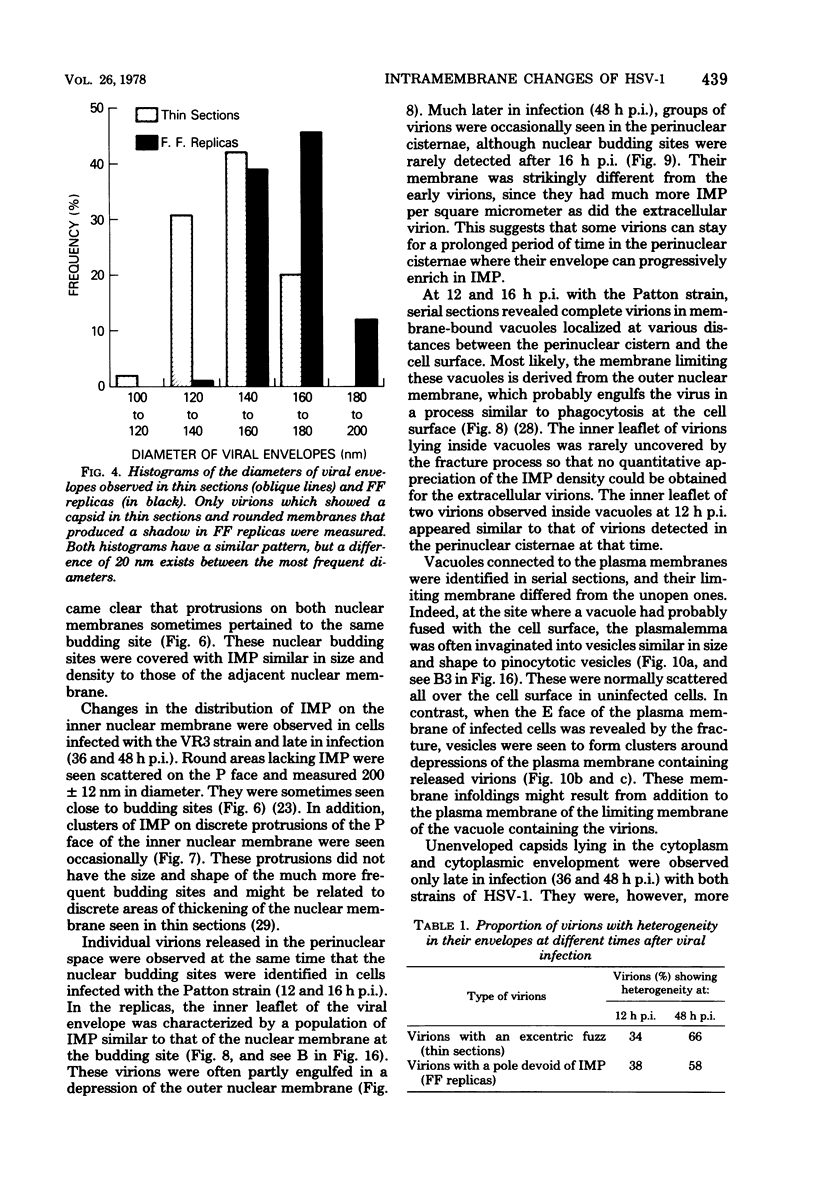
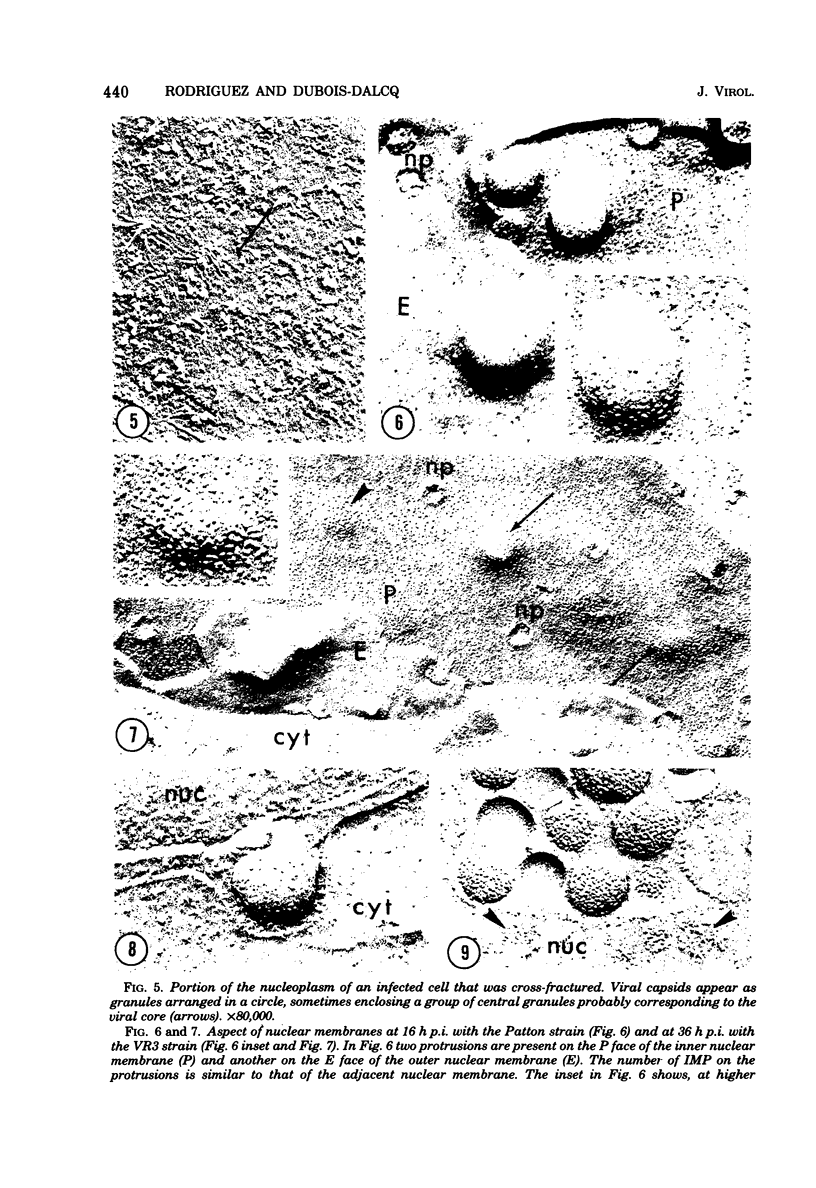
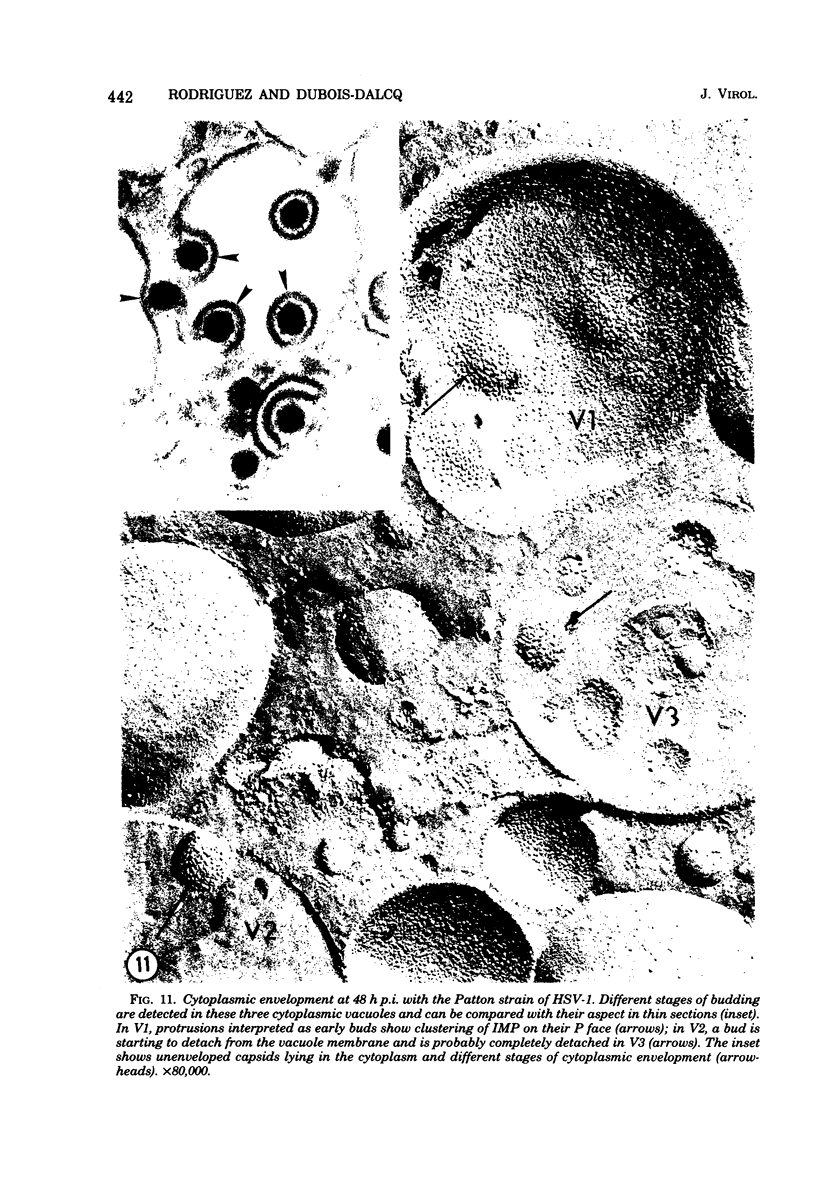
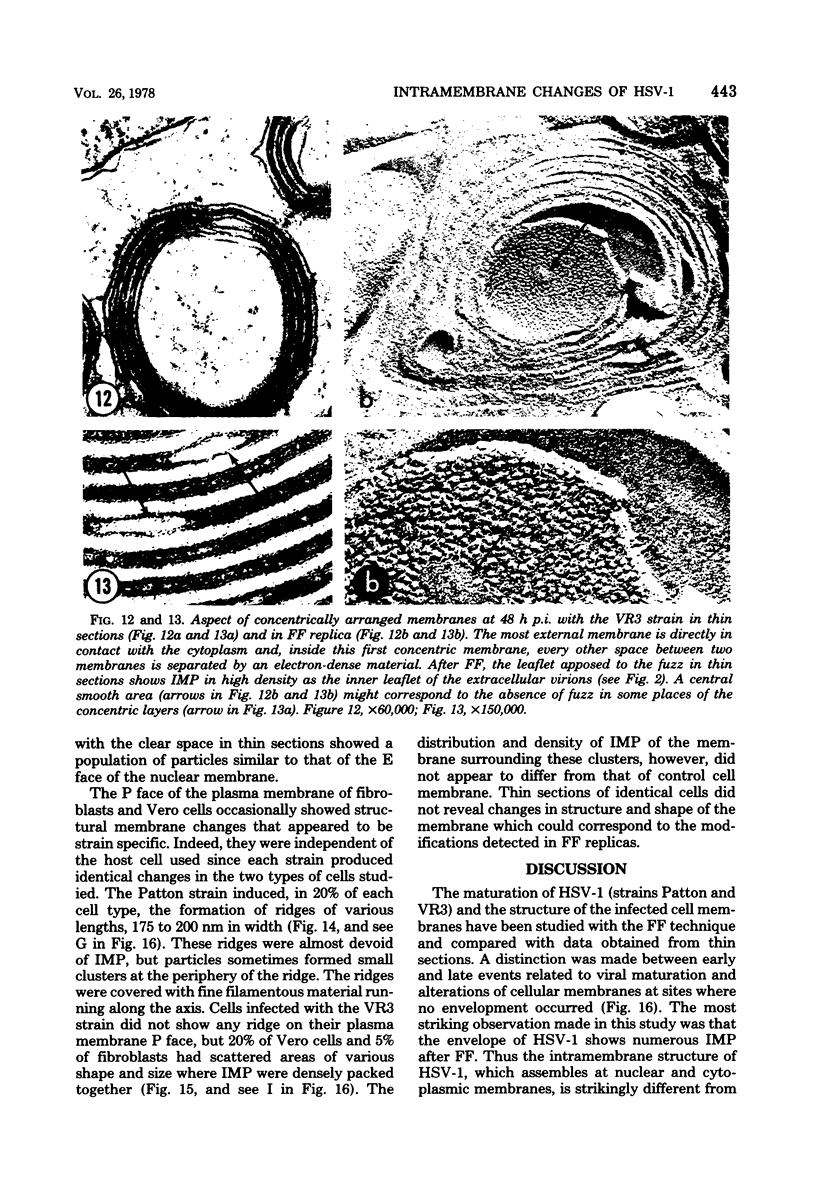
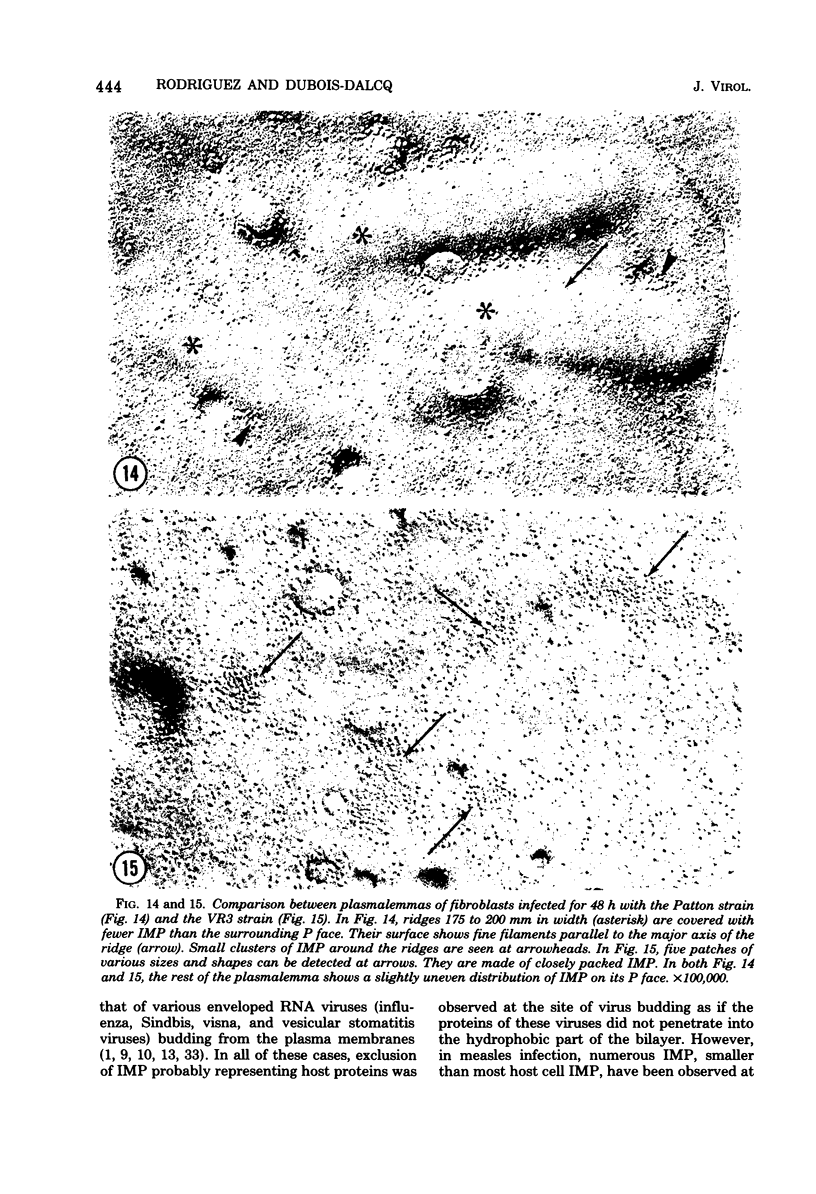
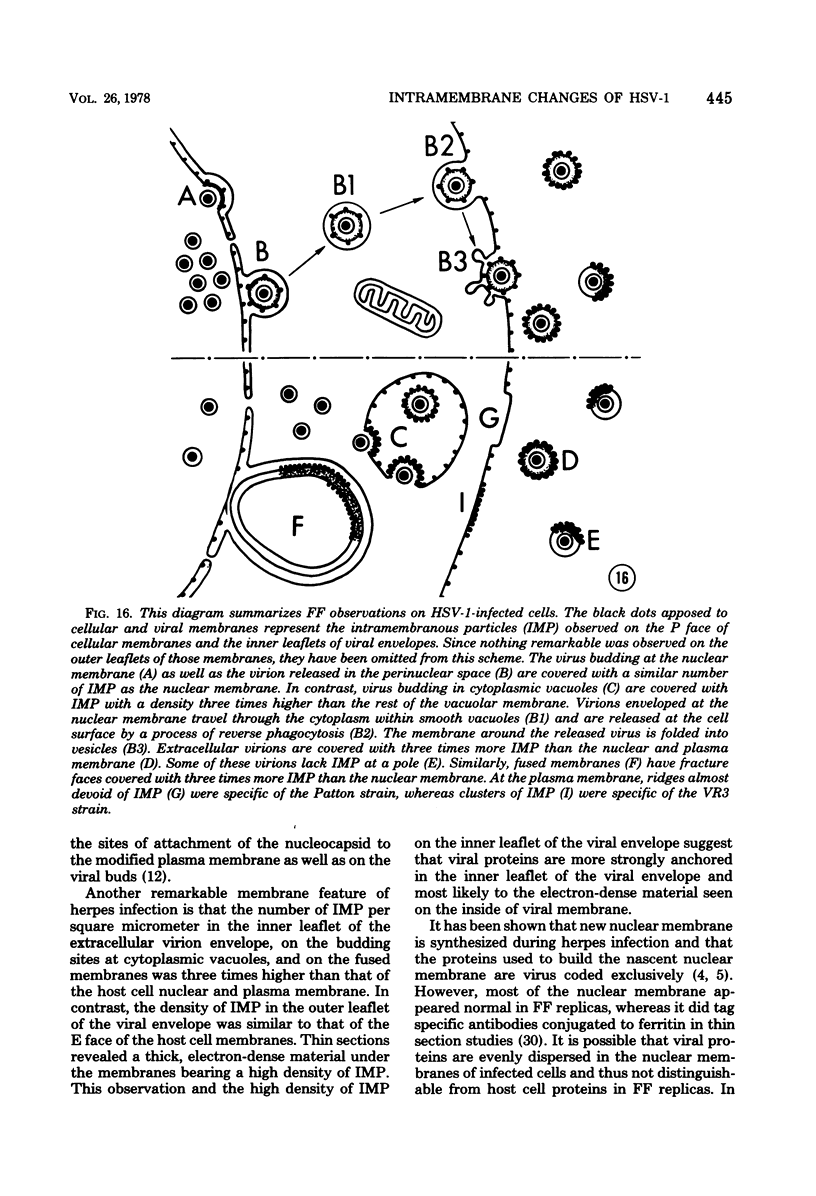
During the maturation of two strains of herpes simplex virus type 1 (VR3 and Patton), intramembrane changes were detected with the freeze-fracture technique in the viral envelope and the infected cell plasma membrane, and these changes were compared with data obtained from thin sections. Regardless of the strain, the inner leaflet of the viral envelope of extracellular virions was characterized by a density of intramembrane particles (IMP) three times larger than the host nuclear and plasma membrane. Addition of IMP, which probably represent virus-coded