Abstract
Dynamic visual acuity (DVA) may be a useful indicator of the function of the vestibulo-ocular reflex (VOR) but most DVA tests involve active head motion in the yaw plane. During gait the passive, vertical VOR may be more relevant and passive testing would be less likely to elicit compensatory strategies. The goal of this study was to determine if testing dynamic visual acuity during passive vertical motion of the subject would differentiate normal subjects from patients with known vestibular disorders. Subjects, normals and patients who had been diagnosed with either unilateral vestibular weaknesses or were post-acoustic neuroma resections, sat in a chair that could oscillate vertically with the head either free or constrained with a cervical orthosis. They viewed a computer screen 2 m away that showed Landholt C optotypes in one of 8 spatial configurations and which ranged in size from 0.4 to 1.0 logMAR. They were tested while the chair was stationary and while it was moving. Scores were worse for both groups during the dynamic condition compared to the static condition. In the dynamic condition patients’ scores were significantly worse than normals’ scores. Younger and older age groups differed slightly but significantly; the sample size was too small to examine age differences by decade. The data suggest that many well-compensated patients have dynamic visual acuity that is as good as age-matched normals. Results of ROC analyses were only moderate, indicating that the differences between patients and normals were not strong enough, under the conditions tested, for this test to be useful for screening people to determine if they have vestibular disorders. Modifications of the test paradigm may make it more useful for screening potential patients.
Keywords: Gaze stabilization, vestibulo-ocular reflex, epidemiologic screening, vestibular disorders, vestibular hypofunction
1. Introduction
Objective, clinical, diagnostic tests, such as bi-thermal caloric tests, low frequency rotatory chair tests in darkness, and vestibular evoked myogenic potentials (VEMP), provide good, relatively direct measures of vestibular function. These tests are good indicators of vestibular impairment. Those tests do not, however, indicate how well patients use their vestibular function in daily life. For purposes of screening people for vestibular impairments quickly bi-thermal caloric tests, rotatory chair tests and VEMP may take too long and may require too much expensive, space-occupying equipment. Tests of dynamic visual acuity (DVA), the ability to see clearly while moving, provide a bridge between classical, objective diagnostic testing and clinical, subjective observation of functional motor behavior. Such tests usually take only a few minutes, are not nauseogenic, and are objective.
DVA is an indirect indicator of VOR function. It is a functional test of whole body integration that includes the requirement of good vestibulo-ocular reflex (VOR) function. It is impaired in patients with bilateral loss [6, 7,13]. It may also be impaired in unilateral vestibular impairment during unpredictable head movements [5, 15,16]. Changes in DVA might indicate development of compensatory mechanisms after vestibular impairment. Therefore DVA may be useful for screening. It may also be a useful indicator of compensation
Some experts have recommended screening patients with DVA [1,4]. Goebel [4] has suggested using passive head shaking while the patient reads a Snellen chart. The Snellen chart, however, presents some perceptual disadvantages and passive head shaking presents an uncontrolled stimulus so that results may not be comparable across trials or subjects. Peters and Bloomberg developed a DVA test that used a Landholt C, which appeared in various sizes and orientations on a computer screen, and was tested during treadmill locomotion which provides an active perturbation to the head during each step [10].
Passive movement of the head as part of a vestibular testing paradigm has a long history in clinical vestibular science [2]. Passive, and therefore unpredictable, head motion avoids the potential problem of generating predictive saccades and smooth pursuit during predictable head rotations. Vital et al. developed a DVA test using the Landholt C and either active movement by the patient or passive head thrusts given by the examiner standing behind the patient [18]. Active head movements may confound testing by allowing the patient to predict the position of the head and thus make corrective saccades. Passive head thrusts probably vary across trials, patients and examiners. Also, that stimulus does not occur in daily life.
We have developed a passive DVA test that moves the subject linearly along a vertical axis that bisected the intra-aural line. This procedure mimics the experience of head movement during routine walking and driving. Therefore the test should provide a good indication of how well people use the functional VOR to see clearly during while performing those skills. The chair provides the same, reliable stimulus on every test. The goal of this study was to determine if patients and normals have different scores on this test and to determine if it would be useful as a screening test of vestibular disorders.
