Abstract
The effects of bioactive glass S53P4 or beta-tricalcium phosphate; and bone morphogenetic proteins bone morphogenetic protein-2, bone morphogenetic protein-7, or bone morphogenetic protein-2 + 7 on osteogenic differentiation of human adipose stem cells were compared in control medium, osteogenic medium, and bone morphogenetic protein-supplemented osteogenic medium to assess suitability for bone tissue engineering. Cell amount was evaluated with qDNA measurements; osteogenic differentiation using marker gene expression, alkaline phosphate activity, and angiogenic potential was measured by vascular endothelial growth factor expression. As compared to beta-tricalcium phosphate, cell amount was significantly greater for bioactive glass in control medium after 7 days and in osteogenic medium after 14 days, and alkaline phosphate activity was always significantly greater for bioactive glass in control medium. However, alkaline phosphate activity increased for beta-tricalcium phosphate and decreased for bioactive glass granules in osteogenic medium. For both biomaterials, bone morphogenetic protein supplementation decreased cell amount and osteogenic differentiation of human adipose stem cells, and vascular endothelial growth factor expressions correlated with cell amounts. Effects of culture medium on human adipose stem cells are biomaterial dependent; bioactive glass in control medium enhanced osteogenic differentiation most effectively.
Keywords: bioactive glass, beta-tricalcium phosphate, bone morphogenetic protein-2, bone morphogenetic protein-7, human adipose stem cells, osteogenic differentiation, in vitro
Introduction
The regeneration potential of normal bone is excellent due to extensive vascular supply. However, healing of large bone defects after severe trauma, reconstructive or ablative surgery is still challenging despite recent advances in technical and surgical methods.1 Over the past decades, bone grafts and different biomaterials have been employed with variable results.1 Therefore, there is still a need to enhance fracture or bone defect repair. Autologous bone grafts are still considered the gold standard for reconstructive bone surgery due to low immunogenicity, simultaneous presence of stem cells, and growth factors as well as their osteoinductive/osteoconductive properties. However, donor site morbidity and limited availability are of concern.1
Therefore, suitability of bone tissue engineering and cell-based therapies utilizing stem cells, co-incubated with biological factors, and seeded on biomaterials have recently been explored to regenerate bone.2 We and others have treated successfully patients with bone defects in craniomaxillofacial area using autologous human adipose stem cells (hASCs) in combination with biomaterials.3,4 Among the stem cells used, hASCs have gained popularity as alternative source of mesenchymal stem cells (MSCs) to human bone marrow stromal cells (hBMSCs) due to abundant availability, ease of harvesting, simple processing, and proven ability to differentiate into multiple lineages including osteoblasts.5
Human ASCs are traditionally cultured and expanded in medium supplemented with animal-derived fetal bovine serum (FBS) posing the risk for zoonotic diseases and allergic reactions. Hence, the use of FBS is one concern for its direct clinical application in humans.6 Therefore, standardized human serum (HS) has recently being investigated, and research is directed toward the development of even xeno-free culture media, produced according to good manufacturing practice.7,8 It is also possible to use autologous HS for cell expansion.3,4 Isolated hASCs have also co-incubated with osteogenic nutrients to promote osteogenic differentiation in vitro and, finally to stimulate new bone formation in vivo.3 Among the additives, the osteogenic potential of bone morphogenetic protein-2 and -7 (BMP-2 and BMP-7) on hASCs has been investigated over the last years.9 Currently, the osteoinductive growth factors BMP-2 and BMP-7 are in clinical use.3,10,11 However, controversial results were recently reported regarding their beneficial role in osteogenesis requiring further clarification.11
Implanted biomaterials serve as initial scaffolds, cell attachment bases and may induce signals for cell differentiation, and in the past, several bioactive materials were thoroughly investigated.12 Among those, bioactive glass (BAG) and beta-tricalcium phosphate (β-TCP) have been used widely in oral–maxillofacial and orthopedic surgery due to good biocompatibility and ability to support osteoblastic growth and maturation.13–15 Customized implants that contain osteogenic cells (e.g. hASCs), osteoinductive factors (e.g. BMPs) along with a synthetic osteoconductive matrix (e.g. BAG or β-TCP) represent an attractive alternative to autografts and allografts while uniting all three bone-forming properties in a more controlled and effective combination.
To our knowledge, the response of hASCs to BMP-2/7 and BAG or β-TCP after expansion in HS has not been reported yet. Therefore, the aim of the current study was to evaluate and compare the effect of (1) BAG and β-TCP and (2) BMP-2 and BMP-7 or both on osteogenic differentiation of hASCs when maintained in medium containing HS.
Materials and methods
Ethics statement, hASCs isolation, and culture
The study was conducted in accordance with the Ethics Committee of the Pirkanmaa Hospital District, Tampere, Finland (R03058), and the Declaration of Helsinki 1975, revised Hong Kong 1989. Adipose tissue samples were harvested as by-products of open surgical procedures from six patients (age = 39 ± 18 years, one male and five female patients) at the Tampere University Hospital, Finland. A written consent form was obtained from each patient before the procedure. Subsequently, hASCs were isolated and cultured as described elsewhere.5 Briefly, samples were washed with Dulbecco’s phosphate-buffered saline (DPBS) (Invitrogen, UK), minced manually into smaller pieces, digested with 1.5 mg/mL collagenase type I (Life Technologies, UK) and were incubated in a water bath at 37°C for 90 min. Subsequently, the digested tissue was centrifuged (600g, 10 min) in consecutive steps achieving sufficient segregation of hASCs from connective tissue. The supernatant was discarded, the cell pellet resuspended in 10% HS and, finally, filtered through a 100 µm strainer. Subsequently, isolated cells were maintained and expanded in polystyrene flasks (Nunc, Denmark) in control medium (CM) containing Dulbecco’s modified Eagle medium (DMEM)/Ham’s nutrient mixture F-12 (F-12 1:1; Invitrogen) that was supplemented with 1% l-glutamine (GlutaMAX; Invitrogen), 1% antibiotics (100 U/mL penicillin, 0.1 mg/mL streptomycin; Invitrogen), and 10% HS (PAA Laboratories GmbH, Austria) at 37°C and 5% CO2. The cells were passaged when the flasks approached about 80% confluency and were detached enzymatically using trypsin (TrypLE Select™, Invitrogen). Finally, expanded hASCs were cryopreserved using liquid nitrogen in a freezing solution containing 10% dimethyl sulfoxide (Hybri-Max, Sigma–Aldrich, USA) and 10% HS. Before experiments, hASCs were thawed and expanded in CM, and cell passages 1–4 were used.
Flow cytometric analysis of hASC surface marker expression
After primary culture for cell passages 1–2, hASCs were harvested and characterized by flow cytometry (FACSAria™; BD Biosciences, Belgium) as described previously.16 Monoclonal antibodies against CD14–phycoerythrin–cyanine (PECy7), CD19–PECy7, CD90–allophycocyanin (APC) (BD Biosciences), CD34–APC, HLA-DR–phycoerythrin (PE) (ImmunoTools GmbH, Germany), and CD105–PE (R&D Systems Inc., USA) were employed. Analysis was performed on 10,000 cells per sample, and unstained cell samples were used to compensate for the background autofluorescence levels.
