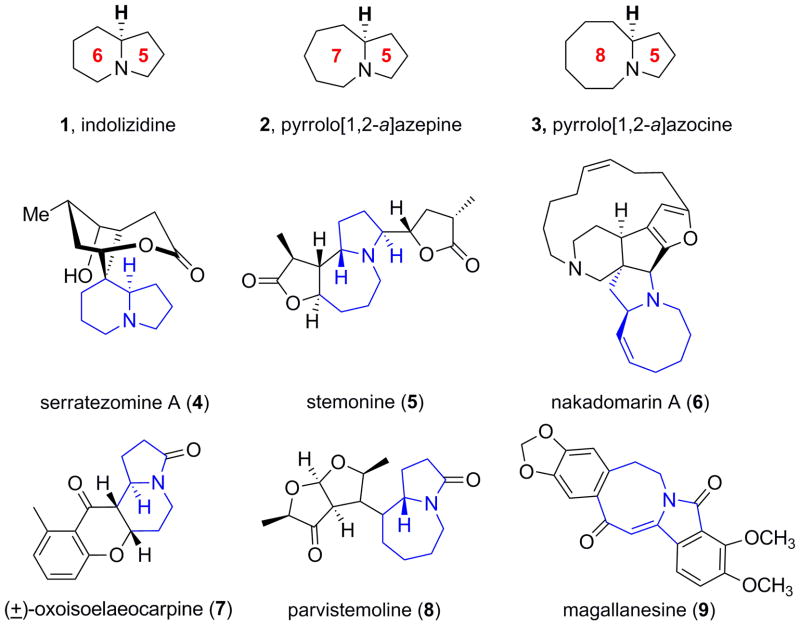
Azabicyclic ring skeletons are common structural subunits present in numerous alkaloid natural products and serve as important scaffolds in biologically active and pharmaceutically significant compounds.[1] Of particular interest in our lab (Figure 1), are the indolizidine 1, pyrrolo[1,2-a]azepine 2, and pyrrolo[1,2-a]azocine 3 azabicyclic systems,[1] that are found in a number of natural products such as the indolizidine alkaloid, serratezomine (4),[2] the stemona alkaloid, stemonine (5),[3] and the manzamine alkaloid, nakadomarin A (6),[4] to highlight but a few representative examples. Other related natural products contain the corresponding lactam moiety, such as 7–9.[5–7] More recently, these azabicyclic cores are key pharmacophores of HTS hits from our MLPCN probe discovery efforts,[8] requiring robust methodology to rapidly access the parent azabicyclic ring sytems as well as flexiblity to incorporate structural diversity for analogue synthesis.[9]
Figure 1.
Structures of indolizidine 1, pyrrolo[1,2-a]azepine 2, and pyrrolo[1,2-a]azocine 3 alkaloid cores and natural products 4–9 that possess these azabicyclic ring systems.[1–7]
Many synthetic strategies have been developed for the construction of azabicyclic ring systems,[10] such as 1 and 2, including Staudinger-aza-Wittig approaches,[11] 7-exo-tet-cyclizations,[12] [4+2] and [2+2+2] cycloadditions,[13] ring-closing metathesis (RCM) strategies,[14] nitrone rearrangements[15] and intramolecular Schmidt rearrangements.[16] General approaches for the synthesis of the pyrrolo[1,2-a]azocine 3 system are rare and most lack stereocontrol.[10,17] The majority of these were developed in the context of natural product target-oriented synthesis, and our lab required a general synthetic strategy to access 1–3 with considerable flexibility for both the synthesis of unnatural analogues and the ease of scale-up. Here, we detail our contributions to this dynamic field with the development of a rapid, general protocol for the enantioselective construction of azabicyclic ring systems 1–3 and the application of this new methodology to a concise total synthesis of (+)-grandisine D, the formal total synthesis of (+)-grandisine B, and unnatural analogues.
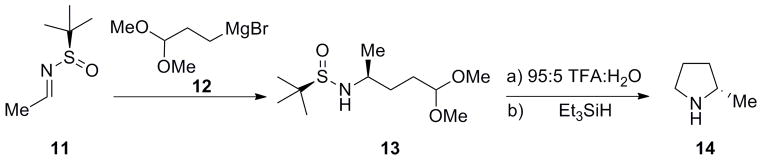
Our work was inspired by the chiral sulfinamide work of Ellman and co-workers for the synthesis of chiral 2-substituted pyrrolidines (Scheme 1).[18] Addition of a Grignard reagent 12, bearing a latent aldehyde moiety, to a chiral aldimine 11 provides 13 in high yield and excellent diastereoselectivity. A one-pot deprotection/acetal hydrolysis facilitates an intramolecular reductive amination reaction to produce chiral pyrrolidine 14 in high enantiomeric excess.[18] In our hands,[19] the addition of organometallic reagents such as 12 to aldimines 11 always proceed with high diastereoselectivity, but the relative stereochemistry can be substrate dependent based on the ability of the dioxolane oxygens atoms to coordinate the metal, and the addition may proceed through either a metal-chelated or non-metal-chelated transition state.[20,21]
Scheme 1.