2. Methods
2.1. Subjects
Subjects were 112 normal subjects and 45 subjects with vestibular impairments with either a chronic vestibular impairment as indicated by a unilateral weakness score on bi-thermal caloric testing of ≥20% (UW) (n = 27) in our laboratory or post-operative acoustic neuroma resection (AN) (n = 18) by neurotologists in the Department of Otolaryngology at Baylor College of Medicine. See Table 1. Normal subjects had no history of hearing loss, vestibular impairment, vertigo, gait abnormality, artificial joints, or neurologic problems. Head shaking in yaw rotations did not elicit vertigo, head thrusts and Dix-Hallpike maneuvers were all negative, bilaterally, and gait showed no ataxia.
Table 1.
Demographic details. Mean age (standard deviation, ranges) and length of illness are reported in years
| Group | Age | Length of illness | Females/Males |
|---|---|---|---|
| Normal | 51.1 (15.4, 23.3 to 79.6) | 58F, 54 M | |
| AN | 55.6 (10.7, 35.2 to 72.9) | 5.5 (5.8, 0.27 to 27) | 12 F, 6 M |
| UW | 54.9 (17.9, 21.4 to 75.2) | 4.0 (9.0, 0.07 to 40) | 18 F, 9 M |
This study was approved by the Institutional Review Board for Human Subjects Research for Baylor College of Medicine and Affiliated Hospitals. Subjects gave informed consent prior to participation.
2.2. Apparatus
Subjects sat in a comfortable chair, consisting of an automobile-type bucket seat atop upon a torque motor. They were strapped in using a standard automobile lap belt. During head free conditions the head was unconstrained. During head constrained conditions the head was stabilized with reference to the trunk with a cervical orthosis (Lerman Minerva), which stabilized the head to the maximum extent possible [11]. The chair was able to move linearly along rails in the vertical direction. An electric motor and gear mechanism were positioned beneath the chair. When the motor was turned on the chair oscillated vertically at a frequency of 2 Hz with maximal displacements of ± 2.5 cm, which simulated the vertical displacement of the head during locomotion. A personal computer set upon a tripod 2 m away from the chair was used to display visual acuity optotypes. For each subject the center of the computer screen was set at eye level when the chair was stationary, in the resting position, at the lowest point of it’s range.
2.3. Procedure
Visual acuity was assessed using custom-written software that sequentially presents Landholt C optotypes on a computer screen. Unlike an earlier use of the software [10] the presentation of the optotypes in the dynamic condition was triggered based on the motion of the chair. In both the static and dynamic testing phases the Landholt C flashed on the computer screen for 75 ms. When the chair was in motion, a mechanical switch on the chair caused the optotype to be presented in a time period centered around the peak velocity of the chair. The display was triggered to occur before the peak velocity and it remained on for an equivalent time after the peak velocity occurred. The number of presentations was equal for both upward travel and downward travel and the presentation were randomized. Due to the randomization and slight inconsistency by staff from trial to trial in typing the subject’s responses on the keypad, the time between successive presentations varied unpredictably.
Optotypes in one of 8 configurations – up, down, right, left, up-right, up-left, down-right and down-left –ranged in size from 0.4 to 1.0 logMAR (log of the Minimum Angle Resolvable) or 20/8 to 20/200 Snellen ratios. Subjects were instructed to state the orientation of each C. After the first, the optotype size used for each successive presentation was based on the accuracy of the subject’s responses to all previous presentations. A PEST (parameter estimation by sequential testing) algorithm was used to estimate the subject’s acuity after each presentation and the next presentation size corresponded with that calculated threshold. A slightly modified version of the same algorithm was used in data post processing to determine the subject’s acuity threshold for each condition. The modification allowed the final measure, which was based on 32 presentations, to be calculated with a resolution of 0.02 logMAR. Therefore the dependent measures, as the raw scores, were the logMAR scores.
2.4. Statistical analyses
Correlation coefficients were used to examine the level of association between age and DVA score at each condition (static, dynamic) within patients and normals. Differences in acuity scores between groups (normal vs vestibular patients) and across conditions (static vs. dynamic) were tested via multilevel and mixed effect models using maximum likelihood estimation techniques. In each model, interactions were included and tested for significance. Adjustments were made for multiple comparisons. p < 0.05 was considered statistically significant. All analyses were performed in SAS Statistical software (SAS, Cary, NC).