Biomaterials and growth factors
Commercially available biomaterials accepted for clinical use were selected for the study. BAG granules (S53P4, 23% Na2O, 20% CaO, 53% SiO2, 4% P2O5, BoneAlive granules, 1.0–2.0 mm; BoneAlive Biomaterials Ltd, Finland) and β-TCP granules (ChronOS granules, 1.4–2.8 mm, porosity 60%; Synthes, Switzerland) were used in the study. Also, clinically used BMP-2 and BMP-7 (Sigma–Aldrich) were chosen as additive for the osteogenic media evaluated.
Seeding and osteogenic differentiation of hASCs on biomaterial combinations
Sterile BAG and β-TCP granules (400 µL biomaterials/well) were incubated with CM in a 24-well plate (Nunc) for 48 h before cell seeding for equilibration purposes. For osteogenic medium (OM), CM was supplemented with l-ascorbic acid-2-phosphate (50 mM), β-glycerophosphate (500 µM), and dexamethasone (10 µM) (all Sigma–Aldrich). Subsequently, individual treatment media (TM) were produced containing OM only, OM + BMP-2 (Sigma–Aldrich, dose 100 ng/mL), OM + BMP-7 (Sigma–Aldrich, dose 100 ng/mL), and OM + BMP-2 + BMP-7 (both 100 ng/mL) resulting in the following combinations: (1) BAG + CM (BAG + CM), (2) BAG + OM (BAG + OM), (3) BAG + OM + BMP-2 (BAG + BMP-2), (4) BAG + OM + BMP-7 (BAG + OM + BMP-7), (5) BAG + OM + BMP-2/7 (BAG + BMP-2/7), (6) β-TCP + CM (TCP + CM), (7) β-TCP + OM (TCP + OM), (8) β-TCP + OM + BMP-2 (TCP + BMP-2), (9) β-TCP + OM + BMP-7 (TCP + BMP-7), and (10) β-TCP + OM + BMP-2/7 (TCP + BMP-2/7) (Table 1). Dosages for BMPs were chosen based on earlier studies.17 Previously isolated hASCs were suspended with each individual treatment medium to initiate osteogenic differentiation and, finally, were seeded on both biomaterials (50,000 cells/well) as described earlier.17 Seeded grafts were maintained in culture at 37.5°C and 5% CO2 changing individual media every 48 h until final analyses.
Table 1.
Overview on experimental design—group assignments
| BAGa + CMb | BAG + OMc | BAG + BMP-2d | BAG + BMP-7e | BAG + BMP-2/7f |
| TCPg + CM | TCP + OM | TCP + BMP-2 | TCP + BMP-7 | TCP + BMP-2/7 |
BAG: bioactive glass; CM: control medium; OM: osteogenic medium; BMP: bone morphogenetic protein; TCP: tricalcium phosphate; β-TCP: beta-tricalcium phosphate.
Bioactive glass granules (BAG group).
Control medium.
Osteogenic medium.
Bone morphogenic protein 2 (BMP-2 medium).
Bone morphogenic protein 7 (BMP-7 medium).
Combination of bone morphogenic protein 2 and 7 (BMP-2/7 medium).
Beta-tricalcium phosphate granules (β-TCP group).
Cell amount
After 1, 7, and 14 days in culture, the amount of hASCs was evaluated quantitatively using CyQUANT®, Cell Proliferation Assay Kit (Molecular Probes, Invitrogen) that measured DNA amounts in samples as described elsewhere.8 Briefly, all cells were lysed using 0.1% Triton-X 100 buffer (Sigma–Aldrich), and the supernatant was collected and stored at −80°C until final analyses. A volume of 20 µL of each sample were mixed with CyQUANT GR dye and lysis buffer in a 96-well plate (Nunc) after a freeze–thaw cycle. Fluorescence signals were measured with a multiple plate reader (Victor 1420 Multilabel Counter; Wallac, Finland) at 480 or 520 nm.
Alkaline phosphatase staining and quantitative alkaline phosphatase analyses
The osteogenic differentiation of isolated hASCs was determined qualitatively by alkaline phosphatase (ALP) staining and quantitatively by ALP measurements. Subsequently, after 1, 7, and 14 days in culture, all samples were stained using a leukocyte ALP kit (Sigma–Aldrich, #86R-1KT). Therefore, cell cultures were washed twice using DPBS and fixed in 4% paraformaldehyde. ALP staining solution was pipetted into each well, incubated for 15 min, washed in deionized water, and finally evaluated macroscopically.
After 1, 7, and 14 days in culture, quantitative alkaline phosphatase (qALP) activities were measured photometrically according to Sigma ALP (Sigma–Aldrich) at 405 nm (Victor 1420). The ALP activities were measured from the same Triton-X 100 lysates as used for the cell numbers.
Quantitative real-time reverse transcription polymerase chain reaction to measure early markers of osteogenic differentiation
The mRNA expression levels of early markers in osteogenesis including osteopontin (OPN), runt-related transcription factor 2 (RUNX-2), collagen type-1 (Col-1), and osteocalcin (OC) were measured 14 days after cell seeding and incubation using quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). The expression of RPLP0 was used to normalize expression levels between samples. Therefore, total RNA was isolated from hASCs using TRIzol reagent (Invitrogen) following the manufacturer’s guidelines. Sequences and accession numbers of all primers (Oligomer Oy, Finland) are displayed in Table 2. All reactions were performed using ABI Prism 7300 Sequence Detection Systems (Applied Biosystems, UK), and the relative gene expression for each individual marker was calculated according to a mathematical model.
Table 2.
Overview on sequences of osteogenic marker genes determined
| Name | Primer direction | Sequences | Product size (bp) |
|---|---|---|---|
| hRPLP0a | Forward | 5′-AAT CTC CAG GGG CAC CAT T-3′ | 70 |
| Reverse | 5′-CGC TGG CTC CCA CTT TGT-3′ | ||
| hCOL1b | Forward | 5′-CCA GAA GAA CTG GTA CAT CAG CAA-3′ | 94 |
| Reverse | 5′-CGC CAT ACT CGA ACT GGA ATC-3′ | ||
| hOCc | Forward | 5′-AGC AAA GGT GCA GCC TTT GT-3′ | 94 |
| Reverse | 5′-GCG CCT GGG TCT CTT CAC T-3′ | ||
| hOPNd | Forward | 5′-GCC GAC CAA GGA AAA CTC ACT-3′ | 71 |
| Reverse | 5′-GGC ACA GGT GAT GCC TAG GA-3′ | ||
| hRUNX2e | Forward | CCCGTGGCCTTCAAGGT | 76 |
| Reverse | CGTTACCCGCCATGACAGTA |
Expression of vascular endothelial growth factor
At days 1, 7, and 14, vascular endothelial growth factor (VEGF) was measured using a human VEGF immunoassay (R&D Systems, UK) according to the manufacturer’s instructions. Briefly, 50 µL of assay diluent and 200 µL cell culture sample supernate were added into each well and incubated for 2 h at room temperature (RT). Subsequently, all samples were aspirated and washed for three times before 200 µL of VEGF conjugate was pipetted into each well and incubated for 2 h at RT. The previous aspiration-washing cycle was repeated followed by addition of 200 µL substrate solution and incubated for 20 min at RT. Finally, the reaction was terminated with 50 µL of stop solution, and optical density of each well was determined using a microplate reader set at a wave length of 450 nm.