Ellman’s approach to the synthesis of chiral 2-substituted pyrrolidines. a) 95:5 TFA:H20, 10 min, then Et3SiH.
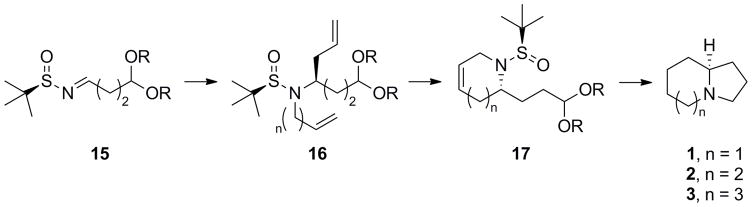
Thus, based on the work of Ellman[18,20] and our experience,[19] we envisioned a protocol involving either diasteroselective Grignard or indium-mediated allylation of a chiral aldimine substrate 15,[18–21] N-alkylation to afford 16, ring-closing methasis (RCM) to provide 17, and finally a one-pot deprotection/acetal hydrolysis/reductive amination sequence to afford enantiopure azabicyclic ring systems 1–3. In this sequence, the stoichiometric chiral auxillary also serves as a protecting group throughout the synthesis.
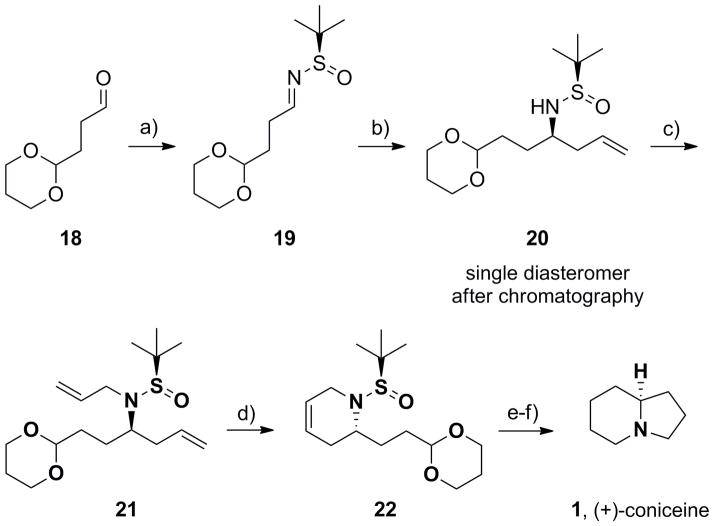
We began by applying the strategy outlined in Scheme 2 to the synthesis of the indolizidine core 1 (Scheme 3). Commercially available aldehyde 18 was converted into the corresponding (R)-N-sulfinyl aldimine 19 under standard conditions[18–20] in 91% yield, followed by an indium-mediated allylation reaction that afforded 20 in >15:1 diastereoselectivity and 87% yield.[19,21] After column chromatography, a single diasteromer of 20 resulted, which was carried forward. Alkylation of the sulfinamide with allyl bromide provided 21,that smoothly underwent an RCM reaction with Grubbs II[22] to deliver 22 in good yield.[19] Hydrogenation to reduce the alkene, followed by a one-pot deprotection/acetal hydrolysis/reductive amination sequence produced the indolizidine alkaloid (−)-coniceine 1 in >98% ee. This approach for the synthesis of (+)-coniceine,[23] the simplest alkaloid possessing the azabicyclic core 1, required only six steps and proceeded in 43% overall yield.
Scheme 2.
Envisioned approach for the rapid, enantioselective synthesis of azabicylic ring systems 1–3.
Scheme 3.
Synthesis of (−)-coniceine (1), representing the basic indolizidine core. a) (R)-tert-butyl sulfinamide, CuSO4, CH2Cl2, 91%; b) In(0), allyl bromide, sat’d aq. NaBr, rt, 16 h, 87% (>15:1 dr); c) LiHMDS, allyl bromide, DMF, −20 °C to rt, 80%; d) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 84%; e) H2, Pd/C; f) i) TFA:H20 (95:5), rt, 45 min; ii) PS-BH(OAc)3, 81% for two steps.