3. Results
3.1. Age-related correlations
Because visual acuity changes with age we tested the results in patients and in normals by age, using Pearson product moment correlations. In normals and in patients the correlations with raw scores were moderate and statistically significant. For the difference scores, calculated as *(Static-Dynamic)*10), correlations were weak and in patients were not statistically significant in the static condition. See Table 2.
Table 2.
Correlations of scores in subtests with age
| Head free
|
Head constrained
|
|||||
|---|---|---|---|---|---|---|
| Static | Dynamic | Difference score | Static | Dynamic | Difference score | |
| Normals | r = 0.55, p < 0.0001 | r = 0.65, p < 0.0001 | r = 0.24, p = 0.009 | r = 0.44, p < 0.0001 | r = 0.58, p < 0.0001 | r = 0.23, p = 0.05 |
| Patients | r = 0.52, p < 0.0002 | r = 0.59, p < 0.0001 | r = 0.17, p = 0.26 | r = 0.50, p = 0.0005 | r = 0.67, p < 0.0001 | r = 0.30, p = 0.05 |
3.2. Differences between diagnostic groups and head conditions
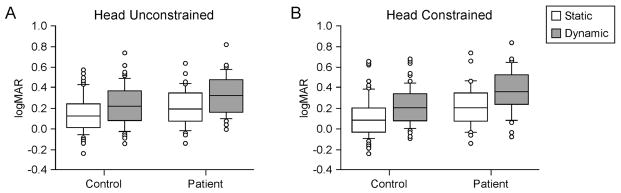
We analyzed the data by condition of the head, either free (unconstrained) or constrained, and by condition of the chair, either stationary (static) or moving (dynamic). Overall normals had lower (better) scores than patients with either head free or head constrained, p < 0.01. Overall, with groups collapsed, regardless of the condition of the head, scores were significantly higher (worse) during dynamic tests than static tests, p < 0.001. Chair condition: For head free tests, within both the patient and the normal groups scores were higher (worse) for the dynamic condition than the static condition, p < 0.001. In the head free, static condition, normals and patients did not differ significantly, p = 0.19. In the head free, dynamic condition, head free, normals had significantly lower scores than patients, p = 0.02. Head condition: Overall, normals and patients had significantly better scores in the head free condition than the head constrained condition, p < 0.001. Differences were also found between static and dynamic conditions by head condition. In the dynamic condition scores were better with head free than head constrained, p < 0.001, for patients and normals. In the static condition normals and patients did not differ significantly, p = 0.07. Scores in the head free condition were, however, significantly better than scores in the head constrained condition, p < 0.0001. See Fig. 1.
Fig. 1.
LogMAR scores for normals and patients, static and dynamic tests. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers. A. Static vs. dynamic conditions. B. Head free vs. head constrained conditions.
3.3. Cuts in age range
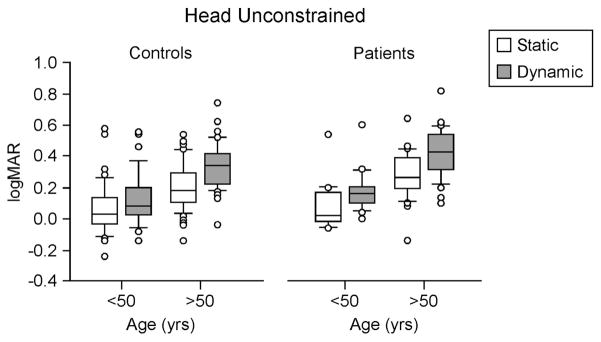
For head free tests, we divided the age range into younger and older subjects with different cut-points in the age range. With the age range cut at age 50, younger subjects showed no differences between the normal and patient groups, p = 0.33, but scores for dynamic tests were higher than static tests for patients, p < 0.0001, and for normals, p < 0.0001. In subjects older than age 50 patients had significantly higher scores than normals, p = 0.02, irrespective of condition. For older subjects, in general, in the dynamic condition scores were higher than in the static condition, p < 0.0001. The interaction was not significant, p = 0.32. See Fig. 2.