Statistical analyses
Quantitative analyses of cell amounts and qALP were run in duplicates per experiment, and experiments were repeated five times. Quantitative measurements of OPN, RUNX-2, Col-1, OC, and VEGF were run in duplicates per experiment, and experiments were repeated three times. All data were presented as mean ± standard deviation (SD). A one-way analysis of variance with the Bonferroni post hoc test for multiple comparisons was used to study statistically significant differences between study groups. The nonparametric Spearman correlation test was used to study correlation between DNA amounts, expression of VEGF, and ALP activity. Values of p < 0.05 were regarded as significant. All graphs and statistics were done using GraphPad Prism 5.01 software.
Results
Cell surface maker profile of hASC in medium containing 10% HS
Human ASCs were isolated and expanded using cell culture medium containing 10% HS. After expansion, cell surface marker expression profile of hASCs was analyzed by flow cytometry. Human ASCs showed positive expression (>70%) for the surface markers CD73 (Ecto-5′-nucleotidase), CD90 (Thy-1), and CD105 (Endoglin) and lacked (<2%) the expression of CD14, CD19, and HLA-DR. Furthermore, moderate expression was recorded for the hematopoietic progenitor marker CD34. Cytometric analyses confirmed the characteristic immunophenotype of hASCs, which was similar to earlier reports.16
Cell amounts in BAG were greater than in β-TCP in OM and CM
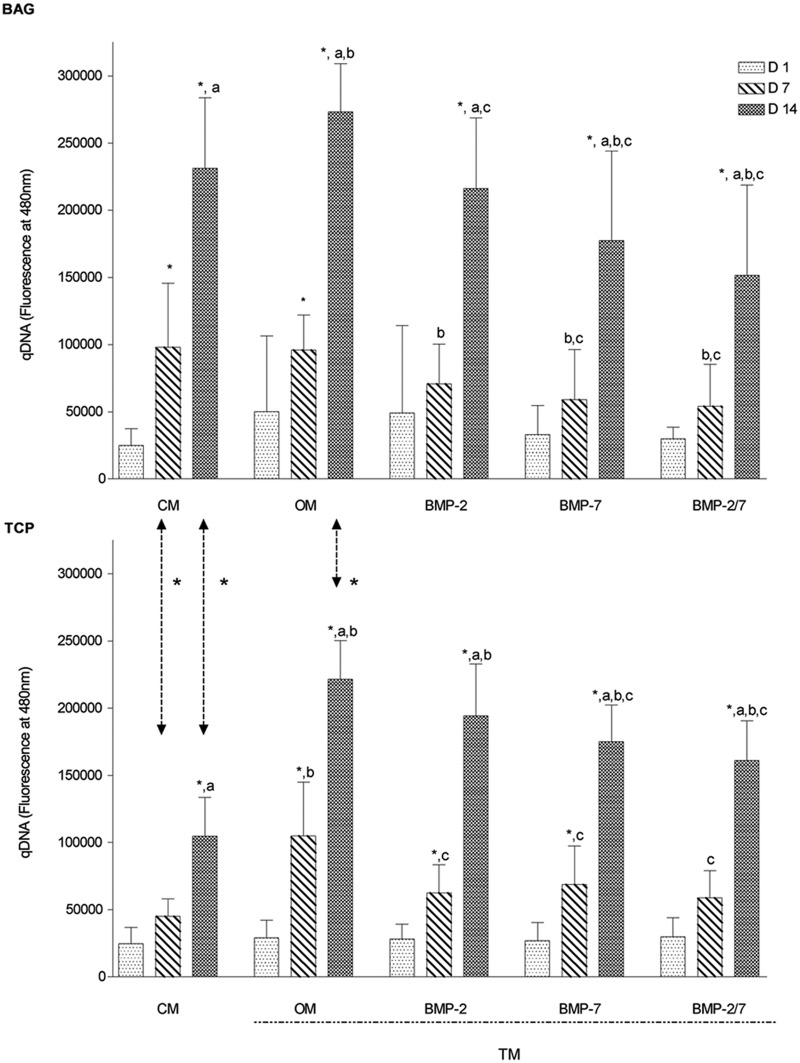
Quantitatively, the DNA amount was analyzed as an indicator of cell amounts at days 1, 7, and 14. Results of both biomaterials, when maintained under the same culture conditions, were compared to each other. In the BAG group, cell number increased continuously and significantly in CM and TM (p < 0.0001). In the TCP group, however, cell amount increased similarly in OM, BMP-2, and BMP-7 medium (Figure 1). DNA amount was significantly greater in the BAG group in CM at days 7 and 14 (p < 0.001) and in OM at day 14 (p < 0.0001) as compared to the β-TCP group, suggesting a greater cell proliferation rate in response to BAG (Figure 1).
Figure 1.
Comparative overview on cell amount over time (N = 10 replicates per time point). Overall, cell amount increased continuously over time independent on biomaterial evaluated. Cell amount was greatest after exposure to BAG in OM. After 7 days, BAG increased cell amount significantly more in CM as compared to β-TCP. Generally, BMP supplementation decreased cell amount independent on biomaterial.
BAG: bioactive glass; β-TCP: beta-tricalcium phosphate; CM: control medium; OM: osteogenic medium; BMP: bone morphogenetic protein; TM: treatment media.
The symbol “*” indicates significant difference between days 1–7 and days 7–14; “a” indicates significant difference between days 1 and 14; “b” indicates significant difference as compared to CM; “c” indicates significant difference as compared to OM; arrows represent significant differences between biomaterials.
BMP-2- and BMP-7-supplemented media decrease cell amount regardless of biomaterial
For the BAG and β-TCP group individually, results of CM and TM were compared among each other to evaluate the effects of different media on cell amount. In the BAG group, when compared to the CM, significantly lower qDNA amounts were measured in BMP-2 medium at day 7 as well as in BMP-7 and BMP-2/7 medium at days 7 and 14 (p < 0.03), but significantly higher qDNA levels were measured in OM at day 14 (p < 0.01) (Figure 1). When compared to OM, cell amount was significantly lower in all BMP media at days 7 and 14 (p < 0.03) except for BMP-2 at day 7, suggesting a negative impact of BMP supplementation on cell amount in BAG (Figure 1). In the β-TCP group, when compared to CM, overall qDNA amounts were significantly higher in OM at days 7 and 14 and in BMP-supplemented media at day 14 (p < 0.05) suggesting enhanced cell proliferation. When compared to OM, cell amount was significantly lower in BMP-2 media at day 7 as well as in BMP-7 and BMP-2/7 at days 7 and 14 (p < 0.03). Overall, BMP supplementation of TM resulted in reduced cell amounts as compared to OM independent of biomaterial (Figure 1).