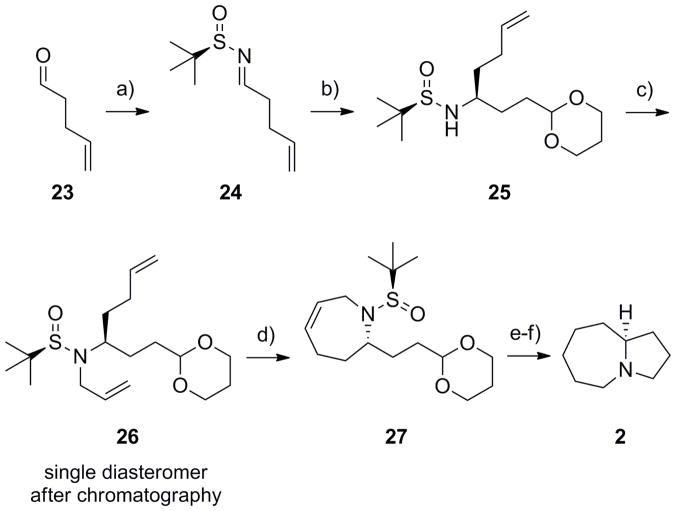
Application of this approach for the enantioselective synthesis of the pyrrolo[1,2-a]azepine 2 proved more challenging. In this instance, we were unable to alkylate 20 with a number of butenyl substrates (Cl, Br, I, OMs, OTS, OTf) in reasonable yield to allow for the RCM to form the seven-membered ring. Therefore, we altered the approach. For azabicyclic core 2, commercially available aldehyde 23 was converted into the corresponding (R)-N-sulfinyl aldimine 24 under Ti(OEt)4-mediated conditions in 95% yield.[18–21] Following the Ellman protocol, addition of Grignard reagent to 24 afforded the desired adduct 25 in 88% yield in >9:1 dr.[18–21] Alkylation of sulfinamide 25 with allyl bromide provided 26 as a single diastereomer after column chromatography in 80% yield. Once again, an RCM reaction with Grubbs II[22] delivered the seven-membered ring 27 in 78% yield. Hydrogenation to reduce the alkene, followed by a one-pot deprotection/acetal hydrolysis/reductive amination sequence produced pyrrolo[1,2-a]azepine 2 in 86% yield for the two steps and in >98% ee. This variation of the approach outlined in Scheme 1 for the synthesis of 2 once again required only six steps and proceeded in 45% overall yield.
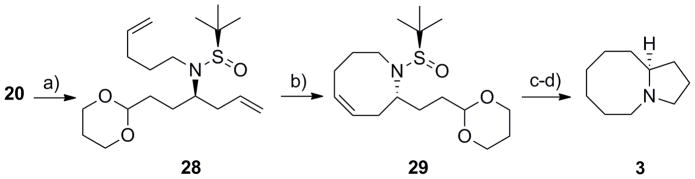
For the more rarely described pyrrolo[1,2-a]azocine azabicyclic system 3, the protocol employed for the synthesis of the indolizidine core 1 (Scheme 3) proceeded smoothly. In this case (Scheme 5), alkylation of sulfinamide 20 with 5-bromo-1-pentene provided 28, that smoothly underwent an RCM reaction with Grubbs II[22] to deliver 29 in 70% yield for the two steps. Hydrogenation to reduce the alkene, followed by a one-pot deprotection/acetal hydrolysis/reductive amination sequence produced pyrrolo[1,2-a]azocine 3 in 87% yield for the two steps and in >98% ee. Thus, from advanced intermediate 20, azabicyclic core 3 could be accessed in four steps and in 60% overall yield. Thus, this new methodology provided rapid, high yielding access to enantiopure azabicyclic rings systems 1–3 from commercial reagents, and either enantiomer of 1–3 can be prepared by the use of either the (R)- or (S)-tert-butyl sulfinamide.
Scheme 5.
Synthesis of the pyrrolo[1,2-a]azocine core 3. a) LiHMDS, 5-bromo-1-pentene, DMF, −20 °C to rt, 85%; b) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 82%; c) H2, Pd/C; d) i) TFA:H20 (95:5), rt, 45 min; ii) PS-BH(OAc)3, 87% for two steps.
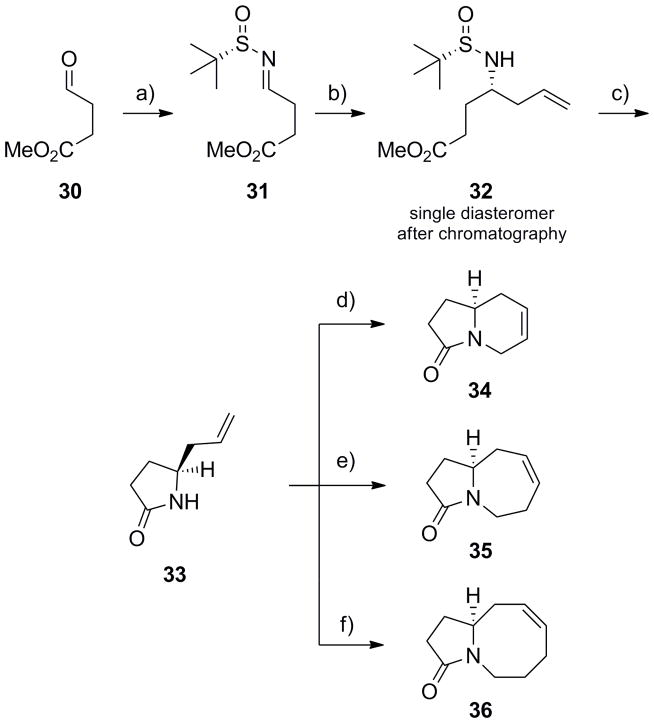
Since many natural products, represented by 7–9, possess the azabicyclic cores 1–3 with a γ-lactam moiety,[5–7] we next applied our methodology to this molecular architecture. Starting from commercial aldehyde 30, a highly convergent approach to the lactam congeners of 1–3 was developed (Scheme 6) wherein 30 was first converted into the corresponding (S)-N-sulfinyl aldimine 31 under standard conditions in 90% yield.[18–21] An indium-mediated allylation reaction afforded 32 in 88% yield with >10:1 diastereoselectivity, and, after column chromatography, a single diasteromer.[19,21] Standard deprotection liberated the primary amine, which in the presence of sodium carbonate, induced cyclization to form key linchpin intermediate (S)-5-allylpyrrolidin-2-one 33 in 97% yield. Allylation of lactam 33 and subsequent RCM with Grubbs II[22] provided indolizinone 34 in 87% yield for the two steps. Similarly, alkylation of 33 with either butenyl bromide or pentenyl bromide, followed by an RCM reaction with Grubbs II,[22] afforded azepinone 35 or azocinone 36, respectively. Thus, azabicyclic lactams 34–36 can be prepared in five steps from commercial aldehyde 30, via linchpin 33, in overall yields ranging from 56–69%. Importantly, the alkene can either be reduced or used as a handle to install additional functionality and chemical diversity.