proteins, was detected in the viral envelope only after budding from the nuclear membrane, whereas it occurred during envelopment of capsids at cytoplasmic vacuoles. Fused membranes also showed one of their fracture faces covered with a high density of IMP similar to that of the mature virion envelope. The internal side of the membrane leaflet bearing these numerous particles was always characterized by the presence of an electron-dense material in thin sections. In addition, the plasma membrane of fibroblasts and Vero cells showed strain-specific changes: patches of closely packed IMP were observed with the VR3 strain, whereas ridges almost devoid of IMP characterized the plasmalemma of cells infected with the Patton strain. These intramembrane changes, however, were not observed as early as herpes membrane antigens. Thus, application of the freeze-fracture technique to herpes simplex virus type 1-infected cells revealed striking structural differences between viral and uninfected cell membranes. These differences are probably related to insertion and clustering of virus-coded proteins in the hydrophobic part of the membrane bilayer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kaplan A. S. Studies on the biogenesis of herpesvirus envelope. Nature. 1972 Jan 21;235(5334):165–166. doi: 10.1038/235165a0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Bentzel C. J., Vermorken A. J., Kibbelaar M., Bloemendal H. A portrait of plasma membrane specializations in eye lens epithelium and fibers. Biochim Biophys Acta. 1976 Dec 14;457(3-4):353–384. doi: 10.1016/0304-4157(76)90004-6. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Diawara A. The organization of the plasma membrane in mammalian cells. Eur J Cancer. 1973 Apr;9(4):263–272. doi: 10.1016/0014-2964(73)90092-3. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol. 1969 Nov;4(5):769–776. doi: 10.1128/jvi.4.5.769-776.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Reese T. S., Narayan O. Membrane changes associated with assembly of visna virus. Virology. 1976 Oct 15;74(2):520–530. doi: 10.1016/0042-6822(76)90357-3. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Reese T. S. Structural changes in the membrane of vero cells infected with a paramyxovirus. J Cell Biol. 1975 Dec;67(3):551–565. doi: 10.1083/jcb.67.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fitzgerald S. C., Fuccillo D. A., Moder F., Sever J. L. Utilization of a further miniaturized serological microtechnique. Appl Microbiol. 1974 Feb;27(2):440–441. doi: 10.1128/am.27.2.440-441.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. K., Tenser R. B., Hsiung G. D., Gross P. A. Ultrastructural studies of the envelopment and release of guinea pig herpes-like virus in cultured cells. Virology. 1973 Apr;52(2):468–477. doi: 10.1016/0042-6822(73)90342-5. [DOI] [PubMed] [Google Scholar]
- Fraser C. E., Melendez L. V., Simeone T. Specificity differentiation of herpes simplex virus types 1 and 2 by indirect immunofluorescence. J Infect Dis. 1974 Jul;130(1):63–66. doi: 10.1093/infdis/130.1.63. [DOI] [PubMed] [Google Scholar]
- Fuccillo D. A., Moder F. L., Catalano L. W., Jr, Vincent M. M., Sever J. L. Herpesvirus hominis types I and II: a specific microindirect hemagglutination test. Proc Soc Exp Biol Med. 1970 Mar;133(3):735–739. doi: 10.3181/00379727-133-34554. [DOI] [PubMed] [Google Scholar]
- Gershon A., Cosio L., Brunell P. A. Observations on the growth of varicella-zoster virus in human diploid cells. J Gen Virol. 1973 Jan;18(1):21–31. doi: 10.1099/0022-1317-18-1-21. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M., Hsu K. C. Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol. 1968 Oct;2(10):1172–1184. doi: 10.1128/jvi.2.10.1172-1184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee Y. C., Talens L. Electron microscopic studies on the development of infectious bovine rhinotracheitis virus in bovine kidney cells. J Gen Virol. 1972 Dec;17(3):333–336. doi: 10.1099/0022-1317-17-3-333. [DOI] [PubMed] [Google Scholar]