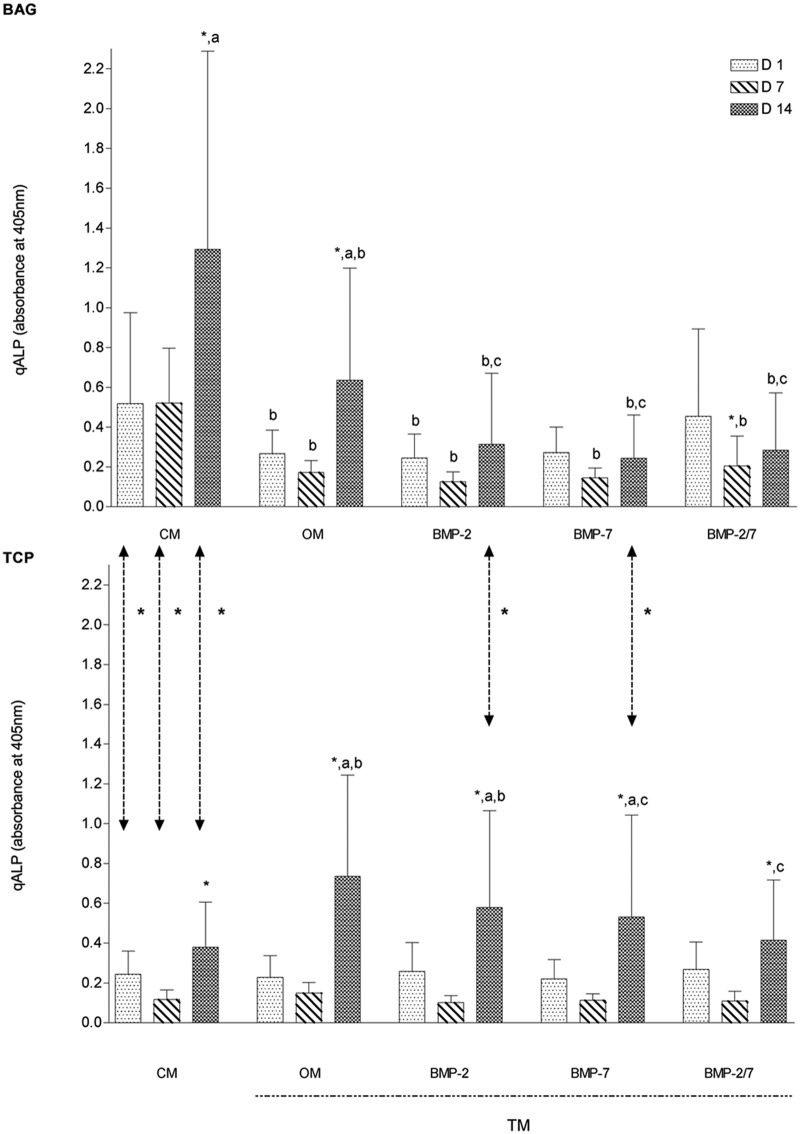
ALP activity in response to CM and OM is differently regulated in hASCs cultured with BAG and β-TCP
Qualitatively, osteogenic differentiation of hASCs was determined by ALP staining and quantitatively by ALP analyses. Results of BAG and β-TCP groups, when maintained in the same media, were compared to evaluate the effects of each biomaterial on osteogenic differentiation. Overall, our qALP measurements were consistent with the ALP staining results.
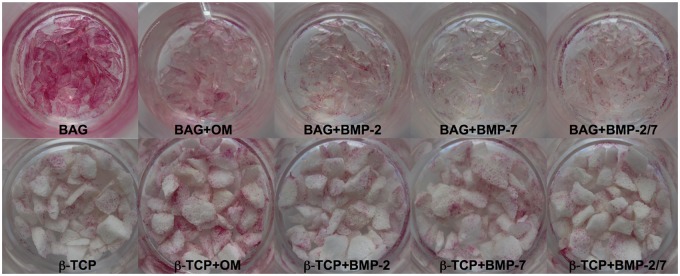
ALP staining indicated osteogenic differentiation of hASCs in BAG and β-TCP groups, but both biomaterials influenced this process differently. BAG exerted the most beneficial effect on differentiation when maintained in CM only (Figure 2), and ALP activity was decreased when OM was used. On the contrary, hASCs cultured with β-TCP required OM for osteogenic differentiation (Figure 2). Although BMP supplementation demonstrated no qualitative enhancing effect on osteogenic differentiation independent of biomaterial, a significantly lower ALP activity was quantitatively measured in BAG group in BMP-2 and BMP-7 medium at day 14 (p < 0.05) when compared to β-TCP group (Figure 3). The superior osteogenic potential of BAG when maintained in plain CM was confirmed by significantly greater qALP activities at days 1, 7, and 14 (p < 0.001) (Figure 3).
Figure 2.
Alkaline phosphatase (ALP) staining at day 14 (N = 5 replicates per group). BAG stimulated osteogenic differentiation in CM most, whereas β-TCP exerted the greatest effects in OM. An increase in color intensity correlates with increased ALP synthesis.
BAG: bioactive glass; CM: control medium; β-TCP: beta-tricalcium phosphate; OM: osteogenic medium; BMP: bone morphogenetic protein.
Figure 3.
Comparative overview on quantitative ALP measurements over time (N = 10 replicates per time point). Human ASCs responded to both biomaterials differently. Bioactive glass promoted greatest ALP gene expression in CM after 14 days whereas β-TCP provoked highest ALP expressions in OM. BMPs decreased ALP activities independent on biomaterial evaluated. Overall, the results were supported by ALP staining.
ALP: alkaline phosphatase; ASC: adipose stem cell; CM: control medium; OM: osteogenic medium; β-TCP: beta-tricalcium phosphate; BMP: bone morphogenetic protein; TM: treatment media.
The symbol ‘*’ indicates significant difference between days 1–7 and days 7–14; “a” indicates significant difference between days 1 and 14; “b” indicates significant difference when compared to CM; “c” significant different when compared to OM; arrows represent significant differences between biomaterials.
BMP-2- and BMP-7-supplemented media decrease ALP activity regardless of biomaterial
Both biomaterials showed an initial reduction in qALP activity from days 1 to 7 that did not correspond with qDNA measurements, followed by an increase of qALP from days 7 to 14 (Figures 1 and 3). We did not find statistically significant correlation between cell amounts and qALP for either biomaterial. Specifically, in the BAG group, an initial decrease in qALP activity was measured on day 7 followed by an increase on day 14 in all TMs. This initial activity decline was significant for OM, BMP-2/7 medium only (p < 0.03), whereas the subsequent rise from days 7 to 14 was significant for CM and OM (p < 0.02). The overall increase from days 1 to 14 was significantly greater in CM and OM only (p < 0.04). When compared to CM, significantly lower activities were measured at day 1 in OM and BMP-2 medium only, but for all TMs at days 7 and 14 (p < 0.05), also suggesting an overall stimulatory effect of plain CM on early osteogenic differentiation in BAG group. When compared to OM, significantly lower qALP activities were measured in all BMP media at day 14 (p < 0.03), which was consistent with ALP staining and was suggestive of an inhibitory effect of BMPs on osteogenic differentiation (Figures 2 and 3). Similarly in the β-TCP group, an initial nonsignificant drop in qALP activity was measured on day 7 followed by a significant rise from days 7 to 14 in CM and all TMs (p < 0.01). The overall increase from days 1 to 14 was significantly greater in OM, BMP-2, and BMP-7 medium (p < 0.05). When compared to the CM, expressions were significantly higher in OM and BMP-2 medium at day 14 (p < 0.01), which was in alignment with our ALP staining for OM (Figures 2 and 3). When compared to OM, significantly lower ALP expressions were measured in BMP-7 and BMP-2/7 medium at day 14 (p < 0.02), suggesting an inhibitory effect of both BMPs in early osteogenic differentiation (Figure 3). Qualitatively, ALP staining was consistent with our qDNA measurements regarding the inhibitory effect of BMP supplementation to TM in both biomaterials.