Scheme 6.
Synthesis of azabicyclic lactams 37–39. a) (S)-tert-butyl sulfinamide, CuSO4, CH2Cl2, 90%; b) In(0), allyl bromide, sat’d aq. NaBr, rt, 16h, 88% (>10:1 dr); c) i) HCl, MeOH, rt, ii) Na2CO3, CH2Cl2, 97%; d) i) LiHMDS, allyl bromide, DMF, −20 °C to rt; ii) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 87% for two steps; e) i) LiHMDS, butenyl bromide, DMF, −20 °C to rt; ii) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 86% for two steps; f) i) LiHMDS, pentenyl bromide, DMF, −20 °C to rt; ii) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 73% for two steps.
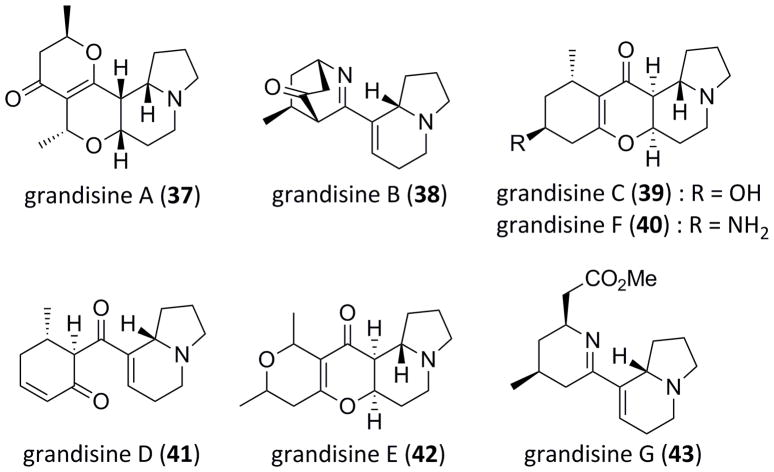
Efforts now focused on the application of this methodology towards targeted natural product total synthesis, with the focus of determining if our streamlined approach for the synthesis of azabicyclic ring systems would offer a tactical advantage. After a perusal of the literature, we were attracted to grandisines A-G (37–46), indolizidine alkaloids isolated by Carroll and co-workers from the leaves of the Australian rain forest tree Elaeocarpus grandis (Figure 2).[24] These alkaloids display selective human δ-opioid receptor affinity. Selective activation of the δ-opioid receptor is an attractive strategy for the development of new analgesics, thus grandisines are potential potent analgesic agents.[25] To highlight our new methodology, we elected to synthesize grandisine D (41). Grandisine D (41) was previously synthesized by Tamura and co-workers in 18 steps (12.5% overall yield) employing a Brønsted acid mediated Morita-Bayis-Hillman ring-closure reaction as the key step; however, two steps suffered from poor stereocontrol.[26] Tamura was also able to convert 41 into grandisine B (38) via a tandem imination/amination reaction sequence.[26] Two years later, Taylor and co-workers improved on the synthesis of 41, requiring only 13 steps (10% overall yield) from commercial starting materials, and featuring a new alkyne-acetal cyclization reaction.[27]
Figure 2.
Structures of the indolizidine alkaloids, the grandisines A–G (37–43).
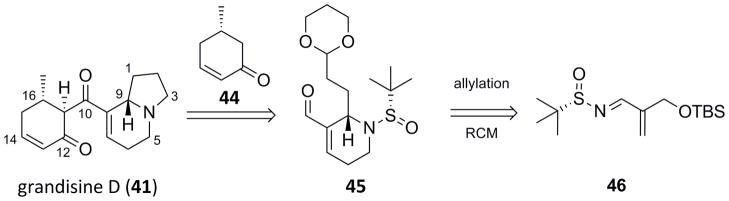
Our retrosynthesis led to the same key aldol chemistry as that employed by Tamura and Taylor,[26,27] but a fundamentally new approach to the indolizidine core (Scheme 7).[28] Thus, 41 would be accessed by an aldol reaction between 8-formylindolizidine 45 and known (S)-5-methylcyclohexanone 44.[29] 8-Formylindolizidine 45 would be prepared from Grignard addition and RCM of (S)-sulfinyl aldimine 46.
Scheme 7.
Retrosynthesis of grandisine D (41).