Fig. 2.
LogMAR scores for normals and patients by age groups. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers.
When the groups were cut at age 45 the finding was not quite a strong. For example, subjects under age 45 showed no differences by group, p = 0.30, but did show lower scores in the static condition compared to the dynamic condition, p < 0.0001. Again, the interaction was not significant, p = 0.18. For subjects over age 45, a group difference was found irrespective of condition, p = 0.04, but the interaction was not significant, p = 0.37. A difference by chair condition was found; scores were significantly higher in the dynamic condition compared to the static condition, p < 0.0001, regardless of the diagnostic group.
When the groups were cut at age 40, however, although the effect of condition remained, p = 0.002, no effect of group was found for younger subjects, p = 0.22. Within both groups scores were significantly lower in the static condition compared to the dynamic condition, p < 0.0001. Subjects older than age 40 showed significant differences by condition and group. In general patients had lower scores than normals, regardless of condition, p = 0.04. Also, within both patients and normals scores were lower at the static condition than the dynamic condition.
3.4. Relationships among scores
We tested the relationships among pairs of scores to determine if they were related. Pearson product moment correlations showed moderately strong, statistically significant correlations between pairs of raw scores, as shown in the Tables 3. The correlation between difference scores in head free and head constrained conditions, however, was weak and not statistically significant: r = 0.19, p = 0.11.
Table 3.
Correlations between pairs of raw data conditions for normals and patients.
| Normals
|
Patients
|
|||||
|---|---|---|---|---|---|---|
| HFS | HFD | HCS | HFS | HFD | HCS | |
| HFD | r = 0.82, p < 0.0001 | r = 0.81, p < 0.0001 | ||||
| HCS | r = 0.84, p < 0.0001 r = | 0.82, p < 0.0001 | r = 0.84, p < 0.0001 r | = 0.81, p < 0.0001 | ||
| HCD | r = 0.85, p < 0.0001 r = | 0.89, p < 0.0001 r = | 0.87, p < 0.0001 | r = 0.79, p < 0.0001 r | = 0.82, p < 0.0001 r = | 0.73, p < 0.0001 |
HFS, Head free static; HFD, head free dynamic; HCS, head constrained, static; HCD, head constrained dynamic. N, normals; P, patients
3.5. Difference scores
As well as using raw scores we also tested difference scores between static and dynamic conditions, in two ways. The difference between the raw scores in each chair condition and the percent change from static to dynamic conditions might have been significantly different between normal and patient groups. Statistical analyses, however, showed that percent change did not differ significantly between groups for either head free, p> 0.05, or for head constrained, p > 0.19.
3.6. Usefulness for screening
We examined the usefulness of this test for screening people with “dizziness”. ROC analyses with all ages, normals and patients, were weak: ROC = 0.51. When we cut the age range at 55 years and examined scores of younger subjects separately from older subjects the results were still weak: < 55 ROC = 0.49, > 55 ROC = 0.51.
4. Discussion
The finding that responses are at least somewhat age-related is not surprising, since the gain of VOR, itself, shows age-related changes [9]. Our sample size was not large enough for finer-grained analyses of age changes by decade. As the data suggest, the change by age is probably gradual across the life span. Vestibular disorders, which are more common in older people, probably exacerbate that difference.
The static condition is less challenging than the dynamic condition. In the dynamic condition normals and patients decreased their scores. Patients’ scores were worse than normals, especially in older subjects. Even in the static condition, however, patients were slightly decreased compared to normals. The decrement in the head constrained condition compared to the head free condition replicates our previous finding that constraining the head even slightly causes decreased visual acuity [3]. Although this idea may seem counterintuitive, the data from our previous work suggest that restricting head motion reduces the ability of the vestibular system to produce a compensatory VOR, or for the nervous system to use other compensatory strategies, to stabilize gaze in space. These findings have implications for driving automobiles and flying airplanes. During head motion or constraint of head motion by head rests decreased visual acuity could affect performance in off-nominal situations.
A strength of the statistical design in this study is the use of ROC analyses. The statistically significant differences between the groups might suggest that this test could be used to differentiate normals from patients. The ROC scores, however, were weak. Thus, despite statistically significant differences between the groups, this test, given under these specific conditions, would not differentiate normals from well-compensated patients with vestibular lesions.