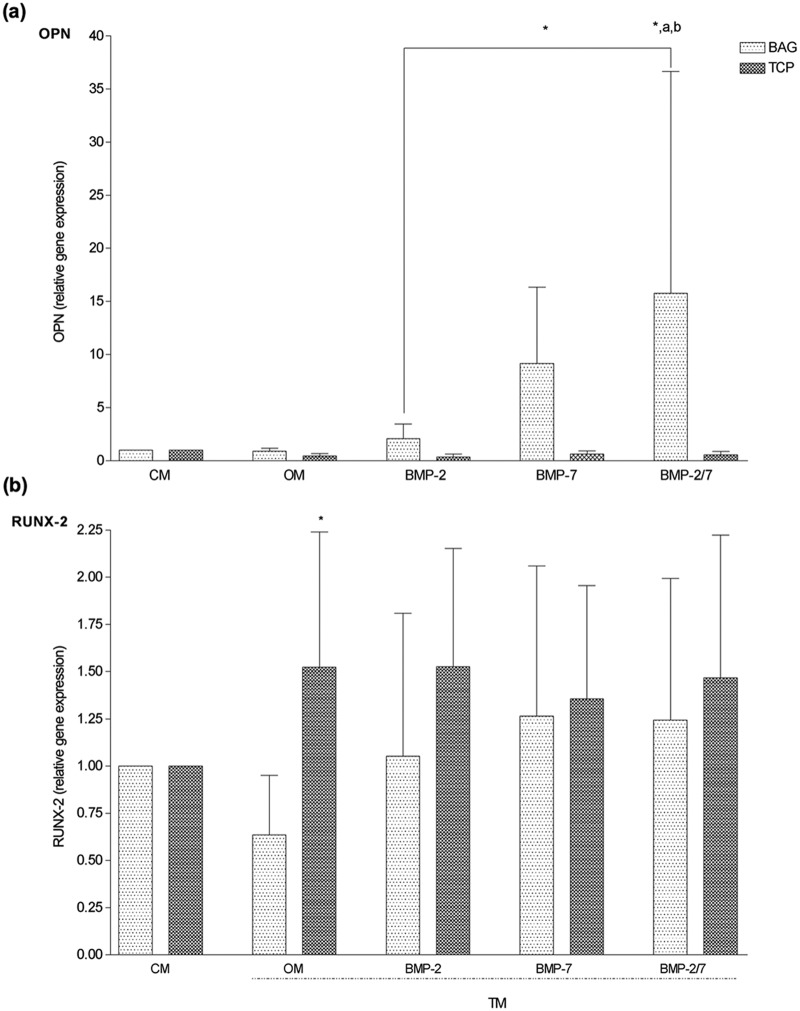
BMP-2/7-supplemented media induces expression of OPN in hASCs cultured on BAG but not on β-TCP
To further analyze the influence of biomaterials and growth factors on osteogenic differentiation of hASCs, the expression of osteogenic marker genes OPN, RUNX-2, Col-1, and OC was measured at day 14.
In the BAG group, mRNA expression of OPN was significantly higher in the BMP-2/7 medium as compared to CM, OM, and BMP-2 medium (p < 0.03) (Figure 4(a)), which was also in alignment with ALP activities recorded for BAG in CM and OM at day 14. Surprisingly, this effect was not seen in the β-TCP group, and mRNA expressions of OPN among TMs were not different. However, OPN gene expression in the BAG group was significantly stronger in BMP-2/7 medium (p < 0.01) as compared to the β-TCP group (Figure 4(a)).
Figure 4.
Comparative overview on gene expression of OPN and RUNX-2 (N = 6 replicates per time point). (a) OPN gene expression was significantly upregulated by BAG in BMP-2/7 medium as compared to β-TCP. Furthermore, BMP-2/7 induced significantly higher OPN expression as compared to BMP-2 in BAG group, suggesting a permissive effect on early osteogenesis. (b) RUNX-2 gene expression was significantly upregulated by β-TCP in OM as compared to BAG. However, additional BMP supplementation failed to enhance the expression. These findings were in alignment with ALP staining.
OPN: osteopontin; RUNX-2: runt-related transcription factor 2; BAG: bioactive glass; CM: control medium; OM: osteogenic medium; BMP: bone morphogenetic protein; β-TCP: beta-tricalcium phosphate; ALP: alkaline phosphatase; TM: treatment media.
The symbol “*” indicates significant difference between biomaterials; “a” indicates significant different as compared to CM; “b” indicates significant different as compared to OM.
In the BAG group and in alignment with the ALP activity, Col-1 expressions were significantly lower in TM as compared to CM (p < 0.01) (Figure 5(a)).
Figure 5.
Comparative overview on gene expression of Col-1 and OC (N = 6 replicates per time point). (a) Our data revealed significantly lower marker expression for Col-1 in all TMs in the BAG group as compared to CM and was in accordance with ALP stainings. However, β-TCP downregulated Col-1 expression in BMP-7 and BMP-2/7 media only as compared to CM. (b) The gene expression for OC was neither different between biomaterials nor among media evaluated.
Col-1: collagen type-1; OC: osteocalcin; CM: control medium; ALP: alkaline phosphatase; β-TCP: beta-tricalcium phosphate; OM: osteogenic medium; BMP: bone morphogenetic protein; TM: treatment media.
“a” indicates significant different as compared to CM.
In the β-TCP group, mRNA expressions for Col-1 were not different among TMs, which was also confirmed by ALP activity measurements. However, Col-1 expressions were significantly lower in the BMP-7 and BMP-2/7 medium when compared to CM (p < 0.009) (Figure 5(a)).
The mRNA expression for RUNX-2 was significantly lower in OM in the BAG group (p < 0.01) than in the β-TCP group (Figure 4(b)), which was in alignment with ALP staining (Figure 2). However, the expression was not regulated by OM or growth factors when compared in the same biomaterial group. Similar expressions of OC were measured in BAG and β-TCP groups in CM and TM. When compared within biomaterial groups, the expression was neither different between CM and TM nor among TMs (Figure 5(b)).