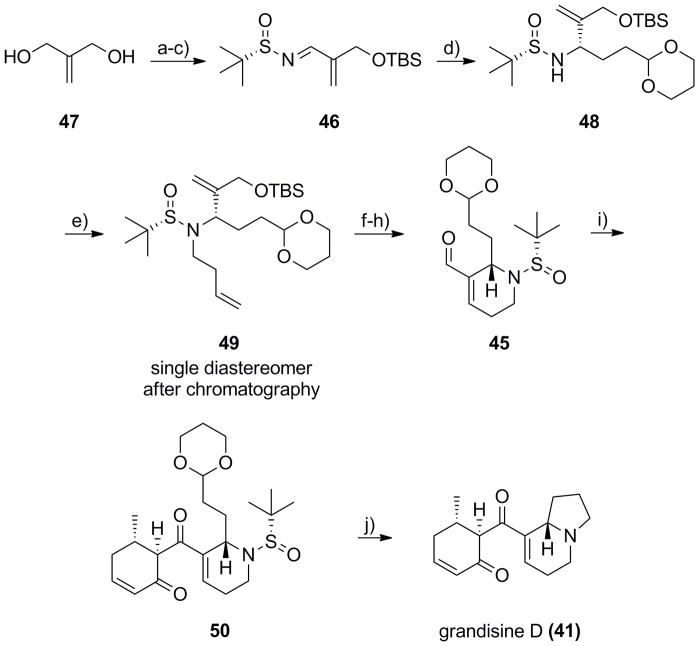
Our synthetic study began with the synthesis of (S)-sulfinyl aldimine 46 (Scheme 8). Starting from commercial diol 47, a mono-silyation and oxidation sequence, followed by conversion into the corresponding (S)-N-sulfinyl aldimine 46 under Ti(OEt)4-mediated conditions proceeded in 74% yield for the three steps.[18–21] Following the Ellman protocol, addition of Grignard reagent to 46 afforded the desired adduct 48 in 79% yield in >10:1 dr.[18–21] Alkylation of sulfinamide 48 with butenyl triflate provided 49 as a single diastereomer after column chromatography in 87% yield. Again, an RCM reaction with Grubbs II[22] delivered the piperidine ring in 96% yield, followed by removal of the TBS group and oxidation to key aldehyde 45 in 93% yield for the two steps. Next, an Evan’s aldol employing boron-enolate methodology[30] with 45 and enone 44,[29] followed by oxidation provided 50 in 77% yield. Finally, application of the one-pot deprotection/acetal hydrolysis/reductive amination sequence produced 41 in 47% yield. Our synthetic 41 was in complete agreement with the reported spectral and rotation data for the natural product[24] as well as the previous synthetic efforts.[26,27] Thus, the total synthesis of grandisine D (41), employing our azabicyclic methodology, required only 11 steps from commercial starting materials in 16.4% overall yield and with excellent stereocontrol throughout. Notably, based on the work of both Tamura[26] and Taylor,[27] the total synthesis of (+)-grandisine (41) also constitutes a formal total synthesis of (−)-grandisine B (38).
Scheme 8.
Synthesis of (+)-grandisine D (41). a) TBSCl, imidazole, CH2Cl2,95%; b) MnO2, CH2Cl2, 90%; c) (S)-tert-butyl sulfinamide, Ti(OEt)4, CH2Cl2, 87%; d) (2(-1,3- dioxan-2-yl)ethyl)magnesium bromide, THF, −78 °C to −45 °C, 79% (>10:1 dr); e) LiHMDS, 3-buteynyl triflate, HMPA:THF, −78 °C, 87%; f) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 96%; g) TBAF, THF, 0 °C, 93%; h) MnO2, CH2Cl2, 100%; i) i) 44, n-Bu2BOTf, i-PrNEt2, CH2Cl2, −78 °C to rt, ii) TFAA, DMSO, CH2Cl2, −78 °C, 77%; j) i) TFA:H20 (95:5), rt, 45 min; ii) PS-BH(OAc)3, DCE, 47%.