Interestingly the difference scores, both percent change and simple difference scores, did not show significant differences between the groups. We had expected that patients with known vestibular disorders, whose diagnostic tests indicated significant decrements in VOR function, should have had obvious differences between the two conditions. They did not. This finding differs from studies in other laboratories. Our paradigm differed from those studies in some important ways. For example we used more subjects than other studies [13,14,16] and a unique passive, vertical stimulus that simulated the vertical component of head movement during gait.
The current results differ from measures of acuity while walking. Previous results indicate that younger normal subjects show no differences between acuity measured when standing and while walking [10]. Also, responses to the static and dynamic conditions in this study differed. Thus, given that the vertical motion perturbations are similar between the two studies, one reason for the difference is that walking is an actively controlled stimulus and the chair motion used in this test provided a passive stimulus. Because the chair motion did not vary over trials, some subjects might have been able to use feed-forward predictive mechanisms if they learned the chair stimulus within 1 to 2 trials. Future research will explore this idea.
In addition to differences in the optotype display time in our previous study (500 ms vs. 75 ms), different target viewing distances were used. In the walking study, no differences were observed between static and dynamic conditions for a 4 m viewing distance. A difference was present for a 0.5 m viewing distance, however, indicating that target distance influences performance. The current study used a 2 m distance.
The influence of the chair motion with vestibular receptors was probably complicated. In the head constrained condition, when little to no head rotation was involved we can assume that the stimulus was purely linear, affecting mostly saccular receptor although possibly some utricular hair cells may have been stimulated, too, as the utricular and saccular maculae are curved surfaces and the utricule may have hair cells that were responsive to the stimulus [17]. In the head free condition, stimulation to the vertical semicircular canals probably occurred, too. Since the chair did not move horizontally, little or no stimulation to the horizontal semicircular canals was involved. We cannot rule out horizontal semicircular canal stimulation because subjects might have inadvertently turned their heads slightly during testing. No doubt the main effect, however, was stimulation to the vertical canals. During the head free condition, as compared to the head constrained condition, the addition of vertical canal signals to otolith signals probably accounted for the improved performance of normals and patients.
When the vertical oscillation of the body is coupled with the pitch motion of the head, theoretically there is a crossover position in space of the head orientation at which no eye movement is required to maintain visual fixation on a target [8]. In other words, theoretically, a vector that represents the orientation of the head crosses itself, if that vector is examined from one time point to another. Therefore, no compensatory eye movements would be required to maintain visual fixation on a target placed at this crossing point. The 2 m viewing distance of visual targets in the current study was near this “head fixation distance” for walking subjects. Note that the head fixation distance is not a fixed value but fluctuates somewhat during the stride cycle (Brian Peters, PhD, unpublished data.) If the crossover position is similar for the passively perturbed subjects in the current study, the VOR might have been suppressed for normal subjects to have performed well. As a result, the conditions used in this study would not have been ideal for differentiating between performance in normals and patients who have vestibular hypofunction. A longer viewing distance might have yielded different results.
The difference score data suggest that chronic, well-compensated patients have functional gaze stabilization, using compensatory mechanisms that are as good as age-matched normals for our given test positions. This idea is supported by research in primates showing that primates compensate reasonably well for canal-plugging within 9 weeks of surgery by increasing the gain of the cervico-ocular reflex [19]. Thus, facilitation of the COR is one mechanism by which patients may have developed functional gaze skills at least 3 months post-operatively. Although this mechanism is inconsistent in humans it may have been involved [12]. Future work will focus on refining the differences between normals and well-compensated patients with vestibular impairments and modifying the test parameters to improve the strength of the test.
Acknowledgments
Supported by NIH grant R01DC009031 (HSC) and grants from the National Space Biomedical Research Institute through NASA NCC 9-58 (APM and JJB). We thank Christopher Miller and Joe Sinka, Wyle Integrated Science and Engineering Group, for the design and assembly of the chair, and the staff of the Center for Balance Disorders, Baylor College of Medicine, for invaluable technical assistance in collecting the data.