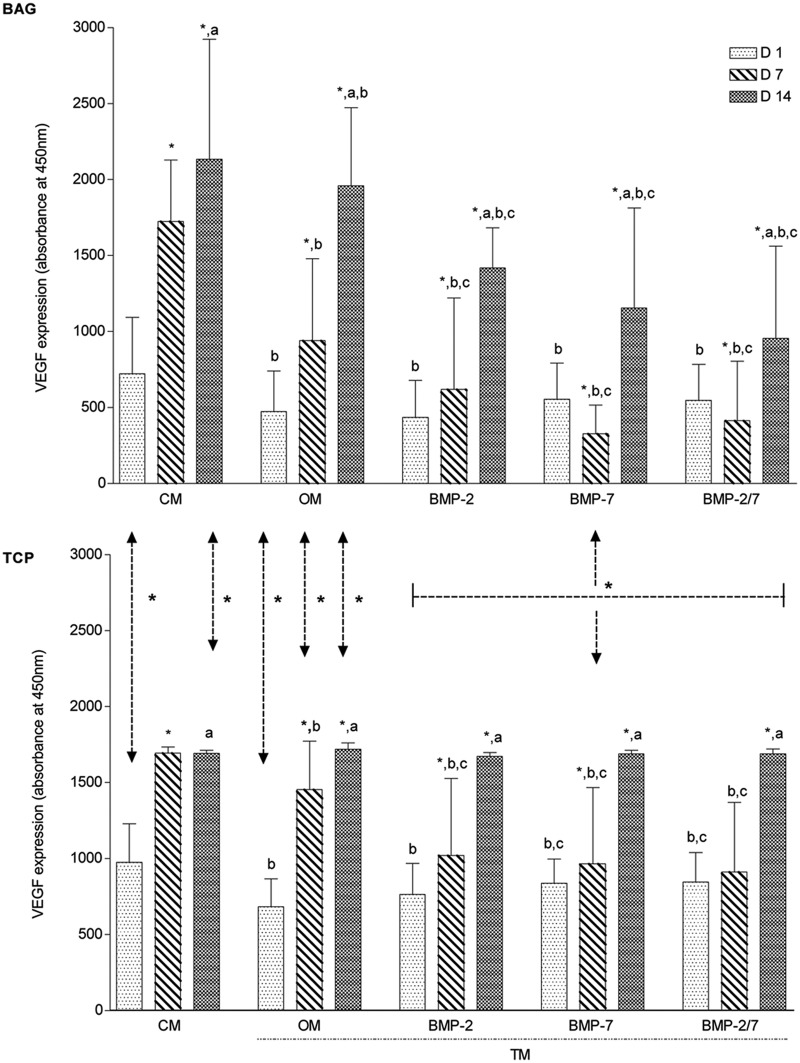
VEGF expression correlated the cell amounts with both biomaterials
The effect of the biomaterial on the angiogenic potential of hASCs was determined by VEGF analyses. The expression of VEGF correlated with the cell amounts in both biomaterials. The Spearman correlation coefficient was 0.75 (p = 0.0012) and 0.56 (p = 0.031) for BAG and β-TCP, respectively.
The results of BAG and β-TCP group were compared to evaluate the effects of each biomaterial when maintained in the same media. In the BAG group, VEGF expression was significantly lower in CM and TM as compared to β-TCP group except for CM at days 7 and 14 as well as in OM at day 14 (p < 0.04). This observation is suggestive for an overall positive effect of β-TCP in combination with BMPs on angiogenic potential when compared to BAG granules (Figure 6).
Figure 6.
Comparative overview on VEGF measurements over time (N = 6 replicates per time point). Overall, a superior effect of β-TCP on early angiogenic potential was detected in BMP-supplemented media as compared to BAG. Supplementation with BMPs decreased early angiogenic potential of BAG. However, BAG had an increased potential in CM as compared to β-TCP.
VEGF: vascular endothelial growth factor; β-TCP: beta-tricalcium phosphate; CM: control medium; OM: osteogenic medium; BMP: bone morphogenetic protein; TM: treatment media.
The symbol “*” indicates significant difference between days 1–7 and days 7–14; “a” indicates significant difference between days 1 and 14; “b” indicates significant difference when compared to CM; “c” indicates significant difference when compared to OM; arrows represent significant differences between biomaterials.
For each biomaterial individually, the results of CM and TM were compared to determine the influence of culture media on angiogenic potential of hASCs. In both biomaterial groups, VEGF levels rose continuously over time and reached highest levels after 14 days in CM and TM (Figure 6). In the BAG group, a continuously significant increase was observed from days 1 to 14 in CM and all TMs (p < 0.002). When compared to CM, VEGF levels were significantly lower in all TMs at all time points (p < 0.04). When compared to OM, significantly reduced VEGF levels were measured in all BMP media at days 7 and 14 (p < 0.04) (Figure 6). In the β-TCP group, the gradual increase of VEGF expression was significant in CM, OM, BMP-2, and BMP-7 media from days 1 to 7 (p < 0.0007), in all TMs from days 7 to 14 (p < 0.01) and in CM and all TMs from days 1 to 14 (p < 0.0005). When compared to CM, VEGF levels were significantly lower in all TMs at days 1 and 7 (p < 0.04). When compared to OM, significantly decreased VEGF levels were measured in BMP-2 medium at day 7 and in BMP-7 and BMP-2/7 at days 1 and 7 (p < 0.03).
Discussion
To our knowledge, our study is the first report evaluating the effect of BAG and β-TCP on osteogenic differentiation of hASCs in HS-supplemented medium. The effects of CM, OM, and growth factor–supplemented (BMP-2, BMP-7, or BMP-2/7) OM on osteogenic differentiation of hASCs on BAG, and β-TCP granules were investigated concurrently.
In the current study, hASCs were used as a stem cell source for bone tissue engineering since they are readily available and show similar osteogenic differentiation potential as compared to hBMSCs.18
In the past, FBS has been used for expansion and differentiation of stem cells but recently, safety concerns have arisen regarding the use of this animal-derived product for clinical application in human medicine. Therefore, alternatives including HS have been investigated.7,16 In theory, substitution of FBS by HS would overcome safety risks and, therefore, peaked interest in orthopedic research.19 Recently, the supportive effect of pooled HS on proliferation of hASCs was demonstrated,7 but the impact of HS on osteogenic differentiation when combined with biomaterials remained to be determined. Therefore, in the current study, hASCs cultured in HS-supplemented medium, and clinically used biomaterials and growth factors were investigated to meet current safety standards while mimicking a feasible clinical application.
The cell amounts were increasing over time with both biomaterials suggesting that HS-supplemented medium in combinations with BAG and β-TCP can provide sufficient amounts of attachment factors for hASCs. The positive effect of BAG and β-TCP on MSC proliferation has been reported previously.13,20–22 Our results were in accordance with these studies; however, BAG appeared to have a superior effect on hASC mount as compared to β-TCP when maintained in CM and OM. The surface roughness and porosity differ between BAG and β-TCP materials used in our study. However, the obtained DNA amount was similar with both biomaterials on day 1 in all groups, suggesting similar amounts of attached cells at the beginning of the experiments.
The effect of BAG and β-TCP on osteogenic differentiation of hBMSCs and hASCs has been previously studied.20,23,24 Similarly to these reports, both biomaterials supported osteogenic differentiation in the current study as indicated by ALP activity. The most enhancing effect of BAG on differentiation was measured in CM, whereas the use of OM decreased ALP activity. In contrast to BAG, osteogenic induction of hASCs was greatest in OM when cultured with β-TCP.