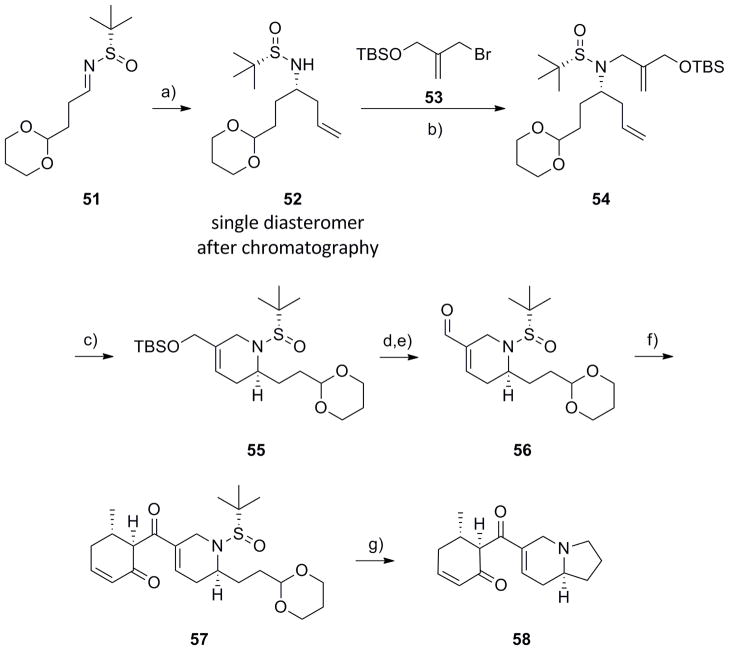
To further highlight the power of this methodology for diversity-oriented synthesis, we applied it towards the synthesis of an unnatural analogue of 41, in which the nitrogen atom was moved from 4-position to the 9-position, resulting in a fundamentally new molecular architecture. Starting with (S)-N-sulfinyl aldimine 51 (the (S)-enantiomer of 19), an indium-mediated allylation reaction afforded 52 (the (S,S) enantiomer of (R,R)-20) in 87% yield with >19:1 diastereoselectivity, and, after column chromatography, a single diasteromer.[18–21] Diol 47 was mono-protected as a TBS ether and the remaining hydroxyl was converted into the corresonding allylic bromide 53 in 85% yield for the two steps. Allylation of 52 with 53 provided 54 in 90% yield, folowed by an RCM reaction with Grubbs II[22] afforded 54 in 81% yield for the two steps. Deprotection fo the TBS ether and oxidation delivered key aldehyde 56, in 95% yield for the two steps. Once again, an Evan’s aldol[30] employing boron-enolate methodology with 56 and enone 44,[29] followed by oxidation provided 57 in 67% yield. Finally, application of the one-pot deprotection/acetal hydrolysis/reductive amination sequence produced the unnatural analogue of (+)-grandisine D 58 in 49% yield. The synthesis of 58 proceeded in 11 steps (9 steps longest linear sequence) from commercial materials in 17.8% overall yield.
In summary, we have developed a novel six step approach for the rapid and enantioselective synthesis of indolizidine 1, pyrrolo[1,2-a]azepine 2, and pyrrolo[1,2-a]azocine 3 azabicyclic systems and their respective lactam congeners 34–36, that are found in a host of natural products as well as pharmaceutical preparations. The methodology described herein allows for either enantiomer to be prepared based on the stereochemistry of the tert-butyl sulfinamide employed and the nature of the transition state of the organometallic used in the initial allylation. To highlight this technology in natural product total synthesis, (+)-grandisine D (41) was prepared in 11 synthetic steps and in 16.4% overall yield, a notable advance over the two previous syntheses, and also constitutes a formal total synthesis of (−)-grandisine B (38). This methodology also lent itself to the rapid synthesis of an unnatural analogue 58, expanding the utility of the methodology for diversity-oriented synthesis. Further refinements, applications to the related pyrrolizidine alkaloids and biological investigations are in progress and will be reported in due course.
Experimental Section
Please see the Supporting Information Section for full experimental details
Supplementary Material
Scheme 4.
Synthesis of the pyrrolo[1,2-a]azepine core 2. a) (R)-tert-butyl sulfinamide, Ti(OEt)4, CH2Cl2, 95%; b) (2(-1,3-dioxan-2-yl)ethyl)magnesium bromide, THF, −45 °C, 88% (>9:1 dr); c) LiHMDS, allyl bromide, DMF, −20 °C to rt, 80%; d) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 78%; e) H2, Pd/C; f) i) TFA:H20 (95:5), rt, 5 min; ii) PS- BH(OAc)3, 86% for two steps.
Scheme 9.
Synthesis of unnatural analogue (58). a) In(0), allyl bromide, sat’d aq. NaBr, rt, 16h, 87% (>19:1 dr); b) LiHMDS, 53, DMF, −20 °C to rt, 90%; c) Grubbs II (5 mol%), CH2Cl2, 40 °C, 1 h, 90%; d) TBAF, THF, 0 °C, 95%; e) MnO2, CH2Cl2, 100%; f) i) 44, n-Bu2BOTf, i-PrNEt2, CH2Cl2, −78 °C to rt, ii) TFAA, DMSO, CH2Cl2, −78 °C, 67%; g) i) TFA:H20 (95:5), rt, 45 min; ii) PS-BH(OAc)3, DCE, 49%.
