Abstract
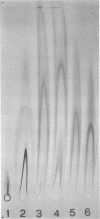
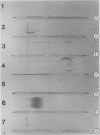
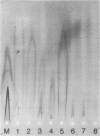
Mouse mammary tumor virus (MMTV) glycoproteins and nonglycosylated polypeptides were purified by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Primary amino groups were labeled with fluorescamine to enable visualization of MMTV polypeptides in the gels. Protein bands were sliced from the gels and eluted with 90 to 95% recovery. Eight MMTV polypeptides, including three of the major viral components as well as five minor proteins, were routinely obtained. Double diffusion assays and immunoelectrophoresis confirmed the retention of antigenicity identical to that of untreated MMTV virions. Antisera obtained from MMTV-free BALB/c mice immunized with these purified proteins reacted with the polypeptide immunogen as well as with detergent-disrupted MMTV virions from mouse milk or cell culture. Double diffusion assays using the specific mouse antisera failed to detect any cross-reactivity among the isolated polypeptides. A hemagglutination-inhibition assay demonstrated that the ability of MMTV virions to inhibit the hemagglutinating properties of influenza virus resides in the glycosylated polypeptides gp52, gp37.7, and gp33.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Compans R. W. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J Virol. 1974 Nov;14(5):1307–1309. doi: 10.1128/jvi.14.5.1307-1309.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Vaidya B., Fout G. S., Moore D. H. Isolation and characterization of RNA-directed DNA polymerase from a B-type RNA tumor virus. J Virol. 1974 Jul;14(1):40–46. doi: 10.1128/jvi.14.1.40-46.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Noon M. C., Wolford R. G., Parks W. P. Expression of mouse mammary tumor viral polypeptides in milks and tissues. J Immunol. 1975 Sep;115(3):653–658. [PubMed] [Google Scholar]
- PREER J. R., Jr A quantitative study of a technique of double diffusion in agar. J Immunol. 1956 Jul;77(1):52–60. [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Scolnick E. M., Oroszlan S., Gilden R. V. Immunochemical characterization of two major polypeptides from murine mammary tumor virus. J Virol. 1974 Jun;13(6):1200–1210. doi: 10.1128/jvi.13.6.1200-1210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi E., Baldi A., Spiegelman S. The purification of a gs antigen of the murine mammary tumor virus and its quantitation by radioimmunoassay. Virology. 1976 Nov;75(1):188–197. doi: 10.1016/0042-6822(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Robinson O. R., Jr, Shibley G. P., Sevoian M. Quantitative immunoelectrophoretic assay for murine oncornavirus p30: noncovalent facilitation by sodium dodecyl sulfate. Infect Immun. 1977 Oct;18(1):60–67. doi: 10.1128/iai.18.1.60-67.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J. B., Zacharchuk C. M., Taraschi N., Daly T. M. Effect of trypsin on mouse mammary tumor virus. J Virol. 1976 Jul;19(1):255–266. doi: 10.1128/jvi.19.1.255-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H. Precursor-product relationship between nonglycosylated polypeptides of A and B particles of mouse mammary tumor virus. Virology. 1977 Feb;76(2):835–850. doi: 10.1016/0042-6822(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Yagi M. J. Characteristics of mammary tumor cultures from four mouse strains infected with mammary tumor virus. Cancer Res. 1975 Feb;35(2):370–373. [PubMed] [Google Scholar]
- Yagi M. J., Compans R. W. Structural components of mouse mammary tumor virus. I. Polypeptides of the virion. Virology. 1977 Feb;76(2):751–766. doi: 10.1016/0042-6822(77)90256-2. [DOI] [PubMed] [Google Scholar]