Interestingly but consistent for both biomaterials independent on culture medium, a discrepancy between cell proliferation and osteogenic differentiation was noticed at day 7. Although cells steadily proliferated, concurrent osteogenic differentiation was not observed within the first seven days as indicated by decreasing qALP activities. This was in contrast with a previous report, in which ALP activity increased consistently over 14 days followed by a decrease after 21 days and requires further investigation.20
Currently, BMP-2 and BMP-7 are in clinical use since their osteoinductive effect has been demonstrated in various studies.10,11 Due to their clinical approval, their potential use for other applications in human medicine is appealing and has resulted in further research.
In our current study, BMP supplementation decreased cell amounts regardless of the used biomaterial. In contrast to our findings, the beneficial effects of BMP-2 and BMP-7 on proliferation of hBMSCs have been demonstrated.25 However, consistent with our findings, the negative impact of BMP-2 on cell growth rates was recently demonstrated in primary immortalized human fetal and primary human osteoblasts.26 Additionally, higher apoptosis rates were recorded after exposure to BMP-2 and BMP-7.26 These observations may suggest decreased cell growth via apoptosis after BMP supplementation but remains to be determined.
Although the supportive effect of BMP-2 and BMP-7 on the osteogenic differentiation of MSC has been well documented in vivo,27,28 conflicting results have been obtained in vitro. Our in vitro findings were suggestive for a negative impact of both growth factors on osteogenic differentiation of hASCs. Over time, decrease in ALP activities was observed in BMP-supplemented OM independent on biomaterial evaluated. Recently,17 it was suggested that the canonical BMP-2 signaling pathway may not be functional in hASCs, and thus, their osteogenic differentiation may not be influenced by BMP-2 even in vivo.29 However, those results were in contrast with a recent study, in which rat bone marrow stromal cells (BMSCs) differentiated in the presence of BMP-2 and BAG into more rounded osteoblast-like cells resulting in greater ALP expression.28 Similarly, when β-TCP containing collagen sponges were used as carrier for rat BMSCs and BMP-2, an enhanced osteogenic differentiation was confirmed by increased ALP and OC gene expression and histology.27
The negative effect on differentiation may also originate in the autoregulatory negative feedback loop of BMP preventing excessive stimulation. BMPs exert their effects via receptors that activate Smad-dependent and Smad-independent mechanisms resulting in linear expression of target genes including early markers of osteogenesis such as OC, ALP, or Col-1.30 Several factors may be involved in this negative feedback loop including negative nonsignaling pseudoreceptors (BAMBI), inhibitory Smads (Smad 6 or 7), Smad-binding proteins (Ski and Tob), or degradation of Smads and extracellular BMP antagonists.31 Among those, continuous exposure to BMP may have resulted in Smad 6 or 7 and/or Ski accumulation operating the negative feedback loop. This assumption is supported by a recent study in which no BMP-induced nuclear translocation of Smad 1/Smad 4 complexes was observed, suggesting a nonfunctional BMP-2 signaling pathway in hASCs.17 However, a consistent downregulation of all osteogenic markers should have been observed. The exact timing after BMP stimulation of each individual marker remains to be determined.
The discrepancy between our results and previous observations may also originate in the BMP delivery method. In general, growth factors can be delivered exogenously via frequent media changes or endogenously via gene therapy. The effect of BMP-2 and BMP-7 on hASCs was investigated using frequent medium changes in the current study. The osteogenic differentiation of BMP-2 transfected rat BMSCs cultured with β-TCP scaffolds were recently investigated in vivo. Contrary to our findings, mRNA expression of OPN and OC was significantly increased, and ALP staining was more intensive.21 In another study, MSCs were co-transfected with BMP genes, resulting in endogenous BMP synthesis. A prolonged effect for weeks was achieved after transfection.32 These data may suggest that gene therapy is a more efficient method in supporting osteogenic differentiation of MSCs than regular media changes. Those contradictory results highlight the importance of delivery systems for BMPs and call for additional investigations. Interestingly, in our study, OPN was the only marker of which expression was increased by BMPs but this was only detected in BAG and requires further investigations.
Formation of vasculature is essential for bone formation. In the current study, VEGF was measured since its validity as early marker for angiogenic potential is well documented and the VEGF secretion is considered as a permissive factor for bone formation.33 In our study, both biomaterials consistently supported VEGF expression in all samples. This is in accordance with recent studies, in which the beneficial effects of BAG and β-TCP on VEGF secretion were reported.34,35 Our results suggest a significantly better effect of β-TCP on the VEGF secretion of hASCs in BMP-supplemented OM when compared to BAG at all times. These findings are supported by a previous study, in which the efficacy of MSCs seeded on β-TCP scaffolds was investigated in rats.36 An upregulation of VEGF mRNA and protein levels was measured over time, which also resulted in greater bone formation in vivo. Potentially, β-TCP granules in BMP-2- and BMP-7-supplemented OM may support the angiogenic potential of hASCs, whereas BAG under similar culture conditions may even decrease the VEGF expression but this requires further clarification.
Although the positive effects of both BMPs on angiogenesis have been well reported using different cell lines, media, and biomaterials,37 this is the first in vitro study investigating the VEGF secretion of hASCs when exposed to BAG or β-TCP and supplemented with BMPs. Contrary to those well-known supportive effects, BMP-2 and BMP-7 supplementation of BAG decreased the angiogenic potential of hASCs in our study.
The VEGF secretion solely followed the pattern of cell amount. Interestingly, a recent study demonstrated an inhibitory effect of BMPs on cell proliferation and angiogenesis. Recently, the negative effect of BMP-2 on oral squamous cell carcinoma proliferation and angiogenesis was demonstrated.38 Also, the interaction of VEGF and BMP-2 was investigated in BMSC in vitro and in vivo. Those coculture experiments revealed VEGF as a potent inhibitor of BMP-2 expression and, thus, may inhibit osteogenesis.39 Similarly, in our study, inhibition of early osteogenesis may have been indicated by reduced qALP activity secondary to VEGF expression. These findings call for studies elucidating the exact role of BMP-2, BMP-7, and VEGF on the angiogenic and osteogenic potentials of hASCs.
In conclusion, hASCs can be successfully isolated and expanded in HS but the response differs on BAG S53P4 and β-TCP granules. In the current study, BAG S53P4 stimulated osteogenic differentiation of hASCs in CM, whereas β-TCP required OM for osteogenic induction. Overall, supplementation of OM with BMP-2 and BMP-7 decreased early osteogenic differentiation independent on biomaterial evaluated. Based on our measurements, a combination of BAG S53P4 and CM may be an applicable way in enhancing proliferation and osteogenic differentiation of hASCs while minimizing safety and regulatory concerns in bone tissue engineering. Further studies elucidating the individual role of growth factors and biomaterials are required to design an effective bone tissue engineered implant suitable for clinical use. This study is a preliminary step forward in achieving this goal.
Acknowledgments
The authors thank Anna-Maija Honkala und Laura Tirkkonen for technical assistance.
Footnotes
Funding: The work was supported by TEKES, the Finnish Funding Agency for Technology and Innovation as well as the competitive research funding of the Pirkanmaa Hospital District (9M058, 9N042), Tampere, Finland.