Acknowledgments
The authors acknowledge the Vanderbilt Department of Pharmacology, VUMC and the NIH for funding our research, and in particular the MLPCN (U54MH084659) for the Vanderbilt Specialized Cheimstry Center.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
References
- 1.a) Michael JP. Nat Prod Rep. 2005;22:603–626. doi: 10.1039/b413748p. [DOI] [PubMed] [Google Scholar]; b) Mitchinson A, Nadin A. J Chem Soc Perkin Trans 1. 2000:2862–2892. [Google Scholar]; c) Ohagan D. Nat Prod Rep. 1997;14:637–651. [Google Scholar]; d) Sakata K, Oki KA, Chang CF, Sakurai A, Tamura S, Murakoshi S. Agric Biol Chem. 1978;42:457–465. [Google Scholar]; e) Ye Y, Qin GW, Xu RS. Phytochemistry. 1994;37:1205–1215. [Google Scholar]; f) Shinozaki H, Ishida M. Brain Res. 1985;334:33–39. doi: 10.1016/0006-8993(85)90564-5. [DOI] [PubMed] [Google Scholar]; g) Pilli RA, Rosso GB, De Oliveira MDCF. Nat Prod Rep. 2000;17:117–127. doi: 10.1039/a902437i. [DOI] [PubMed] [Google Scholar]; h) Pilli RA, Rosso GB, De Oliveira MDCF. Nat Prod Rep. 2010;27:1908–1937. doi: 10.1039/c005018k. [DOI] [PubMed] [Google Scholar]; i) Seger C, Mereiter K, Kaltenegger E, Pacher T, Greger H, Hofer O. Chem Biodiversity. 2004;1:265–279. doi: 10.1002/cbdv.200490023. [DOI] [PubMed] [Google Scholar]; j) Gregor H, Schinnerl J, Vajrodaya S, Brecker L, Hofer O. J Nat Prod. 2009;72:1708–1711. doi: 10.1021/np900294c. [DOI] [PubMed] [Google Scholar]
- 2.Morita H, Arisaka M, Kobayashi J. J Org Chem. 2000;65:6241–6245. doi: 10.1021/jo000661e. [DOI] [PubMed] [Google Scholar]
- 3.a) Zou C, Li J, Lei H, Fu H, Lin W. J Chin Pharm Sci. 2000;9:113–115. [Google Scholar]; b) Williams DR, Shamim K, Khalida R, Reddy J, Amato GS, Shaw SM. Org Lett. 2003;5:3361–3364. doi: 10.1021/ol035368q. [DOI] [PubMed] [Google Scholar]
- 4.a) Kobayashi J, Watanabe D, Kawasaki N, Tsuda M. J Org Chem. 1997;62:9236–9239. [Google Scholar]; b) Nagatam T, Nakgawa M, Nishida A. J Am Chem Soc. 2003;125:7484–7485. doi: 10.1021/ja034464j. [DOI] [PubMed] [Google Scholar]; c) Ono K, Nakagawa M, Nishida A. Angew Chem Int Ed. 2004;43:202–2023. doi: 10.1002/anie.200453673. [DOI] [PubMed] [Google Scholar]; d) Jakubec P, Cockfield DM, Dixon DJ. J Am Chem Soc. 2009;13:16632–16633. doi: 10.1021/ja908399s. [DOI] [PubMed] [Google Scholar]; e) Martin DBC, Vanderwal CD. Angew Chem Int Ed. 2010;49:2830–2832. doi: 10.1002/anie.201000045. [DOI] [PubMed] [Google Scholar]; f) Nilson MG, Funk RL. Org Lett. 2010;12:4912–4915. doi: 10.1021/ol102079z. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Cheng B, Wu F, Yang X, Zhou Y, Wan X, Zhai H. Chem Eur J. 2011;52:6094–6097. [Google Scholar]
- 5.a) Johns SR, Lamberton JA, Sioumis AA. Chem Commun. 1968;21:1324–1325. [Google Scholar]; b) Johns SR, Lamberton JA, Sioumis AA. Aust J Chem. 1969;22:793–800. [Google Scholar]
- 6.Lin W, Xu R, Zhong Q. Huaxue Xuebao. 1991;49:927–931. [Google Scholar]
- 7.a) Valencia E, Fajardo V, Freyer AJ, Shamma M. Tetrahedron Lett. 1985;26:993–996. [Google Scholar]; b) Fang FG, Feigelson GB, Danishefsky SJ. Tetrahedron Lett. 1989;30:2743–2746. [Google Scholar]
- 8.For information on the MLPCN and the Vanderbilt Center, please see: http://www.mc.vanderbilt.edu/centers/mlpcn/index.html or http://mli.nih.gov/mli/mlpcn/
- 9.Kennedy JP, Williams L, Bridges TM, Daniels RN, Weaver CD, Lindsley CW. J Combi Chem. 2008;10:345–354. doi: 10.1021/cc700187t. [DOI] [PubMed] [Google Scholar]
- 10.For reviews on the synthesis of azabicyclic ring systems, see: Michael JP. Beilstein J Org Chem. 2007:3. doi: 10.1186/1860-5397-3-27.Hodgson DM, Winning LH. Org Biomol Chem. 2007;5:3071–3082. doi: 10.1039/b707566a.Toure BB, Hall DG. Chem Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p.Michael JP. Nat Prod Rep. 2008;25:139–165. doi: 10.1039/b612166g.Pyne SG, Davis AS, Gates N, Hartley JP, Lindsay KB, Machan T, Tang M. Synlett. 2004;15:625–649.Enders D, Thiebes T. Pure & Appl Chem. 2001;73:573–578.Pili RA, Rosso GB, De Oliviera F, da Conceicao M. Nat Prod Rep. 2011;27:1908–1937. doi: 10.1039/c005018k.Aibes R, Figueredo M. Eu J Org Chem. 2009;15:2421–2435.Khim SK, Schultz AG. J Org Chem. 2004;69:7734–7736. doi: 10.1021/jo049083i.