References
- 1.Baloh RW. Approach to the evaluation of the dizzy patient. Otolaryngology – Head and Neck Surgery. 1995;112:3–7. doi: 10.1016/S0194-59989570299-7. [DOI] [PubMed] [Google Scholar]
- 2.Cohen B. Erasmus Darwin’s observations on rotation and vertigo. Human Neurobiology. 1984;3:121–128. [PubMed] [Google Scholar]
- 3.Cohen HS, Bloomberg JJ. Modified dynamic visual acuity tests after acoustic neuroma resection. Acta Otolaryngologica. 2007;127:825–828. doi: 10.1080/00016480601002047. [DOI] [PubMed] [Google Scholar]
- 4.Goebel JA. The ten-minute examination of the dizzy patient. Seminars in Neurology. 2001;21:391–398. doi: 10.1055/s-2001-19410. [DOI] [PubMed] [Google Scholar]
- 5.Herdman S, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129:819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- 6.Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001;127:1205–1210. doi: 10.1001/archotol.127.10.1205. [DOI] [PubMed] [Google Scholar]
- 7.Hillman EJ, Bloomberg JJ, McDonald VP, Cohen HS. Dynamic visual acuity while walking in normals and labyrinthine deficient patients. J Vestib Res. 1999;9:49–57. [PubMed] [Google Scholar]
- 8.Moore ST, Hirasaki E, Cohen B, Raphan T. Effect of viewing distance on the generation of vertical eye movements during locomotion. Exp Brain Res. 1999;129:347–361. doi: 10.1007/s002210050903. [DOI] [PubMed] [Google Scholar]
- 9.Paige GD. Senescence of human visual-vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res. 1992;2:133–151. [PubMed] [Google Scholar]
- 10.Peters BT, Bloomberg JJ. Dynamic visual acuity using “far” and “near” targets. Acta Otolaryngol. 2005;125:353–357. doi: 10.1080/00016480410024631. [DOI] [PubMed] [Google Scholar]
- 11.Schneider AM, Hipp JA, Nguyen L, Reitman CA. Reduction in head and intervertebral motion provided by 7 contemporary cervical orthoses in 45 individuals. Spine. 2007;32:E1–E6. doi: 10.1097/01.brs.0000251019.24917.44. [DOI] [PubMed] [Google Scholar]
- 12.Schubert MC, Das V, Tusa RJ, Herdman SJ. Cervico-ocular reflex in normal subjects and patients with unilateral vestibular hypofunction. Otol Neurotol. 2004;25:65–71. doi: 10.1097/00129492-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Schubert MC, Herdman SJ, Tusa RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol. 2002;23:372–377. doi: 10.1097/00129492-200205000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Schubert MC, Migliaccio AA, Della Santina CC. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7:329–338. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian J-R, Shubayev I, Demer JL. Dynamic visual acuity during yaw rotation in normal and unilaterally vestibulopathic humans. Ann NY Acad Sci. 2001;942:501–504. doi: 10.1111/j.1749-6632.2001.tb03781.x. [DOI] [PubMed] [Google Scholar]
- 16.Tian J-R, Shubayev I, Demer JL. Dynamic visual acuity during passive and self-generated transient head rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res. 2002;142:486–495. doi: 10.1007/s00221-001-0959-7. [DOI] [PubMed] [Google Scholar]
- 17.Uzun-Coruhlu H, Curthoys IS, Jones AS. Attachment of the utricular and saccular maculae to the temporal bone. Hear Res. 2007;233:77–85. doi: 10.1016/j.heares.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Vital D, Hegemann SCA, Straumann D, Bergamin O, Bockisch CJ, Angehrn D, Schmitt KU, Probst R. A new dynamic visual acuity test to assess peripheral vestibular function. Arch Otolaryngol Head Neck Surg. 2010;136:686–691. doi: 10.1001/archoto.2010.99. [DOI] [PubMed] [Google Scholar]
- 19.Yakushin SB, Kolesnikova OV, Cohen B, Ogorodnikov DA, Suzuki JI, Della Santina CC, Minor LB, Raphan T. Complementary gain modifications of the cervico-ocular (COR) and angular vestibulo-ocular (aVOR) reflexes after canal plugging. Exp Brain Res. 2011;210:549–560. doi: 10.1007/s00221-011-2558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]