Conflict of interest: The authors declare that there are no conflicting interests.
References
- 1. De Long WG, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am 2007; 89(3): 649–658 [DOI] [PubMed] [Google Scholar]
- 2. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000; 21(24): 2529–2543 [DOI] [PubMed] [Google Scholar]
- 3. Mesimäki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 2009; 38(3): 201–209 [DOI] [PubMed] [Google Scholar]
- 4. Thesleff T, Lehtimaki K, Niskakangas T, et al. Cranioplasty with adipose-derived stem cells and biomaterial. A novel method for cranial reconstruction. Neurosurgery 2011; 68(6): 1535–1540 [DOI] [PubMed] [Google Scholar]
- 5. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7(2): 211–228 [DOI] [PubMed] [Google Scholar]
- 6. Tekkatte C, Gunasingh GP, Cherian KM, et al. “Humanized” stem cell culture techniques: the animal serum controversy. Stem Cells Int 2011; 504723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kocaoemer A, Kern S, Kluter H, et al. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25(5): 1270–1278 [DOI] [PubMed] [Google Scholar]
- 8. Lindroos B, Boucher S, Chase L, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 2009; 11(7): 958–972 [DOI] [PubMed] [Google Scholar]
- 9. Kim M, Choe S. BMPs and their clinical potentials. BMB Rep 2011; 44(10): 619–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haid RW, Branch CL, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 2004; 4(5): 527–538; discussion 538–539. [DOI] [PubMed] [Google Scholar]
- 11. Giannoudis PV, Tzioupis C. Clinical applications of BMP-7: the UK perspective. Injury 2005; 36(Suppl. 3): S47–S50 [DOI] [PubMed] [Google Scholar]
- 12. Jones JR, Hench LL. Regeneration of trabecular bone using porous ceramics. Curr Opin Solid State Mater Sci 2003; 7(4–5): 301–307 [Google Scholar]
- 13. Hench LL. Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res 1998; 41(4): 511–518 [DOI] [PubMed] [Google Scholar]
- 14. Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed 2004; 15(4): 543–562 [DOI] [PubMed] [Google Scholar]
- 15. Knop C, Sitte I, Canto F, et al. Successful posterior interlaminar fusion at the thoracic spine by sole use of beta-tricalcium phosphate. Arch Orthop Trauma Surg 2006; 126(3): 204–210 [DOI] [PubMed] [Google Scholar]
- 16. Lindroos B, Aho KL, Kuokkanen H, et al. Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Eng Part A 2010; 16(7): 2281–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuk P, Chou YF, Mussano F, et al. Adipose-derived stem cells and BMP2: part 2. BMP2 may not influence the osteogenic fate of human adipose-derived stem cells. Connect Tissue Res 2011; 52(2): 119–132 [DOI] [PubMed] [Google Scholar]
- 18. Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 2011; 7(2): 269–291 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T, Watanabe H, Yanagawa T, et al. Motility and growth of human bone-marrow mesenchymal stem cells during ex vivo expansion in autologous serum. J Bone Joint Surg Br 2005; 87(10): 1426–1433 [DOI] [PubMed] [Google Scholar]
- 20. Gomide VS, Zonari AAC, Breyner NM, et al. Attachment and proliferation of human-adipose-tissue-derived stem cells on bioactive glass/PVA hybrid scaffolds. ISRN Mater Sci 2011; 240864 [Google Scholar]
- 21. Zhao J, Hu J, Wang S, et al. Combination of beta-TCP and BMP-2 gene-modified bMSCs to heal critical size mandibular defects in rats. Oral Dis 2010; 16(1): 46–54 [DOI] [PubMed] [Google Scholar]
- 22. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater 2011; 7(6): 2355–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haimi S, Gorianc G, Moimas L, et al. Characterization of zinc-releasing three-dimensional bioactive glass scaffolds and their effect on human adipose stem cell proliferation and osteogenic differentiation. Acta Biomater 2009; 5(8): 3122–3131 [DOI] [PubMed] [Google Scholar]
- 24. Liu G, Zhao L, Cui L, et al. Tissue-engineered bone formation using human bone marrow stromal cells and novel beta-tricalcium phosphate. Biomed Mater 2007; 2(2): 78–86 [DOI] [PubMed] [Google Scholar]
- 25. Pountos I, Georgouli T, Henshaw K, et al. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma 2010; 24(9): 552–556 [DOI] [PubMed] [Google Scholar]
- 26. Gautschi OP, Cadosch D, Zellweger R, et al. Apoptosis induction and reduced proliferation in human osteoblasts by rhBMP-2, -4 and -7. J Musculoskelet Neuronal Interact 2009; 9(1): 53–60 [PubMed] [Google Scholar]
- 27. Tadokoro M, Matsushima A, Kotobuki N, et al. Bone morphogenetic protein-2 in biodegradable gelatin and beta-tricalcium phosphate sponges enhances the in vivo bone-forming capability of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med 2011. DOI: 10.1002/term.427 [DOI] [PubMed] [Google Scholar]
- 28. Radin S, Reilly G, Bhargave G, et al. Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res A 2005; 73(1): 21–29 [DOI] [PubMed] [Google Scholar]
- 29. Chou YF, Zuk PA, Chang TL, et al. Adipose-derived stem cells and BMP2: part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect Tissue Res 2011; 52(2): 109–118 [DOI] [PubMed] [Google Scholar]
- 30. Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Adv Immunol 2000; 75: 115–157 [DOI] [PubMed] [Google Scholar]
- 31. Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 2003; 24(2): 218–235 [DOI] [PubMed] [Google Scholar]
- 32. Liu SB, Hu PZ, Hou Y, et al. Recombinant human bone morphogenetic protein-2 promotes the proliferation of mesenchymal stem cells in vivo and in vitro. Chin Med J (Engl) 2009; 122(7): 839–843 [PubMed] [Google Scholar]
- 33. Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109(10): 1292–1298 [DOI] [PubMed] [Google Scholar]
- 34. Ghanaati S, Barbeck M, Orth C, et al. Influence of beta-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater 2010; 6(12): 4476–4487 [DOI] [PubMed] [Google Scholar]
- 35. Gorustovich AA, Roether JA, Boccaccini AR. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B Rev 2010; 16(2): 199–207 [DOI] [PubMed] [Google Scholar]
- 36. Deckers MM, Van Bezooijen RL, Van der Horst G, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 2002; 143(4): 1545–1553 [DOI] [PubMed] [Google Scholar]
- 37. Shoji T, Mifune Y, Matsumoto T, et al. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest 2010; 90(4): 637–649 [DOI] [PubMed] [Google Scholar]
- 38. Gao Q, Tong W, Luria JS, et al. Effects of bone morphogenetic protein-2 on proliferation and angiogenesis in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2010; 39(3): 266–271 [DOI] [PubMed] [Google Scholar]
- 39. Schonmeyr BH, Soares M, Avraham T, et al. Vascular endothelial growth factor inhibits bone morphogenetic protein 2 expression in rat mesenchymal stem cells. Tissue Eng Part A 2010; 16(2): 653–662 [DOI] [PMC free article] [PubMed] [Google Scholar]