- 11.a) Williams DR, Brown DL, Benbow JW. J Am Chem Soc. 1989;111:1923–1925. [Google Scholar]; b) Williams DR, Fromhold MG, Early JD. Org Lett. 2001;3:2712–2724. doi: 10.1021/ol016336a. [DOI] [PubMed] [Google Scholar]; c) Williams DR, Shamim K, Reddy JP, Amato GS, Shaw SM. Org Lett. 2003:3361–3364. doi: 10.1021/ol035368q. [DOI] [PubMed] [Google Scholar]
- 12.Kohno Y, Narasaka K. Bull Chem Soc Jpn. 1996;69:2063–2070. [Google Scholar]
- 13.a) Jacobi PA, Lee K. J Am Chem Soc. 1997;119:3409–3410. [Google Scholar]; b) Jacobi PA, Lee K. J Am Chem Soc. 2000;122:4295–4303. [Google Scholar]; c) Yu RT, Rovis T. J Am Chem Soc. 2006;128:12370–12371. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]
- 14.a) Alibes R, Figueredo M. J Org Chem. 2009;74:2421–2435. doi: 10.1021/jo802492g. [DOI] [PubMed] [Google Scholar]; b) Torssell S, Wanngren E, Somafi P. J Org Chem. 2007;72:4246–4249. doi: 10.1021/jo070498o. [DOI] [PubMed] [Google Scholar]; c) Sibi MP, Subramanian T. Synlett. 2004:1211–1214. [Google Scholar]; d) Olivo HF, Tovar-Miranda R, Barragan E. J Org Chem. 2006;71:3287–3290. doi: 10.1021/jo052364l. [DOI] [PubMed] [Google Scholar]; e) Hoye AT, Wipf P. Org Lett. 2011;13:2634–2637. doi: 10.1021/ol200743u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cid P, Closa M, De March P, Figueredo M, Font J, Sanfeliu E, Soria A. Eur J Org Chem. 2004:4215–4233. [Google Scholar]
- 16.Kapat A, Nyfeler E, Giuffredi GT, Renaud J. J Am Chem Soc. 2009;131:17746–17747. doi: 10.1021/ja908933s. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Y, Smith BT, Hershberger J, Aubé J. J Org Chem. 2003;68:8065–8067. doi: 10.1021/jo035004b. [DOI] [PubMed] [Google Scholar]
- 18.a) Brinner KM, Ellman JA. Org Biomol Chem. 2005;3:2109–2113. doi: 10.1039/b502080h. [DOI] [PubMed] [Google Scholar]; b) Coogan DA, Ellman JA. J Am Chem Soc. 1999;121:269–270. [Google Scholar]; c) Tang TP, Ellman JA. J Org Chem. 2002;67:7819–7832. doi: 10.1021/jo025957u. [DOI] [PubMed] [Google Scholar]
- 19.Schulte ML, Lindsley CW. Org Lett. 2011;13:5684–5687. doi: 10.1021/ol202415j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coogan DA, Liu G, Ellman JA. Tetrahedron. 1999;55:8883–8904. [Google Scholar]
- 21.Sun XW, Liu M, Xu MH, Lin GQ. Org Lett. 2008;10:1259–1262. doi: 10.1021/ol8001514. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn KM, Champange TM, Hong SH, Wei WH, Nickel A, Lee CW, Virgil SC, Grubbs RH, Pederson RL. Org Lett. 2010;125:984–987. doi: 10.1021/ol9029808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoda H, Katoh H, Ujihara Y, Takabe K. Tetrahedron Lett. 2001;42:2509–2512. [Google Scholar]
- 24.a) Carroll AR, Arumugan G, Quinn RJ, Guymer G, Grimshaw P. J Org Chem. 2005;70:1889–1892. doi: 10.1021/jo048525n. [DOI] [PubMed] [Google Scholar]; b) Katavic PL, Venables DA, Forster PI, Guymer G, Carroll AR. J Nat Prod. 2006;69:1295–1299. doi: 10.1021/np060179c. [DOI] [PubMed] [Google Scholar]
- 25.Pradham AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Brigitte BL. Trends Pharm Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Kurasaki H, Okamoto I, Morita N, Tamura O. Org Lett. 2009;11:1179–1181. doi: 10.1021/ol900032h. [DOI] [PubMed] [Google Scholar]; b) Kurasaki H, Okamoto I, Morita N, Tamura O. Chem Eur J. 2009;15:12754–12763. doi: 10.1002/chem.200901843. [DOI] [PubMed] [Google Scholar]
- 27.Cuthbertson JD, Godfrey AA, Taylor RJK. Org Lett. 2011;13:3976–3979. doi: 10.1021/ol2014939. [DOI] [PubMed] [Google Scholar]
- 28.See Experimental Section for full details.
- 29.Carlone A, Margio M, North C, Landa A, Jorgensen KA. Chem Commun. 2006:4928–4930. doi: 10.1039/b611366d. [DOI] [PubMed] [Google Scholar]
- 30.a) Evans DA, Bartroli J, Shih TL. J Am Chem Soc. 1981;103:2127–2129. [Google Scholar]; b) Evans DA, Nelson JV, Taber TR. Top Stereochem. 1982;13:1–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.